Abstract
Purpose
To explore the potential complementary value of PET/CT and dynamic contrast-enhanced MRI in predicting pathological response to neoadjuvant chemotherapy (NAC) of breast cancer and the dependency on breast cancer subtype.
Methods
We performed 18F-FDG PET/CT and MRI examinations before and during NAC. The imaging features evaluated on both examinations included baseline and changes in 18F-FDG maximum standardized uptake value (SUVmax) on PET/CT, and tumour morphology and contrast uptake kinetics on MRI. The outcome measure was a (near) pathological complete response ((near-)pCR) after surgery. Receiver operating characteristic curves with area under the curve (AUC) were used to evaluate the relationships between patient, tumour and imaging characteristics and tumour responses.
Results
Of 93 patients, 43 achieved a (near-)pCR. The responses varied among the different breast cancer subtypes. On univariate analysis the following variables were significantly associated with (near-)pCR: age (p = 0.033), breast cancer subtype (p < 0.001), relative change in SUVmax on PET/CT (p < 0.001) and relative change in largest tumour diameter on MRI (p < 0.001). The AUC for the relative reduction in SUVmax on PET/CT was 0.78 (95 % CI 0.68–0.88), and for the relative reduction in tumour diameter at late enhancement on MRI was 0.79 (95 % CI 0.70–0.89). The AUC increased to 0.90 (95 % CI 0.83–0.96) in the final multivariate model with PET/CT, MRI and breast cancer subtype combined (p = 0.012).
Conclusion
PET/CT and MRI showed comparable value for monitoring response during NAC. Combined use of PET/CT and MRI had complementary potential. Research with more patients is required to further elucidate the dependency on breast cancer subtype.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neoadjuvant chemotherapy (NAC), also referred to as ‘preoperative’ or ‘primary systemic’ therapy, is the standard treatment for locally advanced breast cancer. NAC has several advantages. First, by reducing the size of the tumour, it may allow breast-conserving surgery instead of mastectomy in about 16 % [1] up to 37 % [2] of all patients. Second, monitoring the effects treatment during NAC enables adaptation of the treatment in case of an unfavourable tumour response. Third, NAC offers an excellent platform for translational research, since the molecular characteristics of breast cancer can be directly related to chemosensitivity.
Results from several studies have demonstrated superior disease-free survival in patients who achieve a pathological complete response (pCR) [3, 4]. Hence, achieving pCR is an important treatment objective, particularly in triple-negative tumours and tumours positive for human epidermal growth factor receptor 2 (HER2) [3]. In theory, response monitoring during treatment may help to predict which patients will achieve the desired pCR at a time when a change of chemotherapy regimen would still be practical. With this strategy administration of ineffective treatment can be limited, unnecessary drug toxicity may be decreased, and more cycles of effective chemotherapy can be administered before surgery. Moreover, some studies have suggested an improvement in outcome after treatment modification during NAC [5–7]. Dynamic contrast-enhanced MRI is frequently used to evaluate the effects of treatment, but its predictive value is not perfect and it performs relatively poorly in oestrogen receptor (ER)-positive/HER2-negative disease [8–10]. This limitation has led to the investigation of other imaging strategies. In this context, the role of 18F-FDG PET/CT is under investigation. Promising but varying results have been reported [11–13], but the patient populations studied have been relatively small and/or heterogeneous.
PET/CT visualizes changes in glucose metabolism, whereas contrast-enhanced MRI depicts changes in morphology and perfusion. The rationale behind the possible complementary value of PET/CT and MRI for monitoring tumour response is based on this difference in visualization of underlying tumour characteristics. If this complementary value could be exploited effectively, new strategies might be developed to improve the accuracy of evaluating response during NAC.
The value of breast cancer response monitoring using either PET/CT or MRI alone has been previously reported. The performance of each of these techniques was shown to differ markedly among breast cancer subtypes [10, 14, 15]. Moreover, several investigators have reported higher baseline FDG uptakes in triple-negative tumours than in tumours of other breast cancer subtypes [14, 16].
The aim of the present study was to investigate the complementary value of the combined use of PET/CT and MRI for monitoring response during NAC. In this context, the differences among breast cancer subtypes were also considered.
