Abstract
Multiple chemical sensitivity (MCS) patients usually react to odour compounds and the majority of neuroimaging studies assessed, especially at the cortical level, many olfactory-related correlates. The purpose of the present study was to depict sub-cortical metabolic changes during a neutral (NC) and pure (OC) olfactory stimulation by using a recently validated 18F-2-fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography/computer tomography procedure in 26 MCS and 11 healthy (HC) resting subjects undergoing a battery of clinical tests. Twelve subcortical volumes of interest were identified by the automated anatomical labeling library and normalized to thalamus FDG uptake. In both groups, when comparing OC to NC, the within-subjects ANOVA demonstrated a relative decreased metabolism in bilateral putamen and hippocampus and a relative increased metabolism in bilateral amygdala, olfactory cortex (OLF), caudate and pallidum. The between-groups ANOVA demonstrated in MCS a significant higher metabolism in bilateral OLF during NC. As in HC subjects negative correlations were found in OC between FDG uptake in bilateral amygdala and hippocampus and odor pleasantness scale, the latter positively correlated with MCS subjects’ bilateral putamen FDG uptake in OC. Besides FDG uptake resemblances in both groups were found, for the first time a relative higher metabolism increase in OLF in MCS subjects at rest with respect to HC was found. When merging this aspect to the different subcortical FDG uptake correlations patterns in the two groups, the present study demonstrated to describe a peculiar metabolic index of behavioral and neurological aspects of MCS complaints.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple chemical sensitivity (MCS)/idiopathic environmental illness (IEI) is a relatively common clinical diagnosis in western populations (Alessandrini et al. 2013a). The prevalence of self-reported chemical sensitivity symptoms in population based studies ranges from 9 to 33 % (Dantoft et al. 2014), whereas physician diagnosed MCS or reports of disabling consequences in the form of social and occupational disruptions are much lower, ranging from 0.5 to 6.3 % (Dantoft et al. 2014).
MCS patients usually react to a wide range of everyday chemical compounds, such as petrol, perfume or pesticides (Das-Munshi et al. 2006) by complaining of a wide spectrum of symptoms ranging form headache, fatigue, respiratory symptoms, dizziness, nausea and, especially, dysosmia (Alessandrini et al. 2013a; Kärnekull et al. 2011).
Major criteria to define the clinical spectrum of MCS were introduced by Cullen (1987); however, the discussion on definition and nomenclature reflects the fact that the aetiology of MCS is still unclear and a matter of debate (Das-Munshi et al. 2007; Alessandrini et al. 2013a). Most frequently discussed etiologies include neurogenic inflammation (Bascom et al. 1997), classical conditioning (Winters et al. 2003) and biochemical disruptions (Pall 2003). According to the neural sensitization theory, MCS is attributed to a pathological hyper-reactivity of neurons in olfactory and limbic areas of the brain (Bell et al. 1992). Other MCS theories have also suggested hyper-reactivity in the central nervous system (CNS) as an important aspect (Bascom et al. 1997). The altered CNS responses are hypothesized to be paralleled by increased anxiety, avoidance, anticipatory stress (Bell et al. 1992) and attention bias (Bascom et al. 1997) to chemical exposure. However, it remains unclear which degree of MCS smell perception is dependent on sniffing behaviors and/or attentional mechanism (Siegel and Kreutzer 1997).
A recent investigation reporting 18F-2-fluoro-2-deoxy-d-glucose (FDG)-positron emission tomography (PET)/computer tomography (CT) cortical changes after a pure olfactory stimulation in MCS patients (Chiaravalloti et al. 2015) demonstrated to overcome that possible unintended sensory stimulation (Alessandrini et al. 2014a; Benjamin et al. 2010; Hillert et al. 2007; Orriols et al. 2009, Azuma et al. 2013; Andersson et al. 2014) related to such neuroimaging techniques coupling the olfactory task with the acquisition phase. These aspects could have constituted a possible common bias to the interpretation of the majority of existing data. Thus, it remains unclear to what extent discrepancies in the results of different studies may reflect unwanted attentional behaviors, sensory-specific engagement of different brain regions rather than methodological discrepancies (Alessandrini et al. 2014a).
Besides, the majority of existing studies pointed at assessing only those correlates of olfactory-related changes that could underpin—at cortical level—MCS patients complaints. Due to the fact that primary olfactory areas in the brain exist outside the cerebral cortex and the neural pathways that subserve olfactory processes are strictly related to olfactory-limbic areas (Yeshurun and Sobel 2010), studies in this field could be useful to unravel possible interferences involving peculiar neural olfactory (and related hedonic) pathways before reaching cortical level.
