Abstract
Purpose
We sought to determine whether metabolic volume-based measurements on FDG PET/CT scans could provide additional information for predicting outcome in patients with stage III non-small-cell lung cancer (NSCLC) treated with induction chemotherapy.
Methods
Included in the study were 32 patients with stage III NSCLC who were treated with induction platinum-based chemotherapy followed in 21 by surgery. All patients had an FDG PET/CT scan before and after the induction chemotherapy. Tumours were delineated using adaptive threshold methods. The SUVmax, SUVpeak, SUVmean, tumour volume (TV), total lesion glycolysis (TLG), and volume and largest diameter on the CT images (CTV and CTD, respectively) were calculated. Index ratios of the primary tumour were calculated by dividing the follow-up measurements by the baseline measurements. The prognostic value of each parameter for event-free survival (EFS) was determined using Cox regression models.
Results
The median follow-up time was 19 months (range 6–43 months). Baseline PET and CT parameters were not significant prognostic factors. After induction therapy, only SUVmax, SUVpeak, SUVmean, TV, TLG and CTV were prognostic factors for EFS, in contrast to CTD. Of the index ratios, only TV and TLG ratios were prognostic factors for EFS. Patients with a TLG ratio <0.48 had a longer EFS than those with a TLG ratio >0.48 (13.9 vs. 9.2 months, p = 0.04). After adjustment for the effect of surgical treatment, all the parameters significantly correlated with EFS remained significant.
Conclusion
SUV, metabolic volume-based indices, and CTV after induction chemotherapy give independent prognostic information in stage III NSCLC. However, changes in metabolic TV and TLG under induction treatment provide more accurate prognostic information than SUV alone, and CTD and CTV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most common malignant tumour in western countries and one of the main causes of death from cancer [1]. Lung cancer usually has a poor prognosis because most patients present with advanced or metastatic disease at the time of diagnosis [2]. Patients with locally advanced non-small-cell lung cancer (NSCLC) are a heterogeneous group of patients regarding prognosis [3]. The treatment of stage III NSCLC has evolved over the last two decades, the current standard of care being combined-modality therapy including chemotherapy, radiotherapy and surgery [3]. Induction treatment before surgery can produce tumour downstaging that increases the likelihood of complete resection, organ preservation, and long-term survival. However, the potential benefit of surgical resection following chemotherapy (with or without radiotherapy) in stage III NSCLC is still controversial [4–6]. Stage III patients with a minimal disease burden and low tumour volume (TV) after induction treatment could benefit from surgical treatment [3, 7]. Thus, it is of utmost importance to find prognostic factors in this setting to identify patients who will benefit from an intensive treatment such as surgery.
Induction chemotherapy provides an opportunity to assess the in vivo chemosensitivity of the primary lesion and nodal metastases. During recent years, FDG PET/CT has emerged as an essential tool for NSCLC staging. One recent meta-analysis suggested that a high standardized uptake value (SUV) might be a poor prognostic factor in NSCLC, especially in early stages [8]. However, the prognostic role of FDG PET/CT in evaluation of tumour response in patients with NSCLC after induction treatment remains unclear and controversial. Few studies have indicated a potential role for FDG PET imaging and SUV measurements in this setting, but results have shown discrepancies in terms of prognostic value [9, 10]. In this context, we sought to determine whether volume-based measurements could provide additional information in comparison to SUV measurements alone for predicting outcome in patients with potentially resectable stage III NSCLC.
Materials and methods
Patient selection and induction chemotherapy
This study was approved by our institutional review board. Between January 2009 and June 2011, 32 consecutive patients with potentially resectable biopsy-proven stage III NSCLC who were treated with induction chemotherapy and with an FDG PET/CT scan before and after the induction treatment were prospectively included. Initial routine staging procedures consisted of a clinical examination including lung function tests, bronchoscopy with or without mediastinoscopy, thoracic enhanced multidetector CT and brain imaging (enhanced CT or MRI). Disease had been staged according to the 7th edition of the UICC (International Union Against Cancer) staging system [11]. Operability was assessed by the patient’s performance status, lung function status and cardiac status. Induction treatment was composed of three cycles of platinum-based chemotherapy given every 3 weeks.