Patients and methods
Patient selection
From September 2008 we included patients who were scheduled to receive NAC in a prospective single-institution study of response monitoring. All patients had primary invasive breast cancer of at least 3 cm in diameter and/or at least one tumour-positive axillary lymph node. The institutional review board approved this study and written informed consent was obtained from all patients.
Pretreatment pathology
Three core biopsies from the primary tumour were taken before NAC to determine the histological type and for immunohistochemical staining. All biopsies were reviewed by an experienced breast pathologist (J.W.). Samples were scored as positive for ER and progesterone receptor (PR) on immunohistochemistry when at least 10 % of the tumour cells showed staining. Samples were scored as HER2-positive when either strong membrane staining (3+) was observed on immunohistochemistry or if chromogenic in situ hybridization revealed amplification of the HER2 gene. We categorized breast cancer subtypes as HER2-positive (ER and PR either positive or negative), ER-positive/HER2-negative, and triple-negative (ER-negative, PR-negative and HER2-negative). Grade was determined using the criteria of Bloom and Richardson with modification [17].
Treatment
Patients with HER2-positive tumours were treated with a trastuzumab-based regimen consisting of paclitaxel (70 mg/m2/week), trastuzumab (2 mg/kg/week) and carboplatin (AUC 3 mg.h/ml/week; PTC) in three cycles of 8 weeks. In week 7 and 8 of each course only trastuzumab was given [18]. Patients with HER2-negative tumours began NAC with three courses of ddAC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 on day 1, every 14 days, with PEG-filgrastim on day 2). In the context of a larger study, three courses of docetaxel and capecitabine (docetaxel 75 mg/m2 on day 1, every 21 days, and capecitabine 2 × 1,000 mg/m2 on days 1–14; DC) were administered when an ‘unfavourable response’ was detected by MRI evaluation after the three initial courses. When a ‘favourable response’ was achieved, three further courses of ddAC were administered. The findings from this neoadjuvant programme have been reported previously [19]. Criteria for favourable and unfavourable MRI responses have been published elsewhere [20]. According to institutional guidelines, based on consensus in a multidisciplinary meeting, the chemotherapy regimen was changed to a theoretically noncross-resistant regimen in patients with an unfavourable response [20] after three courses. The second-line regimen consisted of capecitabine (850 mg/m2 twice daily orally on days 1–14) and docetaxel (75 mg/m2 intravenously on day 1; CD). Three courses were given, every 3 weeks. After NAC, all patients underwent breast-conserving surgery or a mastectomy.
PET/CT and MRI
Tumour response was monitored with both PET/CT and MRI. PET/CT and MRI examinations were performed before the start of chemotherapy (baseline examinations), and repeated at the end of the first of three 8-week courses of chemotherapy for HER2-positive tumours and after three of six cycles of chemotherapy for HER2-negative tumours (interim examinations). For the PET/CT scans, patients were prepared with a 6-h fasting period. Blood glucose levels were required to be <10 mmol/l. An FDG dose of 180–240 MBq was given intravenously, depending on body mass index. The PET/CT scan was performed after a resting period of 60 ± 10 min using a whole-body PET/CT scanner (Gemini TF; Philips, Cleveland, OH). With the patient in prone position (“hanging breast” configuration identical to positioning for MRI), a PET scan (3 min per bed position) of the chest was performed with image reconstruction to 2 × 2 × 2 mm voxels. PET acquisition was preceded by a low-dose CT scan (40 mAs, 2-mm slices). Subsequently, as a baseline staging procedure, a standard supine whole-body PET/CT scan (1.30 min per bed position, 5.0-mm CT slices) was performed from the base of the skull to the upper half of the femora. During NAC, only the breast PET/CT was repeated for response monitoring using a similar acquisition, time after FDG injection and patient positioning as those used in baseline imaging. All PET/CT examinations in the current study were performed using the same scanner. A panel of experienced readers (B.K., W.V. and R.V.O.) evaluated the images. We have described this procedure previously in more detail [14].
