Abstract
Purpose
The aim of this study was to determine if warming patients prior to and during 18F-FDG uptake by controlling the room temperature could decrease uptake by brown adipose tissue (BAT).
Methods
A group of 40 children underwent 18F-FDG PET after being kept in the injection room at a constant temperature of 24°C for half an hour before and 1 hour after intravenous tracer administration. The rate of uptake by BAT in this group was compared to the uptake in a control group of 45 patients who underwent PET when the injection room temperature was 21°C.
Results
Uptake by BAT occurred in 5% of studies in the temperature-controlled room compared to 31% of studies performed when the injection room temperature was 21°C (p<0.002).
Conclusion
Maintaining room temperature at a constant 24°C, half an hour prior to and during the period of FDG uptake significantly decreases accumulation of FDG in BAT in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
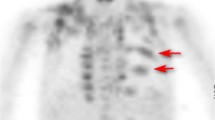
With the development of hybrid PET/CT scanners, localization of metabolic activity to regional anatomy has led to an evolving appreciation of normal variants. In particular, 18F-FDG uptake may be seen in metabolically active brown adipose tissue (BAT). This physiologic tracer accumulation may be a source of false-positive interpretations or alternatively may obscure adjacent metabolically active disease [1–4]. FDG-avid BAT is most commonly seen in thin patients, women and children [3–6]. Often symmetric, sites of FDG-avid BAT include among others, the neck, mediastinum, paravertebral region and retroperitoneum especially in perinephric or perihepatic locations.
Previous reports have suggested that FDG accumulation in BAT is related to low environmental temperature [6, 7]. This is consistent with a thermoregulatory role for BAT which becomes metabolically activated following sympathetic stimulation upon exposure to a cold environment, such as during the winter months in New England [7, 8]. Several interventions have been employed to reduce FDG accumulation in BAT. Mainly pharmacologic, these include patient premedication with propranolol, fentanyl, or a benzodiazepine [9–12]. An attractive alternative to drug administration is controlling environmental temperature prior to imaging. Prior reports have suggested this may be helpful in reducing FDG uptake by BAT [13–15]. This is an easily tolerated intervention particularly when a pharmacologic approach is undesirable. The aim of this study was to determine if keeping a child in a controlled, warm room prior to, during and following tracer administration could decrease the rate of uptake by BAT.
Methods
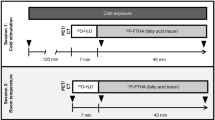
Based on prior reports of the effect of temperature on FDG uptake by BAT, the temperature in the PET injection room was increased to a constant 24°C (75°F) [13, 14]. All patients referred to the Division of Nuclear Medicine for whole-body 18F-FDG PET between December 2007 and March 2008 were kept in this warmed room for half an hour before until 1 h following tracer administration. A total of 40 patients underwent 18F-FDG PET according to this protocol (warmed conditions). To ascertain the effect of this, a retrospective review was conducted. The rate of uptake by BAT in the group that underwent PET under warmed conditions was retrospectively compared to the rate of uptake by BAT in a control group of 45 patients who underwent PET when the injection room temperature was 21–22°C (70–72°F).
Patient population
Between December 2007 and March 2008, 54 patients were referred to the Division of Nuclear Medicine for a whole-body 18F-FDG PET scan. Only patients aged 21 years or younger were included in the study, and 11 patients who received sedation were excluded from the study. None of the 40 remaining patients received premedication known to reduce FDG uptake by BAT, and all were warmed prior to and during tracer uptake. The age range of those included in the study was 5–21 years (mean about 13 years) and their average body mass index (BMI) was 21 kg/m2. All were referred for evaluation of oncological conditions. There were 15 (38%) girls and 25 (62%) boys.
The control group included 52 patients (aged 21 years or younger with an average BMI of 23 kg/m2) who were referred between December 2006 and March 2007 for whole-body 18F-FDG PET scan for oncological evaluation. Seven of these patients (aged 17 months to 5 years) received sedation and were excluded. None of the remaining 45 patients aged 2–21 years (mean about 14 years) received premedication. There were 19 (42%) girls and 26 (58%) boys. Patients in both groups were similar with respect to age, gender, and BMI.
