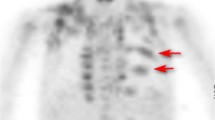
Abstract
Purpose
It has been shown that warming patients prior to and during 18F-FDG uptake by controlling the room temperature can decrease uptake by brown adipose tissue (BAT). The aim of this study is to determine if this effect is subject to seasonal variation.
Methods
A retrospective review was conducted of all patients referred for whole-body 18F-FDG PET between December 2006 and December 2008. After December 2007, all patients were kept in the PET injection room at a constant 24°C for 30 min before and until 1 h following FDG administration. Patients over 22 years of age and those who received pre-medication known to reduce FDG uptake by BAT were excluded. One hundred and three patients were warmed to 24°C prior to scanning. The number of patients showing uptake by BAT in this group was compared to a control group of 99 patients who underwent PET prior to December 2007 when the injection room temperature was 21°C.
Results
Uptake by BAT occurred in 9% of studies performed after patient warming (24°C), compared to 27% of studies performed on the control group (21°C) (p < 0.00001). The effect of warming on decreasing FDG accumulation in BAT was statistically significant in the winter (p < 0.005) and summer (p < 0.001). However, in the spring and autumn, though the effect of warming on decreasing FDG accumulation in BAT was evident, it was not statistically significant (p > 0.05).
Conclusion
Maintaining room temperature at a constant 24°C for 30 min prior to and 1 h after IV tracer administration significantly decreases FDG uptake by BAT in children. This effect is greatest in the summer and winter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown adipose tissue (BAT) was first described by Gessner in 1551 [1] and is associated with increased energy expenditure, insulin sensitivity, and decreased susceptibility to weight gain [2–6]. Present in approximately 5% of the adult population, it is more commonly found in women, thin patients, and children [7–9]. Thought to play a thermogenic role, brown adipocytes may be metabolically activated by sympathetic stimulation following exposure to cold [10–13].
18F-fluorodeoxyglucose (18F-FDG) is a radioactive glucose analog taken up by metabolically active BAT and may be seen on positron emission tomography (PET) [14, 15]. Sites of FDG-avid BAT on PET include the neck, mediastinum, paravertebral region, and retroperitoneum [15, 16]. The intensity of radiotracer uptake is variable and may depend on seasonal variation in ambient temperature [12, 13]. Physiologic radiotracer accumulation in metabolically active BAT may be a source of false-positive interpretations or may obscure underlying metabolically active disease when present [17, 18]. Interventions that have been used to decrease 18F-FDG uptake by BAT include pre-medication with propranolol, fentanyl, and benzodiazepines [19–23]. Alternatively, patient warming has been proposed as an attractive alternative to drug administration to reduce 18F-FDG uptake by BAT [24, 25]. To date, there is considerable variability in performing nuclear medicine studies between different institutions. In particular, departmental temperatures may vary and patient warming prior to PET/CT is not standardized.
In December 2007, we adopted a new protocol for patient warming prior to whole-body PET/CT. It has been shown that warming patients at a constant temperature of 24°C (75°F) for 30 min prior to and for 1 h after intravenous tracer administration can decrease FDG uptake by BAT [26]. The aim of this study is to determine if this effect is subject to seasonal variation.
Materials and methods
In accordance with the Institutional Review Board approval, a retrospective review was conducted to ascertain the seasonal variation in the effect of patient warming on 18F-FDG uptake by BAT. Based on prior reports, the temperature in the PET injection room was increased from approximately 21°C to a constant 24°C in December 2007 [24, 25].
Patient population
All patients referred to the Division of Nuclear Medicine at Children’s Hospital in Boston for whole-body 18F-FDG PET in 2008 were kept in the PET injection room at a constant 24°C (75°F) for 30 min before and until 1 h following tracer administration. There were 103 patients 21 years of age and younger (46% girls and 54% boys; mean age 14) who had an 18F-FDG PET in 2008 and who did not receive sedation or pre-medication known to alter FDG uptake by BAT. The average body mass index (BMI) was 22 kg/m2. The majority of patients were referred for the evaluation of oncologic conditions with a small minority referred for non-oncologic conditions such as infection. In total, these 103 patients completed 185 FDG PET scans in the 12-month period between 1 January 2008 and 31 December 2008.
All patients referred to the Division of Nuclear Medicine for whole-body 18F-FDG PET prior to December 2007 underwent PET when the injection room temperature was approximately 21°C (70°F). There were 99 patients 21 years of age and younger (40% girls and 60% boys; mean age 14) who had an 18F-FDG PET between December 2006 and December 2007 and who did not receive pre-medication known to reduce FDG uptake by BAT. The average BMI was 22 kg/m2. The majority of patients were referred for the evaluation of oncologic conditions with a small minority of studies done for other indications such as the work-up of suspected infection. In total, the 99 patients in the control group completed 168 FDG PET scans in the 12-month period between 1 December 2006 and 30 November 2007.