MRI was performed with a 3.0-T scanner (Achieva, Philips, Best, The Netherlands) using a dedicated seven-element SENSE breast coil. Both breasts were simultaneously imaged in prone orientation. An unenhanced coronal 3-D THRIVE SENSE T1-weighted sequence was acquired. A bolus (15 mL) of gadolinium-containing contrast agent (Dotarem 0.5 mmol/ml; Guerbet; Aulnay-sous-Bois, France) was administered intravenously at 3 mL/s using a power injector followed by a bolus of 30 mL of saline solution. Subsequently, five consecutive series were acquired with a voxel size of 1.1 × 1.1 × 1.1 mm. The following scanning parameters were used: acquisition time 90 s, TR/TE 4.4/2.3 ms, flip angle10°, FOV 360 mm.
All images were assessed according to a previously described procedure [20, 21] by a radiologist (C.L.) experienced in breast MRI. In brief: a viewing station that permitted simultaneous viewing of two series reformatted and linked in three orthogonal directions was used for the interpretation of the breast MR images. The viewing station displayed all image series (unenhanced and contrast-enhanced), comprising subtraction images at initial enhancement (90 s after contrast agent injection), at late enhancement (450 s after contrast agent injection) and maximum intensity projections of both breasts. The subtraction images were also colour-coded, representing different rates and shapes of enhancement curves. These colour codes were categorized from red (initial enhancement ≥100 % with washout late enhancement) to green (initial enhancement <100 % with persisting late enhancement). In accordance with Kuhl et al. [22], we categorized the enhancement curves as type 1, 2 and 3, where type 1 represented a persisting shape, type 2 a plateau and type 3 a wash-out enhancing shape. The largest tumour diameter was assessed in the three reformatted planes (sagittal, axial and coronal) at initial and late enhancement.
Postsurgery pathology
The surgical resection specimens were assessed according to EUSOMA (European Society of Breast Cancer Specialists) guidelines [23, 24] by an experienced breast pathologist (J.W.). Complete absence of residual invasive tumour cells irrespective of carcinoma in situ was defined as pCR. A small number of scattered tumour cells left on microscopy was considered near pCR. A combination of both near pCR and pCR was classified as (near-)pCR. The presence of viable residual disease in the resection specimen due to tumour progression, stable disease or partial response to NAC, was classified as non-pCR. Pathological response was assessed dichotomously: (near-)pCR versus non-pCR. Radiographs of the specimens were obtained and the pathologist had access to the presurgery breast images. Axillary response was not evaluated in the current study.
Statistics
The relative change in maximum standardized uptake value (SUVmax) was calculated using an equation comparable to that used by Hatt et al. [25]:
The relative change in tumour size on MRI was calculated using the equation:
With these equations, a negative value indicates a reduction, a value of zero no change, and a positive value tumour progression on imaging. For example, the relative change of a tumour with an SUVmax of 12 at the start of treatment and a value of 3 during treatment would be −75 % (i.e. 75 % reduction).
SPSS (version 20.0; SPSS Chicago, Illinois) was used for all analyses. Univariate analyses were done using Student t test for normally distributed continuous variables and the Mann Whitney U test for nonnormally distributed variables. Multivariate binary logistic regression was performed using backwards step-wise feature selection with a probability for entry 0.05 and a probability for removal 0.10. Features that were significant in the univariate analysis (p ≤ 0.05) were entered in the multivariate analysis.
Receiver operating characteristics (ROC) curve analysis with area under the curve (AUC) measurement were used to investigate relationships between patient, tumour characteristics and imaging characteristics and the tumour response on pathology after surgery. In addition, these relationships were studied separately for the different breast cancer subtypes (HER2-positive, ER-positive/HER2-negative and triple-negative).
Results
Included in the study were 93 women with breast cancer of stage 2 or higher. Their mean age was 47.8 years (25.8–68.1 years). The vast majority of the tumours (91 %) were invasive ductal cancers. The baseline characteristics of the cohort are presented in Table 1.
Of the 93 patients, 43 (46.2 %) achieved a (near-)pCR and 50 (53.8 %) had residual disease (non-pCR) as shown in Table 2.
Age
Age was significantly associated with (near-)pCR (p = 0.033) as shown in Table 2. Of the 43 patients with (near-)pCR and of the 50 patients with non-pCR, 28 (65.1 %) and 27 (54 %), respectively, were younger than 50 years (p = 0.28). This distribution, however, varied among the different breast cancer subtypes: of the patients with a HER2-positive, ER-positive and triple–negative tumour who achieved (near-)pCR 8 of 19 (59.7 %, p = 0.55), 2 of 5 (40 %, p = 0.63) and 17 of 19 (89.5 %, p = 0.001), respectively, were younger than 50 years.