Staging was repeated after completion of induction chemotherapy, and included a thoracic enhanced CT scan and an FDG PET/CT scan. Patients with downstaging of the tumour and/or mediastinal lymph nodes (LN) according to RECIST 1.1 criteria underwent surgery. Patients without mediastinal LN downstaging or progression of disease underwent thoracic radiotherapy with or without chemotherapy.
FDG PET/CT protocol
The baseline FDG PET/CT scans were performed within 1 month prior to the start of the chemotherapy. The follow-up FDG PET/CT scans were performed in all patients approximately 2 weeks after administration of the last dose of chemotherapy. PET/CT images were acquired 60 min after intravenous injection of 3 MBq/kg of FDG. The serum glucose level was <1.4 g/L at the time of injection in all patients. All FDG PET/CT images were obtained using a Gemini TF PET/CT scanner (Philips Medical Systems, The Netherlands). The Gemini TF is a TOF-capable, fully three-dimensional PET scanner together with a 16-slice Brilliance CT scanner [12, 13]. CT images were obtained without injection of contrast medium using the following settings: 120 kV, 100 mA, collimation 16 × 1.5 mm, pitch 0.69, slice thickness 3 mm, and increment 1.5 mm. PET images were reconstructed using a BLOB-OS-TF list-mode iterative algorithm with two iterations and 33 subsets. A single-scatter simulation model was used for scatter correction [14]. Attenuation correction was performed based on the CT data. No post-reconstruction smoothing filter was used. The reconstructed spatial resolution was 5 mm in the centre of the field of view. The image voxel size was 4 mm × 4 mm × 4 mm for the PET images and 1.17 mm × 1.17 mm × 1.5 mm for the CT images. SUVs were calculated from the counts-per-pixel and normalized to body weight (BW):
with injected activity decay-corrected from the delay between injection and image acquisition.
Image analysis
PET/CT and enhanced CT scans were used to characterize the lung primary tumour and mediastinal LN before and after induction chemotherapy. The largest diameter of each primary tumour on CT images (CTD) was measured from a single enhanced CT transverse image as described in the RECIST 1.1 guidelines [15]. The volume of each primary tumour on CT imaging (CTV) was computed using a commercially available lung analysis software package (CT Oncology Engine; Siemens, Forchheim, Germany) [16]. This semiautomated method of segmentation only required the identification of the largest diameter of the tumour. Software tools allowing additional manual 3-D editing were used to avoid the inclusion of adjacent structures, including blood vessels, LN and chest wall, and unrelated conditions such as atelectasis and consolidation.
In PET images, for each tumour, the SUVmax and SUVpeak were calculated within a large manually defined volume of interest containing the primary tumour (DOSIsoft software). SUVpeak was computed as follows. First, a sphere of 1 mL was defined and, given the PET image sampling, the contribution of each voxel to this sphere was calculated. For each possible location of the sphere in the large TV of interest, the average SUV in the sphere was then calculated by weighting each voxel belonging to the sphere as a function of the fraction of the voxel included in the sphere. Finally, the highest average SUV obtained for each possible location of the sphere was defined as SUVpeak.
Two methods were then used for primary tumour delineation. The first method was as described by Nestle et al. [17], called “TVN”. In this method, the TV is delineated using a threshold depending on the activity surrounding the tumour and on the mean activity in an initial TV defined as the voxels with an intensity higher than 0.7 SUVmax [17]. The second method (fit method) described by Tylski et al. [18], called “TVF”, assumes that the tumour image can be modelled as the convolution of the actual TV of uniform activity with a 3-D gaussian function describing the local spatial resolution (5 mm) in the reconstructed image. SUVmean was calculated in the volume segmented by TVN (SUVmeanN) and by TVF (SUVmeanF). SUVmeanF was intrinsically corrected for partial volume effects as the sampling and resolution effects were modelled in the TV estimation method [18]. SUVmeanN was not corrected for partial volume effects.