Overall, patient characteristics in the warmed group and control group were similar with respect to age, gender, and BMI.
Image acquisition
Prior to having the 18F-FDG PET all patients were asked to fast for at least 4 h, to avoid caffeine, nicotine, or alcohol for 12 h, and not to perform strenuous exercise for 24 h. Warm and comfortable clothing was suggested for the day of the scan. The PET injection room temperature was maintained constant using an individualized thermostatic control and checked regularly using a thermometer. Patients warmed prior to the FDG PET were kept in the warmed, quiet, dimly lit injection room for 30 min before tracer administration and remained in this room for 1 h following intravenous radiopharmaceutical injection. For all patients, the radiopharmaceutical dose was calculated according to the patient weight using 5.55 MBq/kg (150 μCi/kg) with minimum and maximum administered dose of 18.5 MBq (500 μCi) and 370 MBq (10 mCi), respectively. Whole-body FDG PET was obtained on a GE Advance NXi PET scanner (GE Healthcare Technologies, Waukesha, WI, USA, a stand-alone PET scanner) using 2-D mode with 5-min emission and 3-min transmission scans per bed position. Patients were scanned from the skull base to mid-thigh; however, the axial field of view was extended to include sites of suspected or known tumor involvement outside this region.
Data analysis
The National Weather Service Forecast Office [27] was used to obtain daily outdoor temperatures for Boston, MA in 2006, 2007, and 2008. During the 12-month period between January and December 2008, the minimum outside temperature range on the days the 18F-FDG PET studies were performed was −13 to 22°C (9–72°F); the average outside temperature range was −11 to 27°C (12–81°F); the maximum outside temperature range was −10 to 35°C (14–95°F) (Fig. 1). During the 12-month period between December 2006 and November 2007, the minimum outside temperature range on the days the 18F-FDG PET studies were performed was −14 to 24°C (7–75°F); the average outside temperature range was −10 to 30°C (12–86°F); the maximum outside temperature range was −7 to 35°C (19–95°F) (Fig. 2).
Two nuclear medicine physicians and one radiologist independently evaluated each PET scan for FDG uptake by BAT, and the final result was based on consensus. For each study, FDG accumulation in BAT was determined to be either present or absent. Tracer uptake in BAT was considered to be present when the uptake in characteristic areas of brown fat localization was greater than background soft tissue activity. Tracer uptake in BAT was considered to be absent if the uptake in the same areas was the same as in soft tissue. In each case, the pattern of uptake in BAT was consistent with the typical appearance described in the literature. A two-tailed Fisher exact probability test was used to compare the rate of uptake by BAT in patients who were warmed prior to scanning with those who were not.
Results
FDG uptake by BAT occurred in 16 of 185 (9%) FDG PET scans completed following preparation in the temperature-controlled room at 24°C (75°F). By comparison, FDG uptake by BAT occurred in 45 of 168 (27%) FDG PET scans performed when the injection room temperature was 21°C (70°F). This is statistically significant (p < 0.00001). Furthermore, 14 of 36 patients who had FDG uptake in BAT when the injection room temperature was 21°C (70°F) were also imaged when the injection room temperature was 24°C (75°F). Of these 14 patients, 13 (93%) did not have FDG uptake in BAT when the injection room temperature was 24°C (75°F), while 1 patient had persistent FDG uptake in BAT.
Meteorologists generally define four seasons demarcated by their average monthly temperature with the three warmest months constituting summer, the three coldest months constituting winter and the intervening months representing spring and autumn. For the purposes of this study, the winter months were considered to be December, January, and February. The spring months were considered to be March, April, and May. The summer months were considered to be June, July, and August. The fall months were considered to be September, October, and November.