PET/CT
SUVmax on interim PET/CT and relative change in SUVmax on PET/CT were significantly associated with (near-)pCR (p = 0.007 and <0.001, respectively; Table 2). Residual disease was found on pathology in 21 of 58 tumours (36.2 %) with ≥50 % reduction in SUVmax and in 5 of 19 tumours (26.3 %) with ≥80 % reduction in SUVmax.
MRI
The largest tumour diameters at initial as well as late enhancement were significantly associated with (near-)pCR (p < 0.001 for both). Accordingly, there was a significant association between relative change in largest tumour diameter at both initial and late enhancement and (near-)pCR (p < 0.001 for both; Table 2). Of 93 tumours, 23 (24.7 %) showed a reduction of 75 % or more in largest tumour diameter at initial enhancement on interim MRI. Of these 23 tumours, 20 (87.0 %) achieved a (near-)pCR on pathology.
Of the 43 tumours with a (near-)pCR, 18 (42 %) had no residual enhancement on the interim MRI, 9 (21 %) had a type 1 curve, 14 (33 %) a type 2 curve, and 2 (5 %) a type 3 curve. Of the 50 tumours with a non-pCR, 3 (6 %) had no more enhancement on the interim MRI, 5 (10 %) a type 1 curve, 29 (58 %) a type 2 curve, and 13 (26 %) a type 3 curve.
Breast cancer subtypes
There was a higher rate of (near-)pCR in HER2-positive and triple-negative tumours than in ER-positive tumours: 76.0 %, 67.9 % and 12.5 %, respectively (p < 0.001). Typical responses to NAC for various breast cancer subtypes are shown in Fig. 1.
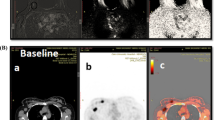
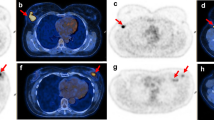
Typical examples of images obtained in patients with tumours of various breast cancer subtypes. a A 39-year-old woman with a HER2-positive tumour showing a good response on MRI and PET/CT and complete response on final pathology. b A 36-year-old woman with an ER-positive/HER2-negative tumour showing a moderate response on MRI and PET/CT and Residual disease on final pathology. c A 32-year-old woman with a triple-negative tumour showing a good response on MRI and PET/CT and complete response on final pathology. Maximum intensity projection MR images (left), MR subtraction images colour-coded for time-vs.-intensity curve type (centre), and SUV on PET/CT (right). The images in the upper rows were obtained prior to treatment and the images in the lower rows during treatment. The colour codes on the subtraction images reflect contrast uptake kinetics: red to green initial enhancement ≥100 % with washout late enhancement to initial enhancement <100 % with persisting late enhancement
Figure 2 shows the relationship between relative changes on PET/CT and MRI in relation to pathological response in various breast cancer subtypes. For HER2-positive tumours, a 100 % reduction in largest tumour diameter on MRI was associated with a wide range of relative changes in SUVmax on PET/CT (−85 to −15 %). For ER-positive/HER2-negative tumours (near-)pCR was never achieved at relative reductions in SUVmax on PET/CT less than 40 %, independent of the reduction in largest tumour diameter on MRI. For triple-negative tumours, (near-)pCR was related to a relative reduction in SUVmax on PET/CT as well as a relative reduction in largest tumour diameter at initial and late enhancement on MRI (Table 3 and Fig. 2).
Multivariate analysis
In the multivariate analysis, relative change in SUVmax on PET/CT, relative change in largest tumour diameter at late enhancement on MRI and breast cancer subtype retained significant associations with (near-)pCR. The results of the multivariate analysis are presented in Table 4.
ROC-curve
The AUC was 0.78 (95 % confidence interval, CI, 0.68–0.88) for the relative reduction in SUVmax on PET/CT and 0.79 (CI 0.70–0.89) for the relative reduction in tumour diameter at late enhancement on MRI. The AUC increased to 0.90 (CI 0.83–0.96) in the final multivariate model with PET/CT, MRI and breast cancer subtype combined (p = 0.012).