The aim of the present study was to assess the sub-cortical metabolic changes related to a pure olfactory stimulation in resting MCS and healthy (HC) subjects by using a recently validated FDG-PET/CT technique (Alessandrini et al. 2014a; Chiaravalloti et al. 2015) with the purpose of depicting the neural patterns peculiar of MCS.
Subjects and Methods
Participants and Study Design
We included in the study MCS patients admitted to the Regional Center for Diagnosis, Prevention and Treatment of MCS and evaluated at “Tor Vergata” University for those symptoms related to ear–nose–throat complaints. Diagnosis of MCS was achieved according to the US Consensus Criteria for MCS (Anonymous 1999) and the revisions suggested by Lacour et al. (2005) which were operationalized as follows: (1) symptoms are present for at least 6 months; (2) symptoms occur in response to exposure to at least two of 11 common volatile chemicals; (3) co-occurrence of at least one symptom from the CNS and one symptom from another organ system; (4) symptoms cause significant lifestyle changes, (5) symptoms occur when exposed and lessen or resolve when the symptom-triggering agent is removed; (6) symptoms triggered by exposure levels do not induce symptoms in other individuals who are exposed to the same levels.
We also enrolled as control group a population of gender- and age-matched healthy subjects,
Both eligible MCS patients and HC were required to meet the following entry criteria: subjects with diabetes, oncologic or HIV history, neurological and psychiatric or mood disorders, history of surgery, radiation and trauma to the brain were excluded from the study. No patient showed liver or renal abnormalities nor was pregnant or breastfeeding. The peripheral blood of MCS and HC was tested for the usual parameters. A detailed case history was collected for all subjects who underwent ear–nose–throat examination with fiberoptic check of the upper airways. Neurological diseases were excluded with the mini mental state examination and magnetic resonance imaging (MRI). All those conditions that could potentially develop an olfactory dysfunction were considered as exclusion criteria. Thus, patients with sino-nasal disorders or surgery history, head trauma, neuro-psychiatric disorders (Parkinson’s disease, Alzheimer’s disease, schizophrenia, multiple sclerosis and depression), lower airways and/or lung diseases, active hepatitis, cirrhosis, chronic renal failure, Vitamin B12 deficiency, alcohol, tobacco or drug abuse, cerebral vascular accidents, insulin dependent diabetes mellitus, hypothyroidism and Cushing syndrome were not included in the study. Finally, we excluded all subjects taking drugs possibly impacting on FDG uptake and distribution in the brain (Varrone et al. 2009).
The Ethics Committee of ‘‘Tor Vergata’’ University School of Medicine approved the protocol research. The study adhered to the principles of the Declaration of Helsinki and all of the participants provided written informed consent after receiving a detailed explanation of the study.
Experimental Procedure
According to previous studies of this research group (Alessandrini et al. 2014a; Chiaravalloti et al. 2015), all subjects underwent FDG-PET/CT after both a neutral (neutral condition—NC) and pure olfactory stimulation (olfactory condition—OC) by using a ordinary aerosol facial mask containing in its ampoule: only 5 ml of saline sodium chloride (NaCl) 0.9 % in NC and a solution of 1.5 ml of Vanilline 100 % (Dacor Ltd|Sarandrea Ltd) and 5 ml NaCl 0.9 % in OC.
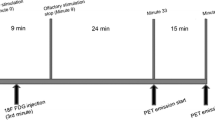
In order to avoid a close radiation exposure and possible expectations related to the presence of an odor, same individuals underwent the second procedure after 1 month by using a computer-generated randomization schedule. Under both conditions the oxygen flow rate was 3.5 l/min. Before the stimulation period each patient lay for 30 min with the eyes closed in a semidarkened and quiet room without any artificial stimulation. Taking into account the kinetics of FDG in the brain and the fact that during olfactory experiments subjects may automatically control their olfactory exposure by altering their respiration and attention to olfaction during the task condition (Savic 2002; Chiaravalloti et al. 2015), FDG was injected after 3 min of a continuous 9-min block stimulation with instructions not to sniff but only inhale the supplied air constantly. The duration of the brain PET acquisition was 15 min and was started 24 min from the end of the olfactory stimulation in all the patients.