Two tumour lesion glycolysis (TLG) indices were also calculated as follows: TLGN = SUVmeanN × TVN and TLGF = SUVmeanF × TVF.
Changes in the primary tumour features were calculated by calculating index ratios obtained by dividing the follow-up measurements by the baseline measurements. Thus, index ratios corresponding to changes in CTD, CTV, TVN, TVF, SUVpeak, SUVmeanN, SUVmeanF, SUVmax, TLGN and TLGF were obtained for each lesion.
The staging of the regional LN (N stage) was evaluated on both the PET/CT and CT images. SUVmax was determined in the dominant mediastinal LN. The “dominant” LN was defined as a LN in an involved mediastinal LN region with increased FDG uptake (i.e. uptake above the background level) as the first criterion and with the maximum short axis as a subsequent criterion in cases where several LN had similar SUV. The dominant LN was identified on the baseline FDG PET/CT scan. Index ratios for LN were also calculated by dividing by the follow-up measurements by the baseline measurements.
Follow-up and statistical analysis
All patients were followed in our centre. Patients who underwent surgical treatment after induction chemotherapy were followed by clinical examination and by CT scan every 6 months for 5 years. Patients who underwent combination radiotherapy with or without chemotherapy after induction chemotherapy were closely followed depending on treatment protocol and evolution of disease. Treatment decisions were based on clinical, laboratory or imaging data, including the PET/CT results, discussed during multidisciplinary ward rounds. Event-free survival (EFS) and overall survival (OS) were calculated since all patients were followed even after progression. In patients who underwent surgical treatment, EFS was defined as the time from histological diagnosis to recurrence. In patients who underwent radiotherapy and/or chemotherapy, EFS was defined as the time from histological diagnosis to disease progression. OS was defined as the time from histological diagnosis to death from any cause. Predictive factors for survival were identified using univariate and multivariate Cox regression analyses. Each factor whose p value was less than 0.1 in the univariate analysis was included in the multivariate analysis. OS and EFS were estimated using the Kaplan-Meier method and groups were compared using a log-rank test. A two-tailed p value < 0.05 was considered as statistically significant. Statistical analyses were carried out using ORIGIN PRO® software.
Results
Patients and treatments
The patient characteristics are given in Table 1. After the course of induction chemotherapy, 21 of the 32 patients underwent surgical treatment (lobectomy in 16, pneumonectomy in 5). All these patients had a complete resection of the primary tumour with negative margins and complete thoracic lymphadenectomy. Five of the 21 patients had N2 disease on histological analysis after surgery and underwent mediastinal radiotherapy. The 11 patients who did not undergo surgery received thoracic radiotherapy with or without chemotherapy.
The median follow-up time was 19 months (range 6–43 months). The median OS time was 18 months (6–41 months). The median of EFS was 11.6 months (3–41 months). Figure 1 illustrates outcomes in patients at the time of the last follow-up.
Univariate survival analysis
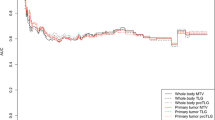
Imaging parameter values at baseline and at follow-up and their ratios are presented in Table 2. The ratios of CTV and metabolic volume-based measurements were smaller than the ratios of SUV and CTD under chemotherapy. The median ratios of CTV, TV and TLG were less than 0.5 in all patients, whereas the median ratios of CTD and SUV indices were more than 0.7.
Univariate survival analysis was performed using the following criteria: age, WHO status, N stage (N2 vs. N0/1), UICC stage (IIIA vs. IIIB), surgical treatment, imaging parameters measured at baseline and follow-up, and index ratios (Table 3). The best cut-off imaging parameters for separating two prognostic subgroups for EFS (log-rank tests) are shown in Table 4.