Discussion
We explored the potential complementary value of PET/CT and contrast-enhanced MRI for monitoring the response of breast cancer to NAC. We identified changes in imaging features during NAC that were associated with the pathological response after NAC in relation to breast cancer subtype. In the multivariate analysis a large relative reduction in SUVmax on PET/CT, a large relative reduction in the largest tumour diameter at late enhancement on MRI, and breast cancer subtype were independent markers for a (near-)pCR (Table 4). A combination of these features in 93 patients led to an increased AUC, suggesting an improved ability to differentiate between responders and nonresponders to NAC by applying both modalities in combination with knowledge of the breast cancer subtype.
Several studies have investigated PET/CT and MRI in the setting of NAC. These studies focused on comparison between the two techniques rather than on the assessment of their complementary value [26–29]. Moreover, all studies were performed in relatively small patient groups, with the exception of the study by Tateishi et al. [30]. These authors compared MRI and PET/CT in 142 patients and observed a superior accuracy of the latter for predicting pCR to NAC. The potential impact of breast cancer subtype was, however, not reported. To our knowledge no studies have been reported with a design comparable to the current study.
Controversies in NAC imaging studies
Some key issues in NAC imaging studies are currently under investigation. Lack of standardization across studies hampers generalization and comparison of study results. First, there is no consensus with regard to the optimal time-point for performing the examination(s) during NAC. Usually, examinations are done at baseline, but the time-points for the subsequent examination(s) vary: after the first cycle of NAC, after completion of half the NAC course and sometimes after completion of NAC shortly before surgery. The interim examinations in this study were done half way through the treatment. A previous study demonstrated that PET/CT is able to monitor therapy response after one cycle of chemotherapy, but it is less accurate than after completion of half the treatment [31]. Second, it is still under investigation which PET parameter correlates best with response to chemotherapy. The values of parameters such as SUVmean, SUVpeak and total lesion glycolysis are not yet properly validated [25, 32]. In the current study we decided to focus on SUVmax as the most straightforward and reproducible parameter, particular in patients with a good metabolic response. Another consideration was that most other studies have also used SUVmax. This enables generalization and comparison of results among studies.
Third, there is no consensus as to which threshold values should be employed for PET/CT or MRI to assess breast cancer response during NAC. The response criteria for solid tumours (RECIST) are widely applied and recently PERCIST was proposed for PET/CT monitoring [33, 34]. Criteria for response monitoring in breast cancer are not yet standardized, mainly due to varied chemotherapeutic regimens and differences in time-points for response monitoring across studies. In the current study, we employed ROC analysis based on continuous values rather than choosing a specific threshold value on the ROC curve. An important reason for this choice was the relatively large CIs associated with a specific choice of threshold given the current number of our included patients. The AUC indicated, however, that PET/CT and MRI overall provide complementary information. Fourth, different endpoints were used, and different definitions of pCR were applied in NAC studies [9, 35]. An international panel of representatives of breast cancer clinical research groups recommended that pCR should be based on histopathological assessment, including absence of invasive cancer in both breast and lymph nodes [36]. In the current exploratory research, we primarily focused on the ability of interim PET/CT and MRI scans of the breast to detect changes in the primary tumour associated with response on pathology after surgery.
Current limitations and research prospects
There is emerging evidence that breast cancer subtype plays an important role in response monitoring during NAC. Loo et al. reported the relevance of breast cancer subtype in the accuracy of MRI in monitoring response during NAC [10]. In recent studies, Humbert et al. [16] and Koolen et al. [14] reported differences in SUV decrease on PET when stratifying according to breast cancer subtype. In the current study, for some breast cancer subtypes, response on final pathology was primarily associated with response on PET/CT, for others with response on MRI, and for still others with response according to both modalities. These effects can be considered a reflection of differences in the underlying tumour functions (changes in glucose uptake, morphology and perfusion) and to be related to the breast cancer subtypes that are affected by the treatment.
Although the number of patients in the current exploratory study was relatively large, stratification into subgroups of breast cancer subtypes did not provide sufficient statistical power to address the impact of breast cancer subtype on response prediction with PET/CT and MRI. Particularly in the ER-positive/HER2-negative subgroup (the largest subgroup), the number of responders on final pathology was relatively small (5 of the 40, 12.5 %). Nevertheless, our exploratory analyses may generate hypotheses for further research.