PET/CT Scanning
The PET/CT system Discovery ST16 (GE Medical Systems, TN, USA) was used for the whole population in study. This system combines a high-speed ultra 16-detector-row (912 detectors per row) CT unit and a PET scanner with 10,080 bismuth germanate (BGO) crystals in 24 rings with a 128 × 128 matrix. Before and after the FDG injection, hydratation (500 ml of i.v. NaCl) 0.9 %) was performed in order to reduce the pooling of the radiotracer in the kidneys. All subjects had normal serum glucose level and fasted for at least 5 h before the 18F-FDG injection.
Olfactory Assessment and Validated Questionnaires (VQ)
Olfactory performance was assessed in both groups at the time of enrollment with the multiple-forced choice Sniffin’ Sticks Screeinng test (SST; Kobal et al. 1996) in four main domains: odor threshold (OT), odor discrimination (OD), odor identification (OI) and their sum (TDI).
Moreover, all subjects completed the following VQ:
-
Questionnaire of Olfactory Disorders (QOD)—completed only before NC phase—to assess daily life problems due to olfactory impairment. A short modified version of an olfactory-specific QOD developed by Frasnelli and Hummel (Simopoulos et al. 2012) was used. This version consists of 25 statements divided into three general domains: 17 negative (QOD-NS), two positive (QOD-PS), and six socially desired statements (QOD-SD);
-
Zung Self-Rating Anxiety Scale (SAS)—completed only before NC phase—is a 20-item scale with some of the items keyed positively and some negatively on a four-point scale ranging from 1 (none or a little of the time) to 4 (most or all of the time). The final score ranges from 20 to 80, a score between 20 and 44 is considered in the normality range, 45–59 reflects mild to moderate anxiety, 60–74 severe and 75–80 very severe anxiety (Zung and Gianturco 1971);
-
Intensity Visuo-Analogue Scale (IVAS), in which each subject may score from 0 to 10 the subjective intensity of olfactory perception (Lombion-Pouthier et al. 2006) after both NC and OC;
-
Pleasantness Visuo-Analogue Scale (PLEAS), in which each subject may score from 0 to 10 the subjective pleasantness of olfactory perception (Lombion-Pouthier et al. 2006) after the OC.
Statistical Analysis
Differences were analyzed by segmenting sub-cortical regions and comparing 18F-FDG uptake between NC and OC. Anatomical automatic labeling (AAL; Tzourio-Mazoyer et al. 2002) structural VOIs were downloaded from the MarsBaR project download page (http://sourceforge.net/projects/marsbar/) and converted from MarsBaR to nifti format by an in-house modified version of the MarsBaR function mars_roi-s2img.m. The dataset to be analyzed was obtained by an in-house created Matlab-based script that automatically processed mean FDG uptake signal intensities within VOIs and stored them into a matrix in which all values were intra-individually normalized to those of the reference VOI (Pagani et al. 2014). All these calculations were performed by the script in a single step allowing a substantial spare of time and rendering friendly and simple the whole process. The mean FDG uptake values in each of the 12 chosen sub-cortical VOIs (bilateral amygdala, caudate, olfactory, pallidum, putamen and hippocampus) were computed at both NC and OC. Data for each VOI were normalized to the uptake mean of thalami. The choice of thalami as normalizing factor was based on the findings of previous studies in which this region with high gray matter density unlike i.e. cerebellum (Chiaravalloti et al. 2015; Alessandrini et al. 2013b, 2014b) was not found to be affected by MCS.
Statistical differences were calculated by means of a ‘between-groups’ and ‘within-subjects’ analysis of variance (ANOVA). Further, a between-subject ANOVA was performed for all VQ and SST scores, at NC and OC. Age and sex were treated as continuous and categorical predictors, respectively. Bonferroni correction for multiple comparisons was used to test post hoc of significant main effects. Significant cut-off level (α) was set at a P value of 0.01.
In order to assess that data were of Gaussian distribution, D’Agostino K2 normality test has been applied (where the null hypothesis is that the data are normally distributed). Then Spearman’s rank correlation was performed between significant values of subcortical VOIs mean intensity, SST and VQ scores recorded at NC and OC and a Bonferroni correction for multiple comparisons has been applied (STATISTICA 7 package for Windows).
Results
Subjects
Thirty-one consecutive MCS patients were enrolled. Among them, three were using antidepressant drugs, one reported history of alcohol abuse, one of hypothyroidism and were excluded.
Therefore, 26 MCS patients (20 women and 6 men, mean age 46.7 ± 11 years) met the eligible criteria and were included in the study. The control group (HC) consisted of 11 right-handed healthy individuals (six women and five men; mean age 45.7 ± 11 years). Table 1 depicts main sociodemographic variables of HC and MCS subjects.