Baseline indices
Surgical treatment was strongly associated with improved survival (HR 0.2, p = 0.002). Age, N stage, tumour stage and WHO status were not significantly associated with outcome. None of the baseline PET or CT parameters were prognostic factors.
Post-therapeutic indices
Follow-up PET parameters of the primary tumour were prognostic factors for EFS. The higher the SUVmax, SUVpeak, SUVmean, TV and TLG after induction chemotherapy, the shorter the EFS (p < 0.05). Figures 2 and 3 illustrate the effect of TVF and TLGF after induction chemotherapy on EFS. Patients with a TVF <22 ml or TLGF <66 ml after induction therapy had better EFS than those with higher values (p < 0.05). CTV after chemotherapy was also a prognostic factor for EFS, but CTD was not.
Index ratio
Changes in TV and TLG between the baseline and follow-up PET/CT scans were the only significant predictive indices for outcome. The higher the TVF or TLG ratio, the shorter the EFS (p < 0.05). Figure 4 illustrates the effect of TLG ratio on EFS. Patients with a TLG ratio < 0.48 after induction treatment had better EFS than those with a TLG ratio > 0.48 (13.9 vs. 9.2 months, p = 0.04). The other ratios, i.e. CTD, CTV, SUVmean, SUVpeak or SUVmax ratios, were not prognostic factors for EFS. SUVmax ratios on dominant mediastinal LN were not prognostic factor for EFS or OS.
None of the indices at baseline or after induction therapy, or the index ratios, were prognostic factors for OS.
Multivariate survival analysis
After adjustment of the effect of surgical treatment, all the significant parameters at univariate analyses remained significantly correlated with EFS at multivariate analyses.
Discussion
In locally advanced but potentially resectable NSCLC, the therapeutic strategy includes various combinations of chemotherapy and/or radiotherapy aiming at reducing tumour extent in order to reach an operable stage [3, 19]. There is thus a need for an accurate tool to monitor the success of chemotherapy response. FDG PET/CT provides functional information in cancer patients by assessing tumour glucose metabolism related to cell proliferation, metastatic potential and sensitivity of therapy. Metabolic changes after induction chemotherapy may thus predict the prognosis of the patients.
In this study, we showed that FDG PET/CT was relevant for restaging patients with potentially resectable stage III NSCLC after induction chemotherapy. Metabolic information of the primary tumour after induction therapy was more accurate than anatomic response (Table 3). Measurements after therapy including CTV, SUVmax, SUVmean, SUVpeak, TV and TLG of the primary tumour were all independent prognostic factors for outcome (Table 3). Moreover, FDG PET/CT being a whole-body imaging modality, it can assess extrapulmonary progression after induction therapy, even in patients showing a metabolic response in thoracic lesions. This was found in two of our patients. In addition, we showed that changes in metabolic volumes under treatment, including TV or TLG, were more informative than measurements of SUV changes or of CT volume changes for prognostic evaluation. Two semiautomatic methods were used for tumour delineation in this study, and were found to be more reliable than a classical delineation method using a fixed threshold of FDG activity uptake (results not shown).
A meta-analysis suggested that SUV in the primary tumour before therapy has prognostic value in NSCLC [8, 20]. However, this prognostic value does not seem to be so clear in advanced stage lesions [2]. Hoang et al. found that FDG uptake in the primary tumour in advanced stage NSCLC had no prognostic value [21]. Our results are in agreement with those of Hoang et al. in showing that all SUV and volume-based measurements before the start of treatment had no correlation with outcome.
Only a few studies have investigated FDG PET/CT in patients with advanced NSCLC with induction chemotherapy before surgery [22, 23]. Hoekstra et al. showed that FDG PET/CT had additional value over CT in monitoring response to induction treatment in patients with stage IIIA-N2 NSCLC [22]. Residual glucose metabolic rate (measured using Patlak graphical analysis) was found to be the best prognostic factor. Hellwig et al. found that SUVpeak measurements of the primary tumour and LN after induction chemotherapy were prognostic factors [23]. Our results are consistent with the finding of these two studies in showing that SUV indices and CT volume after therapy are of prognostic value. In addition, we found that TV and TLG of the primary tumour were also independent prognostic factors.