In view of these findings, further research in a larger group of patients may enable us to address several subjects that emerged in this exploratory analysis. The relevance of breast cancer subtype will be studied more thoroughly in the context of the combined use of PET/CT and MRI. In particular, if the observed dependency of response on imaging on breast cancer subtype remains consistent in a larger group of patients, we will be able to establish cost-effective imaging strategies based on breast cancer subtype. These cost-effectiveness studies are currently ongoing. In addition, the observed relationship between a good response on interim MRI and (near-)pCR will be integrated in future studies. More precise cut-off values need to be established for SUVmax reduction on PET/CT, in both the breast and the lymph nodes, combined with cut-off values for size reduction on MRI.
There are indications that other MRI techniques such as diffusion-weighted imaging may be of additional value for monitoring response to therapy [37]. We plan to incorporate these analyses in our further research as well. All the above-mentioned efforts may eventually lead to improved patient-tailored treatment.
Conclusion
In this exploratory analysis, the combined use of interim PET/CT and MRI showed potential for improving the ability to predict final tumour response on pathology during NAC. Additional research in a larger group of patients is needed to further elucidate the dependency on breast cancer subtype.
References
Mieog JS, van der Hage JA, van de Velde CJ. Neoadjuvant chemotherapy for operable breast cancer. Br J Surg. 2007;94:1189–200.
Straver ME, Rutgers EJ, Rodenhuis S, Linn SC, Loo CE, Wesseling J, et al. The relevance of breast cancer subtypes in the outcome of neoadjuvant chemotherapy. Ann Surg Oncol. 2010;17:2411–8.
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat. 2012;132:1049–62.
von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013;31:3623–30.
von Minckwitz G, Untch M, Loibl S. Update on neoadjuvant/preoperative therapy of breast cancer: experiences from the German Breast Group. Curr Opin Obstet Gynecol. 2013;25:66–73.
Smith IC, Heys SD, Hutcheon AW, Miller ID, Payne S, Gilbert FJ, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20:1456–66.
Yuan Y, Chen XS, Liu SY, Shen KW. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysis. AJR Am J Roentgenol. 2010;195:260–8.
Marinovich ML, Sardanelli F, Ciatto S, Mamounas E, Brennan M, Macaskill P, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669–77.
Loo CE, Straver ME, Rodenhuis S, Muller SH, Wesseling J, Vrancken Peeters MJ, et al. Magnetic resonance imaging response monitoring of breast cancer during neoadjuvant chemotherapy: relevance of breast cancer subtype. J Clin Oncol. 2011;29:660–6.
Duch J, Fuster D, Munoz M, Fernández PL, Paredes P, Fontanillas M, et al. PET/CT with [18F]fluorodeoxyglucose in the assessment of metabolic response to neoadjuvant chemotherapy in locally advanced breast cancer. Q J Nucl Med Mol Imaging. 2012;56:291–8.
Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41.
Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72.
Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, et al. FDG PET/CT during neoadjuvant chemotherapy may predict response in ER-positive/HER2-negative and triple negative, but not in HER2-positive breast cancer. Breast. 2013;22:691–7.
Straver ME, Loo CE, Rutgers EJ, Oldenburg HS, Wesseling J, Vrancken Peeters MJ, et al. MRI-model to guide the surgical treatment in breast cancer patients after neoadjuvant chemotherapy. Ann Surg. 2010;251:701–7.
Humbert O, Berriolo-Riedinger A, Riedinger JM, Coudert B, Arnould L, Cochet A, et al. Changes in 18F-FDG tumor metabolism after a first course of neoadjuvant chemotherapy in breast cancer: influence of tumor subtypes. Ann Oncol. 2012;23:2572–7.
Bloom HJ, Richardson W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–77.
Sonke GS, Mandjes IA, Holtkamp MJ, Schot M, van Werkhoven E, Wesseling J, et al. Paclitaxel, carboplatin, and trastuzumab in a neo-adjuvant regimen for HER2-positive breast cancer. Breast J. 2013;19:419–26.
Rigter LS, Loo CE, Linn SC, Sonke GS, van Werkhoven E, Lips EH, et al. Neoadjuvant chemotherapy adaptation and serial MRI response monitoring in ER-positive HER2-negative breast cancer. Br J Cancer. 2013;109:2965–72.