Table 1 lists the average outside temperatures for the different seasons as well as the age and BMI of the patients. In the winter months (December–February), uptake by BAT occurred in 3% of studies in the temperature-controlled room at 24°C (75°F) compared with 28% when the injection room temperature was 21°C (70°F) (p < 0.005). In the spring months (March–May), uptake by BAT occurred in 13% of studies in the temperature-controlled room at 24°C (75°F) compared with 26% when the injection room temperature was 21°C (70°F) (p > 0.05). In the summer months (June–August), uptake by BAT occurred in 7% of studies in the temperature-controlled room at 24°C (75°F) compared with 35% when the injection room temperature was 21°C (70°F) (p < 0.001). In the fall months (September–November), uptake by BAT occurred in 11% of studies in the temperature-controlled room at 24°C (75°F) compared with 20% when the injection room temperature was 21°C (70°F) (p > 0.05). Figure 3 illustrates the seasonal variation in the effect of warming patients by controlling the room temperature prior to and during the 18F-FDG uptake period on 18F-FDG uptake by BAT. Figure 3 suggests that when the injection room temperature is 21°C (70°F), the number of PET scans with FDG accumulation in BAT is similar in the winter and summer. However, when the injection room temperature is 24°C (75°F) the number of PET scans with metabolically active BAT is reduced and this effect is most pronounced in the summer and winter.
Discussion
FDG uptake by BAT is variable and may decrease rapidly in response to increased ambient temperature [28], anxiolytic medication [19], or sympathetic blockade [20]. This is in keeping with the theory that cold metabolically activates BAT through sympathetic stimulation and that metabolically active BAT accumulates FDG [11, 15].
Results in the literature suggest that FDG uptake by BAT is related to outdoor temperature. Cohade et al. reported an increased incidence of FDG-avid BAT while outside temperatures were low in a retrospective review of over 1,000 consecutive whole-body PET/CT scans done between July 2001 and June 2002 [12]. Kim et al. reported increased FDG uptake by BAT as an acute response to cold weather in over 1,000 consecutive whole-body PET/CT scans done between March 2000 and November 2003 [13]. Several studies have shown that FDG uptake by BAT may be decreased by warming patients prior to FDG PET. Christensen et al. studied ten patients with a history of hypermetabolic BAT on PET and showed that patient warming through the use of blankets could decrease FDG uptake by BAT [24]. Garcia et al. suggested that preparing patients in a temperature-controlled room prior to FDG PET could decrease tracer accumulation in BAT [25].
In December 2007, we adopted a new protocol for patient preparation prior to whole-body PET. In order to reduce FDG uptake by BAT, all patients referred for whole-body PET were warmed at a constant temperature of 24°C (75°F) for 30 min prior to and for 1 h after intravenous tracer administration. In a prior article, we showed that this simple and standardized protocol significantly decreased FDG uptake by BAT during the winter [26]. The current study provides data on the seasonal variation in the effect of this protocol on FDG uptake by BAT. We included a larger number of patients than previously reported studies and have focused on the pediatric population who, by their age, are at increased risk of FDG uptake in BAT.
Our study demonstrates a significantly decreased incidence of FDG uptake by BAT in patients who had FDG PET following warming in the temperature-controlled room at 24°C prior to and during tracer uptake. These results confirm prior results in the literature and emphasize the utility of ambient temperature control as an alternative approach to pharmacologic intervention [24–26].
Our study also shows that patient warming to 24°C prior to and during tracer uptake is more effective in the winter and summer seasons compared with the spring and fall. In New England, during the spring and fall there are often rapid temperature changes. A possible explanation for the decreased effectiveness of patient warming during the spring and fall could be that rapid temperature changes causing metabolically active BAT result in a more lasting effect. In other words, once the BAT has become metabolically active, it is more resistant to becoming quiescent. However, further controlled studies are warranted.
There are limitations to this study. First, this study was not a randomized clinical controlled trial. The potential for selection bias was minimized by including all patients imaged in the two time frames except for those who were over 22 years of age or who had received pre-medication known to alter FDG uptake by BAT. The two groups of patients included were similar in terms of age, sex, and BMI. All patients were given the same instructions prior to imaging and the only parameter that was altered was exposure to the warmed injection room prior to and following FDG administration. It was assumed that exposure to outdoor temperatures prior to arrival in the department was similar. Second, the protocol for patient preparation prior to FDG PET including patient warming to 24°C was based on results suggested in the literature as well as on practical considerations for the patients. Further research on the most effective protocol for warming optimized for outdoor temperature could be beneficial. In particular, it might prove helpful to increase the temperature in the injection room or the time spent being warmed during the spring and fall. Our study suggests that in children the percentage of PET scans with FDG accumulation in BAT does not significantly change with season (Fig. 3). This is contrary to what has been suggested by many [7, 8, 12, 13] but not all [29] studies conducted in the adult population. The reason for this is not clear; it could be because children might not dress appropriately and are unprepared to deal with the effects of outdoor temperature change or indoor air-conditioning. In fact, it is possible that the total amount of BAT may depend on the ambient outdoor temperature, while the presence of metabolically active BAT depends on patient warming prior to tracer administration [15, 30]. It may therefore be important to report the indoor temperatures at the time the radiotracer was administered as well as the seasonal outdoor temperatures. Our sample size, though larger than previously published results, is still small. A large prospective controlled study in the pediatric population evaluating the warming preparation protocol prior to FDG PET on the presence of metabolically active BAT with respect to BMI and season may be warranted.