The definition of the best threshold to identify patients with a good and a poor prognosis based on PET imaging after therapy remains challenging, be it based on SUV or metabolic volume indices. Dooms et al. found in 30 patients with stage III-N2 NSCLC who underwent induction chemotherapy followed by surgery that FDG PET after therapy may allow selection of candidates for surgery among patients with mediastinal downstaging or persistent minor disease [9]. In that study, the 5-year OS rate in the subgroup of 21 patients with cleared or persistent minor mediastinal LN involvement on histopathological analysis was significantly higher in patients with a decrease in SUVmax in the primary tumour of more than 60 % as compared with patients with a decrease in SUVmax of less than 60 %. However, Tanvetyanon et al. found in 89 patients with stage I/III NSCLC disease that FDG PET after induction therapy was not predictive of survival [10]. Neither visual analysis nor semiquantitative analysis of PET scans showed significant differences in survival between those with or without metabolic response. These findings remained true after adjusting for stage. These discrepancies explain the ongoing debate regarding the FDG parameters that should be measured in determining a therapeutic response, and how a metabolic response should be defined [24, 25].
A few recent studies have suggested a prognostic value of metabolic TV in lung cancer [26–29]. Recently, whole-body TLG before therapy has been found to be a prognostic factor in NSCLC [29]. Nonetheless, to the best of our knowledge, volume-based measurements have never been evaluated in the assessment of therapeutic response in NSCLC. Our data showed that only the decrease in metabolic volume-based measurements of the primary tumour after induction chemotherapy was associated with prognosis, in contrast to decreases in SUVmax, SUVmean or SUVpeak (Fig. 5). This concept of studying the changes in the whole tumour has already been observed with anatomical imaging. Zhao et al. showed that volumetric tumour measurement in CT scans was better than unidimensional tumour measurement at distinguishing tumour sensitive and resistant to gefitinib [30]. TV and TLG enable a global evaluation of tumour shrinkage under treatment and can capture changes in the whole tumour mass. SUVmax measurements alone may be misleading for heterogeneous lung tumours. After treatment, tumour response is also heterogeneous with death of susceptible tumour cells and proliferation of more virulent tumour cells. Moreover, the host inflammatory response under treatment can induce an increase in tumour metabolism [31–33]. All these drawbacks may be mitigated by metabolic volume-based evaluation with TV or TLG. Moreover, CTV changes under chemotherapy failed to predict significantly EFS in our study, emphasizing the strength of metabolic volume changes regarding prognosis.
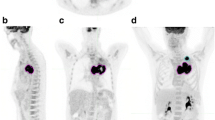
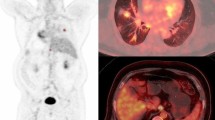
PET/CT images in a 53-year-old woman with adenocarcinoma stage IIIA (T3N1, primary tumour TV in red) illustrating a mismatch between a high decrease in TV and TLG and increasing SUVmax ratios. The baseline (a: coronal view, b: 3D view) and follow-up (c: coronal view, d: 3D view) scans were performed before and after three cycles of induction chemotherapy. SUVmax, TVF and TLGF ratios were respectively 1.25, 0.2 and 0.2. Right upper lobectomy with mediastinal LN dissection was performed. At the most recent follow-up (24 months), the patient was still alive with no evidence of recurrence
Tumour delineation on PET images in this study was based on semiautomatic methods that are fast with minimal interobserver variability. Delineation described by Nestle et al. uses a combination of the mean SUV and the background uptake, while the fit method is based on convolution of the tumour image with the point-spread function corresponding to the spatial resolution of the scanner. These two methods have been previously reported to be reliable and robust in a large variety of configurations [18]. In contrast, fixed-threshold delineation methods have numerous drawbacks given that the value of the threshold to be used for each lesion depends on multiple factors, such as lesion contrast and size and image noise [34].