PET/CT Data
In both MCS and HC subjects a relative decreased metabolism (P < 0.01) in bilateral putamen and hippocampus was found at OC as compared to NC. Conversely, a relative increased metabolism (P < 0.01) in bilateral amygdala, caudate, pallidum and olfactory cortex (OLF)—including olfactory tubercle, anterior olfactory nucleus, piriform cortex, anterior cortical nucleus of the amygdala, periamygdaloid cortex, and entorhinal cortex (Déjerine 1980; Sakamoto et al. 1999; Tzourio-Mazoyer et al. 2002)—was found in OC as compared to NC (Tables 2, 3; Fig. 1).
3D rendering showing in green and red colour the VOIs (with exception of olfactory cortex) in which FDG uptake was significantly lower and higher, respectively, at OC as compared to NC in both MCS and HC subjects. Details of the FDG uptake means and standard deviations in each VOIs are presented for MCS and HC in Tables 1 and 2, respectively (Color figure online)
Further, the between-groups ANOVA with multiple comparisons correction demonstrated that MCS individuals had a significant higher metabolism (P < 0.05) in bilateral OLF during NC, as compared to HC (Fig. 2).
Sagittal (on the top-right), transverse (on the bottom-left) and frontal (on the top-left) view of significant higher metabolism (in red colour) in bilateral olfactory cortex (Olf) during NC in MCS subjects as compared to HC. On the bottom-right, the diagram of the FDG uptake mean and standard deviations in MCS (red colour) and HC (blue colour) subjects showing the between-group approach and significant effect in bilateral Olf. Amy amygdala, Cau caudate, Pall pallidum, Put putamen, Hyp hippocampus, L left, R right (Color figure online)
Olfactory Assessment and Validated Questionnaires (VQ)
A significant statistical increase in QOD NS, SAS, OT, TDI and IVAS OC scores was found in MCS as compared to HC subjects. Conversely, a significant statistical increase in PLEAS scores was found in HC as compared to MCS subjects (detailed means and standard deviations are shown in Table 4).
Correlation Analysis
In HC subjects negative correlations were found in OC between PLEAS scores and FDG uptake in left (Fig. 3a) and right (Fig. 3b) amygdala (r = −0.60 and −0.70, respectively, P < 0.01) and left (Fig. 3c) and right (Fig. 3d) hippocampus (r = −0.68 and −0.65, respectively, P < 0.01).
Diagrams showing HC and MCS correlations analysis (in green and lilac colour, respectively) between VOIs FDG uptake and PLEAS scores during OC: negative correlation between PLEAS scores and FDG uptake in left (a) and right (b) amygdala (yellow colour) and left (c) and right (d) hippocampus (in yellow colour); positive correlation between left (e) and right (f) putamen (in yellow colour) FDG uptake and PLEAS scores (Color figure online)
In MCS subjects Spearman’s rank order correlations proved in OC a significant positive correlation between left (Fig. 3e) and right (Fig. 3f) putamen FDG uptake and PLEAS scores (r = 0.48 and 0.47, respectively, P < 0.01).
Discussion
The first interesting finding of the present study is the significant (P < 0.05) relative higher FDG uptake during NC in MCS OLF with respect to HC (Fig. 2). Beside a relative paucity of information about etiopathological underpinning and olfactory-related brain responses of in MCS, diverse theories have been proposed in previous indexed studies (Andersson et al. 2014; Hillert et al. 2007; Azuma et al. 2013; Chiaravalloti et al. 2015; Orriols et al. 2009).
The relative FDG uptake increase found in the present study in MCS patients OLF at rest when compared to HC is in line with those theories assessing CNS hypereactivity and limbic kindling as key nodes in pathophysiological underpinnings of MCS (Bascom et al. 1997; Winder 2002). Agreeing with those arguments suggesting both attention bias and over-ridden neural behaviour to chemical exposure in MCS patients (Andersson et al. 2009; Witthöft et al. 2006; Winder 2002), this finding could depict for the first time a subcortical resting state correlate of hyper-sensitized neural structure before reaching those secondary/associative olfactory ones (such as OFC, PHG) in which emotional/affective/over-attentional processes could be residing (Qureshy et al. 2000; Kroupi et al. 2012; Keller 2011) and secondary MCS disease-related processes could be nestled (Hillert et al. 2007; Orriols et al. 2009; Hillert et al. 2013).