Loo CE, Teertstra HJ, Rodenhuis S, van de Vijver MJ, Hannemann J, Muller SH, et al. Dynamic contrast-enhanced MRI for prediction of breast cancer response to neoadjuvant chemotherapy: initial results. AJR Am J Roentgenol. 2008;191:1331–8.
Gilhuijs KG, Deurloo EE, Muller SH, Peterse JL, Schultze Kool LJ. Breast MR imaging in women at increased lifetime risk of breast cancer: clinical system for computerized assessment of breast lesions initial results. Radiology. 2002;225:907–16.
Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–10.
Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology. 2007;50:409–17.
EUSOMA – European Society of Breast Cancer Specialists. http://www.eusoma.org. Accessed 13 Apr 2014.
Hatt M, Groheux D, Martineau A, Espié M, Hindié E, Giacchetti S, et al. Comparison between 18F-FDG PET image-derived indices for early prediction of response to neoadjuvant chemotherapy in breast cancer. J Nucl Med. 2013;54:341–9.
Park JS, Moon WK, Lyou CY, Cho N, Kang KW, Chung JK. The assessment of breast cancer response to neoadjuvant chemotherapy: comparison of magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography. Acta Radiol. 2011;52:21–8.
Choi JH, Lim HI, Lee SK, Kim WW, Kim SM, Cho E, et al. The role of PET CT to evaluate the response to neoadjuvant chemotherapy in advanced breast cancer: comparison with ultrasonography and magnetic resonance imaging. J Surg Oncol. 2010;102:392–7.
Partridge SC, Vanantwerp RK, Doot RK, Chai X, Kurland BF, Eby PR, et al. Association between serial dynamic contrast-enhanced MRI and dynamic 18F-FDG PET measures in patients undergoing neoadjuvant chemotherapy for locally advanced breast cancer. J Magn Reson Imaging. 2010;32:1124–31.
Dose-Schwarz J, Tiling R, Avril-Sassen S, Mahner S, Lebeau A, Weber C, et al. Assessment of residual tumour by FDG-PET: conventional imaging and clinical examination following primary chemotherapy of large and locally advanced breast cancer. Br J Cancer. 2010;102:35–41.
Tateishi U, Miyake M, Nagaoka T, Terauchi T, Kubota K, Kinoshita T, et al. Neoadjuvant chemotherapy in breast cancer: prediction of pathologic response with PET/CT and dynamic contrast-enhanced MR imaging – prospective assessment. Radiology. 2012;263:53–63.
Koolen BB, Pengel KE, Wesseling J, Vogel WV, Vrancken Peeters MJ, Vincent AD, et al. Sequential F-FDG PET/CT for early prediction of complete pathological response in breast and axilla during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;;41:32–40.
de Langen AJ, Vincent A, Velasquez LM, van Tinteren H, Boellaard R, Shankar LK, et al. Repeatability of 18F-FDG uptake measurements in tumors: a metaanalysis. J Nucl Med. 2012;53:701–8.
Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50 Suppl 1:122S–50S.
Mukai H, Watanabe T, Ando M, Shimizu C, Katsumata N. Assessment of different criteria for the pathological complete response (pCR) to primary chemotherapy in breast cancer: standardization is needed. Breast Cancer Res Treat. 2009;113:123–8.
Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19:1508–16.
Fangberget A, Nilsen LB, Hole KH, Holmen MM, Engebraaten O, Naume B, et al. Neoadjuvant chemotherapy in breast cancer-response evaluation and prediction of response to treatment using dynamic contrast-enhanced and diffusion-weighted MR imaging. Eur Radiol. 2011;21:1188–99.
Acknowledgments
The authors thank Anita Paape, Inge Kemper, Marjo Holtkamp, Margaret Schot, and Jacqueline van Zyll de Jong for their contribution to this study.
Conflicts of interest
None.
Disclosure
This study was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project Breast CARE (grant 03O-104).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pengel, K.E., Koolen, B.B., Loo, C.E. et al. Combined use of 18F-FDG PET/CT and MRI for response monitoring of breast cancer during neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 41, 1515–1524 (2014). https://doi.org/10.1007/s00259-014-2770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2770-2