Our study was retrospective and included only a small number of patients who did not undergo surgery. Yet our study population was homogeneous including only stage III patients who all received platinum-based induction chemotherapy. While tumour delineation in the lungs was rather easy due to the low background parenchymal activity, delineation of LN involvement was not feasible because of significant mediastinal background activity, especially on follow-up FDG PET/CT scans. Thus, these results should not be extended to other tumours surrounded by high background uptake such as liver tumours.
Based on our results, we have initiated a prospective study in a larger patient cohort. This will allow a more thorough evaluation of the reliability of different tumour features for response assessment, as well as multivariate analysis of different predictive variables, with the final aim of improving the selection of poor prognosis patients who would not benefit from surgery.
Conclusion
FDG PET/CT before and after induction chemotherapy in stage III NSCLC provides prognostic information. Unlike SUV and metabolic volume indices at baseline, SUV and metabolic volume-based indices after induction therapy give independent prognostic information. Changes in metabolic TV and TLG under induction treatment provide more accurate prognostic information than changes in SUV alone or changes in size on CT scans. Thus FDG PET/CT appears to be a promising tool for selecting patients who will benefit from an intensive treatment such as surgery after induction chemotherapy.
References
Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–8.
Berghmans T, Paesmans M, Sculier JP. Prognostic factors in stage III non-small cell lung cancer: a review of conventional, metabolic and new biological variables. Ther Adv Med Oncol. 2011;3:127–38.
Farray D, Mirkovic N, Albain KS. Multimodality therapy for stage III non-small-cell lung cancer. J Clin Oncol. 2005;23:3257–69.
Andre F, Grunenwald D, Pignon JP, Dujon A, Pujol JL, Brichon PY, et al. Survival of patients with resected N2 non-small-cell lung cancer: evidence for a subclassification and implications. J Clin Oncol. 2000;18:2981–9.
Rosell R, Gomez-Codina J, Camps C, Javier Sanchez J, Maestre J, Padilla J, et al. Preresectional chemotherapy in stage IIIA non-small-cell lung cancer: a 7-year assessment of a randomized controlled trial. Lung Cancer. 1999;26:7–14.
Roth JA, Atkinson EN, Fossella F, Komaki R, Bernadette Ryan M, Putnam Jr JB, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung Cancer. 1998;21:1–6.
Albain KS, Swann RS, Rusch VW, Turrisi 3rd AT, Shepherd FA, Smith C, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009;374:379–86.
Paesmans M, Berghmans T, Dusart M, Garcia C, Hossein-Foucher C, Lafitte JJ, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol. 2010;5:612–9.
Dooms C, Verbeken E, Stroobants S, Nackaerts K, De Leyn P, Vansteenkiste J. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–34.
Tanvetyanon T, Eikman EA, Sommers E, Robinson L, Boulware D, Bepler G. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol. 2008;26:4610–6.
Boffa DJ, Detterbeck FC, Smith EJ, Rami-Porta R, Crowley J, Zelterman D, et al. Should the 7th edition of the lung cancer stage classification system change treatment algorithms in non-small cell lung cancer? J Thorac Oncol. 2010;5:1779–83.
Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS. Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nucl Med. 2007;48:471–80.
El Fakhri G, Surti S, Trott CM, Scheuermann J, Karp JS. Improvement in lesion detection with whole-body oncologic time-of-flight PET. J Nucl Med. 2011;52:347–53.
Accorsi R, Adam LE, Werner ME, Karp JS. Optimization of a fully 3D single scatter simulation algorithm for 3D PET. Phys Med Biol. 2004;49:2577–98.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Marten K, Engelke C. Computer-aided detection and automated CT volumetry of pulmonary nodules. Eur Radiol. 2007;17:888–901.