Further, these data appear more relevant especially when merged with the wider FDG uptake found at rest in frontal–prefrontal cortex and ACC in the same sample of HC subjects with respect to the same sample of MCS patients (Chiaravalloti et al. 2015). In particular we could hypothesize the found relative hyperactivation in primary olfactory structures (not counterbalanced by the same HC cortical activity and the same ability in controlling the arousing stimuli) as neural—especially olfactory—behavioural changes related to low-level chemical exposures before emerging at mind level (Winder 2002).
Secondly, a relative decreased metabolism in bilateral putamen and hippocampus and a relative increased metabolism in bilateral caudate, pallidum, amygdala and OLF in OC as compared to NC in both MCS and HC subjects were found (Tables 2, 3; Fig. 1).
This network identified analyzing FDG-PET/CT data by the AAL atlas template (Tzourio-Mazoyer et al. 2002) is known to be in large part the primary target of olfactory bottom-up inflow, and our results are in accordance with previous functional and lesional neuroimaging olfactory studies (Hillert et al. 2007; Gottfried 2006; Bitter et al. 2010) depicting the olfactory pathways as fairly distinct from other senses’ anatomical organization.
As previously reported (Alessandrini et al. 2014a; Chiaravalloti et al. 2015), the improved sensitivity of the state-of-the-art PET camera allowed to explore olfactory neural underpinnings in a defined ecologic condition, possibly avoiding cortical activation related to unwanted attentional processes and odor sensitivity enhancement (Åhs et al. 2013). Upon the present experimental conditions PET studies might be considered the same way as a functional MRI (fMRI) study in which the temporal resolution is in the range of minutes instead of seconds (Scarmeas et al. 2004).
During OC metabolic changes relate mainly to the administered olfactory stimulus and the difference with NC condition can be inferred by regional metabolism intensities differences.
Thus, the relative OC-related FDG uptake increase found in MCS and HC in amygdala, pallidum, OLF and caudate might represent a peculiar subcortical network related to the pure olfactory condition. As OLF (and its sub-regions including olfactory tubercle, anterior olfactory nucleus, piriform cortex, anterior cortical nucleus of the amygdala, periamygdaloid cortex, and entorhinal cortex [Déjerine 1980; Sakamoto et al. 1999; Tzourio-Mazoyer et al. 2002]) OC-related FDG uptake increase in both groups is consistent with many previous pure olfactory studies in healthy and MCS subjects (i.e. Wang et al. 2005; Hillert et al. 2007), amygdala involvement seems to be coherent with its peculiar role in encoding odorant stimuli (Nigri et al. 2013) and with its engagement in the reward-emotion network (Tomasi and Volkow 2011). Furthermore, the OC-related caudate and pallidum relative FDG uptake increase reflects the general trend in literature for which they are thought to be encompassed in the task-positive network which is involved in cognitive control during task performance (Dosenbach et al. 2007), playing an important role in attention (Corbetta and Shulman 2002), alertness (Cavanna 2007), externally driven cognition, and working memory (Corbetta and Shulman 2002).
In line with Magistretti’s observations (2006)—for which the notion of baseline implies that during a particular task not only activation is observed but also deactivation in certain areas resembling the organized default mode network (Raichle et al. 2001)—here we found both in MCS and HC a peculiar putaminal and hippocampal relative decreased FDG uptake during OC as compared to NC. Interestingly, previous elegant works (De Luca et al. 2006; Vincent et al. 2008) assessed these structures as encompassed in task negative network related to patterns described as ‘‘deactivated’’ during active tasks in PET cerebral blood flow studies (Shulman et al. 1997; Mazoyer et al. 2001).
However, discrepancies at cortical and subcortical level are present between this study and previous ones (i.e. hyperactivation under odorant stimulation in prefrontal cortex [Azuma et al. 2013]), cuneus–precuneus and ACC [Hillert et al. 2007]). These inconsistences might derive from diverse technical strategies in olfactory stimulation (i.e. odours delivery, odour compounds), neuroimaging techniques and patients enrollment procedures.
VQ Between-Groups Differences and Correlation Analysis
MCS subjects demonstrated a pattern of responses among VQ that is generally in accordance with the literature (Alessandrini et al. 2013a) and resulted, when compared to HC, in scoring significantly higher VAS OC, lower PLEAS OC, and to be more affected by anxiety and worsened by olfactory-related quality of life (Table 4).
Furthermore, a global increase in SST scoring was demonstrated. However, despite this test might not be properly suited for MCS dysosmia symptoms, it represents the more reliable forced-choice olfactory instrument developed to cluster subjects among their olfactory performance (Kobal et al. 1996).