Conclusion
Obesity may play a role in the development of diabetes, the pathogenesis of vascular disease, and the incidence of cancer [31–33]. BAT is thermogenic and can result in increased energy expenditure, decreased body weight, and a beneficial effect on glucose metabolism. However, FDG uptake in BAT has long been regarded as a source of false-positive and false-negative interpretations on FDG PET scans. Indeed, several methods have been used to reduce FDG uptake by BAT including pharmacologic intervention and patient warming. To date, there is no standardized protocol for preparing patients prior to PET. Our study describes a standardized protocol of maintaining the room temperature at a constant 24°C for 30 min prior to and during the uptake period that significantly decreases FDG uptake by BAT in children. This effect is most pronounced in the summer and winter.
References
Gessner K. Conradi Gesneri medici Tigurini Historiae Animalium: Lib 1—De Quadrupedibus Viviparis. 1551.
Cannon B, Nedergaard J. Developmental biology: neither fat nor flesh. Nature 2008;454:947–8.
Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006;444:847–53.
Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf) 2006;64:355–65.
Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 1993;366:740–2.
Yang X, Enerbäck S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res 2003;11:1182–91.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009;360(15):1509–17.
Au-Yong ITH, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variations in humans. Diabetes 2009;58:2583–7.
Wehrli NE, Bural G, Houseni M, Alkhawaldeh K, Alavi A, Torigian DA. Determination of age-related changes in structure and function of skin, adipose tissue, and skeletal muscle with computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med 2007;37:195–205.
Himms-Hagen J. Thermogenesis in brown adipose tissue as an energy buffer. Implications for obesity. N Engl J Med 1984;311:1549–58.
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 2002;29:1393–8.
Cohade C, Mourtzikos K, Wahl R. “USA-Fat”: prevalence is related to ambient outdoor temperature—evaluation with 18F-FDG PET/CT. J Nucl Med 2003;44:1267–70.
Kim S, Krynyckyi BR, Machac J, Kim CK. Temporal relation between temperature change and FDG uptake in brown adipose tissue. Eur J Nucl Med Mol Imaging 2008;35(5):984–9.
Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 2003;44:170–6.
Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007;293(2):E444–52.
Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972;112:35–9.
Paidisetty S, Blodgett TM. Brown fat: atypical locations and appearances encountered in PET/CT. AJR Am J Roentgenol 2009;193(2):359–66.
Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol 2004;183(4):1127–32.
Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 2005;35(10):984–90.
Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007;32(5):351–7.
Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007;34(7):1018–22.
Sturkenboom MG, Hoekstra OS, Postema EJ, Zijlstra JM, Berkhof J, Franssen EJ. A randomised controlled trial assessing the effect of oral diazepam on 18F-FDG uptake in the neck and upper chest region. Mol Imaging Biol 2009;11(5):364–8.
Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown fat can be reduced pharmacologically. J Nucl Med 2004;45(7):1189–93.
Christensen CR, Clark PB, Morton KA. Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med 2006;31(4):193–6.
Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G, et al. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol 2006;8(1):24–9.
Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, et al. Constant ambient temperature of 24 degrees C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging 2009;36(4):602–6.
National Weather Service Forecast Office. http://www.weather.gov/climate/. Accessed 29 Dec 2009.
Garcia CA, Van Nostrand D, Majd M, Atkins F, Acio E, Sheikh A, et al. Benzodiazepine-resistant “brown fat” pattern in positron emission tomography: two case reports of resolution with temperature control. Mol Imaging Biol 2004;6(6):368–72.
Rousseau C, Bourbouloux E, Campion L, Fleury N, Bridji B, Chatal JF, et al. Brown fat in breast cancer patients: analysis of serial (18)F-FDG PET/CT scans. Eur J Nucl Med Mol Imaging 2006;33(7):785–91.
Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58(7):1526–31.
Lazar MA. How obesity causes diabetes: not a tall tale. Science 2005;307:373–5.
Calle EE, Rodriquez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38.
Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 2006;56:254–81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zukotynski, K.A., Fahey, F.H., Laffin, S. et al. Seasonal variation in the effect of constant ambient temperature of 24°C in reducing FDG uptake by brown adipose tissue in children. Eur J Nucl Med Mol Imaging 37, 1854–1860 (2010). https://doi.org/10.1007/s00259-010-1485-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-010-1485-2