Nestle U, Kremp S, Schaefer-Schuler A, Sebastian-Welsch C, Hellwig D, Rube C, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005;46:1342–8.
Tylski P, Stute S, Grotus N, Doyeux K, Hapdey S, Gardin I, et al. Comparative assessment of methods for estimating tumor volume and standardized uptake value in (18)F-FDG PET. J Nucl Med. 2010;51:268–76.
Eschmann SM, Friedel G, Paulsen F, Reimold M, Hehr T, Budach W, et al. Repeat 18F-FDG PET for monitoring neoadjuvant chemotherapy in patients with stage III non-small cell lung cancer. Lung Cancer. 2007;55:165–71.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12.
Hoang JK, Hoagland LF, Coleman RE, Coan AD, Herndon 2nd JE, Patz Jr EF. Prognostic value of fluorine-18 fluorodeoxyglucose positron emission tomography imaging in patients with advanced-stage non-small-cell lung carcinoma. J Clin Oncol. 2008;26:1459–64.
Hoekstra CJ, Stroobants SG, Smit EF, Vansteenkiste J, van Tinteren H, Postmus PE, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–70.
Hellwig D, Graeter TP, Ukena D, Georg T, Kirsch CM, Schafers HJ. Value of F-18-fluorodeoxyglucose positron emission tomography after induction therapy of locally advanced bronchogenic carcinoma. J Thorac Cardiovasc Surg. 2004;128:892–9.
Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Consensus recommendations for the use of 18F-FDG PET as an indicator of therapeutic response in patients in National Cancer Institute trials. J Nucl Med. 2006;47:1059–66.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–82.
Lee P, Weerasuriya DK, Lavori PW, Quon A, Hara W, Maxim PG, et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:328–33.
Oh JR, Seo JH, Chong A, Min JJ, Song HC, Kim YC, et al. Whole-body metabolic tumour volume of (18)F-FDG PET/CT improves the prediction of prognosis in small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:925–35.
van Loon J, Offermann C, Ollers M, van Elmpt W, Vegt E, Rahmy A, et al. Early CT and FDG-metabolic tumour volume changes show a significant correlation with survival in stage I-III small cell lung cancer: a hypothesis generating study. Radiother Oncol. 2011;99:172–5.
Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–66.
Zhao B, Oxnard GR, Moskowitz CS, Kris MG, Pao W, Guo P, et al. A pilot study of volume measurement as a method of tumor response evaluation to aid biomarker development. Clin Cancer Res. 2010;16:4647–53.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92.
Lee HY, Lee HJ, Kim YT, Kang CH, Jang BG, Chung DH, et al. Value of combined interpretation of computed tomography response and positron emission tomography response for prediction of prognosis after neoadjuvant chemotherapy in non-small cell lung cancer. J Thorac Oncol. 2010;5:497–503.
Poettgen C, Theegarten D, Eberhardt W, Levegruen S, Gauler T, Krbek T, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007;73:316–23.
Biehl KJ, Kong FM, Dehdashti F, Jin JY, Mutic S, El Naqa I, et al. 18F-FDG PET definition of gross tumor volume for radiotherapy of non-small cell lung cancer: is a single standardized uptake value threshold approach appropriate? J Nucl Med. 2006;47:1808–12.
Acknowledgments
We are indebted to Dr. Michel Cymbalista (Department of Radiology, Monfermeil Hospital, France) for his help in using the lung analysis software package, and to Dr. Sébastien Vauclin (DOSIsoft, France) for his help in using the DOSIsoft software.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soussan, M., Chouahnia, K., Maisonobe, JA. et al. Prognostic implications of volume-based measurements on FDG PET/CT in stage III non-small-cell lung cancer after induction chemotherapy. Eur J Nucl Med Mol Imaging 40, 668–676 (2013). https://doi.org/10.1007/s00259-012-2321-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-012-2321-7