Moreover, when performing correlation analysis between VQ scores and FDG uptake in both groups it was possible to highlight two distinct patterns. In MCS a positive correlation was found during OC between FDG uptake in bilateral putamen and PLEAS (Fig. 3e, f). Conversely, in HC a negative correlation was found during OC between FDG uptake in both bilateral amygdala and hippocampus and PLEAS (Fig. 3a–d).
In line with the knowledge postulating amygdala and hippocampus sending dense projections to the olfactory bulb (Carmichael et al. 1994) (supplying numerous physiological routes for modulation of olfactory information processing [Gottfried 2006]) the present study showed a sort of relative competing influences during OC in HC subjects among amygdala and hippocampus on PLEAS.
Besides emotional experience of odor coding results of activation along independent dimensions of intensity and valence (Anderson et al. 2003; Gottfried 2006) amygdala and hippocampus were pointed as key nodes in olfactory-related coding neural stream (i.e. activity in the first structure was previously associated to unpleasant odour compounds [Hudry et al. 2003]) and these regions are thought to be involved in the process of odor-elicited emotion and odor-associated emotional memory (Kadohisa 2013). However, uncertainty in literature did not provide a definitive association of each dimension with a relative brain structure and under the present study conditions their reverse metabolic behavior and relative correlations could depict a neurobiological and psychological pattern distinguishing the HC olfactory-related behavior.
In turn, the peculiar positive correlation in MCS patients during OC between FDG uptake in bilateral putamen and PLEAS could represent a possible retuning of the physiological modulation of olfactory information processing. This aspect could be best grounded when taking into account (i) recent fMRI studies in which the putamen was highlighted as an important structure for stimulus–action coding (Haruno and Kawato 2006) and (ii) previous olfactory SPECT studies in which chemical challenge responses in MCS individuals have been suggested to be related more to their expectations and prior beliefs than to the chemical itself (Das-Munshi et al. 2006).
According to those inferences previously outlined by Carpenter (2003), differences in correlations between odor valence scoring and diverse sub-cortical regions between MCS and HC subjects could point attention on possible neuropathological underpinning of MCS, suggesting a repository of the same olfactory-related function as shifted and hosted in diverse regions.
Conclusion
As compared to previous MCS neuroimaging studies the reliability of the present findings are strengthened by the robust sample of recruited subjects and by the strict MCS patients diagnosis. Moreover, for the first time a relative higher metabolism increase in OLF in MCS subjects with respect to HC was found during resting state. In turn, a different subcortical FDG uptake correlation with odor valence processing was depicted in the two groups.
Even though further investigations in this field are needed, we believe this study to be useful in describing a peculiar metabolic index of behavioral and neurological aspects of MCS/IEI complaints.
References
Åhs F, Miller SS, Gordon AR, Lundström JN (2013) Aversive learning increases sensory detection sensitivity. Biol Psychol 92:135–141
Alessandrini M, Micarelli A, Bruno E, Ottaviani F, Conetta M, Cormano A, Genovesi G (2013a) Intranasal administration of hyaluronan as a further resource in olfactory performance in multiple chemical sensitivity syndrome. Int J Immunopathol Pharmacol 26:1019–1025
Alessandrini M, Pagani M, Napolitano B, Micarelli A, Candidi M, Bruno E et al (2013b) Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS One 8:e57596
Alessandrini M, Micarelli A, Chiaravalloti A, Candidi M, Bruno E, Di Pietro B, Schillaci O, Pagani M (2014a) Cortico-subcortical metabolic correlates of olfactory processing in healthy resting subjects. Sci Rep 4:5146
Alessandrini M, Micarelli A, Chiaravalloti A et al (2014b) Cerebellar metabolic involvement and its correlations with clinical parameters in vestibular neuritis. J Neurol 261:1976–1985
Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N (2003) Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6:196–202
Andersson L, Bende M, Millqvist E, Nordin S (2009) Attention bias and sensitization in chemical sensitivity. J Psychosom Res 66:407–416
Andersson L, Claeson AS, Nyberg L, Stenberg B, Nordin S (2014) Brain responses to olfactory and trigeminal exposure in idiopathic environmental illness (IEI) attributed to smells—an fMRI study. J Psychosom Res 77:401–408
Anonymous (1999) Multiple chemical sensitivity: a 1999 consensus. Arch Environ Health 54:147–149
Azuma K, Uchiyama I, Takano H, Tanigawa M, Azuma M, Bamba I, Yoshikawa T (2013) Changes in cerebral blood flow during olfactory stimulation in patients with multiple chemical sensitivity: a multi-channel near-infrared spectroscopic study. PLoS One 8:e80567
Bascom R, Meggs WJ, Frampton M, Hudnell K, Killburn K, Kobal G, Medinsky M, Rea W (1997) Neurogenic inflammation: with additional discussion of central and perceptual integration of nonneurogenic inflammation. Environ Health Perspect 105:S531–537
Bell IR, Miller CS, Schwartz GE (1992) An olfactory-limbic model of multiple chemical sensitivity syndrome: possible relationships to kindling and affective spectrum disorders. Biol Psychiatry 32:218–242
Benjamin C, Lieberman DA, Chang M, Ofen N, Whitfield-Gabrieli S, Gabrieli JD, Gaab N (2010) The influence of rest period instructions on the default mode network. Front Hum Neurosci 4:218
Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C (2010) Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses 35:407–415
Carmichael ST, Clugnet MC, Price JL (1994) Central olfactory connections in the macaque monkey. J Comp Neurol 346:403–434
Carpenter RHS (2003) Neurophysiology. Arnold, London
Cavanna AE (2007) The precuneus and consciousness. CNS Spectr 12:545–552
Chiaravalloti A, Pagani M, Micarelli A, Di Pietro B, Genovesi G, Alessandrini M, Schillaci O (2015) Cortical activity during olfactory stimulation in multiple chemical sensitivity: a 18F-FDG PET/CT study. Eur J Nucl Med Mol Imaging 42:733–740
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Cullen MR (1987) The worker with multiple chemical sensitivities: an overview. Occup Med State Art Rev 2:655–661
Dantoft TM, Elberling J, Brix S, Szecsi PB, Vesterhauge S, Skovbjerg S (2014) An elevated pro-inflammatory cytokine profile in multiple chemical sensitivity. Psychoneuroendocrinology 40:140–150
Das-Munshi J, Rubin GJ, Wessely S (2006) Multiple chemical sensitivities: a systematic review of provocation studies. J Allergy Clin Immunol 118:1257–1264
Das-Munshi J, Rubin GJ, Wessely S (2007) Multiple chemical sensitivities: review. Curr Opin Otolaryngol Head Neck Surg 15:274–280
De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM (2006) fMRI resting state networks define distinct modes of long-distance interactions in the human brain. NeuroImage 29:1359–1367
Déjerine JJ (1980) Anatomie des centres nerveux. Masson, Paris
Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104:11073–11078
Gottfried JA (2006) Smell: central nervous processing. In: Hummel T, Welge-Lüssen A (eds) Taste and Smell. An Update, Karger, Basel, pp 44–69
Haruno M, Kawato M (2006) Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol 95:948–959
Hillert L, Musabasic V, Berglund H, Ciumas C, Savic I (2007) Odor processing in multiple chemical sensitivity. Hum Brain Mapp 28:172–182
Hillert L, Jovanovic H, Åhs F, Savic I (2013) Women with multiple chemical sensitivity have increased harm avoidance and reduced 5-HT(1A) receptor binding potential in the anterior cingulate and amygdala. PLoS One 8:e54781
Hudry J, Perrin F, Ryvlin P, Mauguiere F, Royet JP (2003) Olfactory short-term memory and related amygdala recordings in patients with temporal lobe epilepsy. Brain 126:1851–1863
Kadohisa M (2013) Effects of odor on emotion, with implications. Front Syst Neurosci 7:66
Kärnekull SC, Jönsson FU, Larsson M, Olofsson JK (2011) Affected by smells? Environmental chemical responsivity predicts odor perception. Chem Senses 36:641–648
Keller A (2011) Attention and olfactory consciousness. Front Psychol 2:380
Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S (1996) ‘‘Sniffin’ sticks’’: screening of olfactory performance. Rhinology 34:222–226
Kroupi E, Yazdani A, Vesin JM, Ebrahimi T (2012) Multivariate spectral analysis for identifying the brain activations during olfactory perception. Conf Proc IEEE Eng Med Biol Soc 2012:6172–6175
Lacour M, Zunder T, Schmidtke K, Vaith P, Scheidt C (2005) Multiple chemical sensitivity syndrome (MCS)—suggestions for an extension of the U.S. MCS-case definition. Int J Hyg Environ Health 208:141–151
Lombion-Pouthier S, Vandel P, Nezelof S, Haffen E, Millot JL (2006) Odor perception in patients with mood disorders. J Affect Disord 90:187–191
Magistretti PJ (2006) Neuron-glia metabolic coupling and plasticity. J Exp Biol 209:2304–2311
Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N (2001) Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54:287–298
Nigri A, Ferraro S, D’Incerti L, Critchley HD, Bruzzone MG, Minati L (2013) Connectivity of the amygdala, piriform, and orbitofrontal cortex during olfactory stimulation: a functional MRI study. NeuroReport 24:171–175
Orriols R, Costa R, Cuberas G, Jacas C, Castell J, Sunyer J (2009) Brain dysfunction in multiple chemical sensitivity. J Neurol Sci 287:72–78
Pagani M, De Carli F, Morbelli S, Öberg J, Chincarini A, Frisoni GB, Caroli A, Perneczky R, Drzezga A, van Berckel BNM, Ossenkoppele R, Guedj E, Didic M, Brugnolo A, Picco A, Arnaldi D, Ferrara M, Buschiazzo A, Sambuceti G, Nobili F (2014) Region of interest-based [18F]fluorodeoxyglucose PET identification of patients with MCI due to Alzheimer disease and healthy controls. An European Alzheimer Disease Consortium (EADC) study. Neuroimage Clin 7:34–42
Pall ML (2003) Elevated nitric oxide/peroxynitrite theory of multiple chemical sensitivity: central role of N-methyl-D-aspartate receptors in the sensitivity mechanism. Environ Health Perspect 111:1461–1464
Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, Itoh M, Schormann T, Zilles K, Fukuda H (2000) Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol 84:1656–1666
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001) A default mode of brain function. Proc Natl Acad Sci USA 98:676–682
Sakamoto N, Pearson J, Shinoda K, Alheid GF, de Olmos JS, Heimer L (1999) The human basal forebrain. Part I. An overview. In: Bloom FE, Bjorklund A, Hokfelt T (eds) Handbook of chemical neuroanatomy: the primate nervous system, Part III, vol 15. Elsevier, Amsterdam, pp 1–13
Savic I (2002) Brain imaging studies of the functional organization of human olfaction. Neuroscientist 8:204–211
Scarmeas N, Habeck CG, Zarahn E, Anderson KE, Park A, Hilton J, Pelton GH, Tabert MH, Honig LS, Moeller JR, Devanand DP, Stern Y (2004) Covariance PET patterns in early Alzheimer’s disease and subjects with cognitive impairment but no dementia: utility in group discrimination and correlations with functional performance. Neuroimage 23:35–45
Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997) Common blood flow changes across visual tasks. II. Decreasesin cerebral cortex. J Cogn Neurosci 9:648–663
Siegel S, Kreutzer R (1997) Pavlovian conditioning and multiple chemical sensitivity. Environ Health Perspect 2:521–526
Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V (2012) Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. Laryngoscope 122:1450–1454
Tomasi D, Volkow ND (2011) Association between functional connectivity hubs and brain networks. Cereb Cortex 9:2003–2013
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K, European Association of Nuclear Medicine Neuroimaging Committee (2009) EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging 36:2103–2110
Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008) Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342
Wang J, Eslinger PJ, Smith MB, Yang QX (2005) Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A 60:510–514
Winder C (2002) Mechanisms of multiple chemical sensitivity. Toxicol Lett 128:85–97
Winters W, Devriese S, Van-Diest I, Nemery B, Veulemans H, Eelen P, Van de Woestijne K, Van den Bergh O (2003) Media warnings about environmental pollution facilitate the acquisition of symptoms in response to chemical substances. Psychosom Med 65:332–338
Witthöft M, Gerlach AL, Bailer J (2006) Selective attention, memory bias, and symptom perception in idiopathic environmental intolerance and somatoform disorders. J Abnorm Psychol 115:397–407
Yeshurun Y, Sobel N (2010) An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. Annu Rev Psychol 61:219–241
Zung WW, Gianturco JA (1971) Personality dimension and the Self-Rating Depression Scale. J Clin Psychol 27:247–248
Acknowledgments
The authors express sincerely thanks to MichelAngela Studio for skillful and creative craftsmanship in preparing illustrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Alessandrini, M., Micarelli, A., Chiaravalloti, A. et al. Involvement of Subcortical Brain Structures During Olfactory Stimulation in Multiple Chemical Sensitivity. Brain Topogr 29, 243–252 (2016). https://doi.org/10.1007/s10548-015-0453-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10548-015-0453-3