Image acquisition
Patients were asked to fast for at least 4 h, to avoid strenuous exercise for 24 h, and to abstain from caffeine, nicotine or alcohol intake for 12 h prior to the study. Warm, comfortable clothing was recommended. These instructions were identical for the two groups. An individualized thermostatic controller was installed to provide accurate and specific temperature control for the PET injection room. A thermometer was used to ensure that the temperature in this room was maintained at a constant 24°C (75°F). Patients scheduled for an 18F-FDG PET scan were kept in the warmed, quiet, dimly lit injection room for half an hour before tracer administration and remained in this room for approximately 1 hour after intravenous administration of the radiopharmaceutical. The radiopharmaceutical dose was calculated based on patient weight, using 5.55 MBq/kg (150 µCi/kg) with minimum and maximum administered doses of 18.5 MBq (500 µCi) and 370 MBq (10 mCi), respectively. For all whole-body scans a GE Advance NXi PET scanner (GE Healthcare Technologies, Waukesha, WI) was used in 2-D mode with a 5-min emission and 3-min transmission scan per bed position. In general, patients were scanned from the base of the skull to mid-thigh (four to six bed positions depending on size). The axial field of view was extended if there were areas of known tumour involvement outside this region.
Data analysis
Historical outdoor temperatures for Boston, Massachusetts, were obtained from the National Weather Service Forecast Office (http://www.weather.gov/climate/).
A total of 40 patients had 18F-FDG PET scans under warmed conditions. The minimum outside temperatures on the days of these studies ranged from −14° to 8°C (7°–46°F) with an average of −2°C (28°F) (Fig. 1). The rate of uptake by BAT on these studies was calculated. These results were compared with those in the control group of 45 patients who underwent 18F-FDG PET scan without warmed conditions. The minimum outside temperatures on the days of these studies was similar to those for the “warmed group”, ranging from −14° to 9°C (6°–48°F) with an average of −2°C (28°F) (Fig. 2). Two nuclear medicine physicians and one radiologist independently evaluated each PET scan for FDG uptake by BAT. For each PET study, FDG uptake by BAT was determined by consensus to be either present or absent. BAT was considered to be present when the uptake in characteristic areas of brown fat localization was greater than background soft-tissue activity. BAT was considered to be absent if the uptake in the same areas was the same as in soft tissue. In each case the pattern of tracer uptake was consistent with the typical appearance of FDG uptake by BAT as described in the literature. A two-tailed Fisher exact probability test was used to compare the rate of uptake by BAT under the two temperature conditions.
Results
FDG uptake by BAT was identified in 2 of 40 studies (5%) performed after using the warmed injection room. One of these patients was a 16-year-old girl who underwent the 18F-FDG PET scan on a day when the minimum outside temperature was −11°C (12°F), and the other patient was an 11-year-old boy who underwent the 18F-FDG PET scan on a day when the minimum outside temperature was −2°C (28°F). By comparison, FDG uptake by BAT was seen in 14 of 45 studies (31%) performed in patients without warm room preparation (p<0.002; Fig. 3).
Of the patients scanned following preparation in the warmed room, 26 had images from prior whole-body PET scans available for review. In 12 of the 26 patients, there was FDG uptake by BAT on the prior study, but in only 1 of these patients was there FDG uptake by BAT when scanned under “warmed conditions” (Fig. 4).
Discussion
BAT was first described in certain mammals more than 450 years ago [16]. In work dating back to 1551, Gessner commented, with reference to BAT, “nec pinguitudo, nec caro” indicating that he felt it was neither fat nor flesh, but something between the two [16, 17]. Thought to play a thermoregulatory role under sympathetic stimulation, BAT is more commonly found in women and children [3–6]. The extent of BAT tends to decrease with age and therefore is particularly prominent in children [5].
Results in the literature suggest that FDG accumulation in BAT is related to environmental factors such as ambient temperature [6, 7]. In a retrospective review of 1,017 consecutive whole-body PET/CT scans done in Baltimore, USA, between July 2001 and June 2002, Cohade et al. reported an increased incidence of FDG-avid supraclavicular BAT from January through March, while outside temperatures were low, compared with the rest of the year [6]. In another retrospective review of 1,495 consecutive whole-body PET/CT scans done between March 2000 and November 2003, Kim et al. reported increased 18F-FDG uptake by BAT as an acute response to cold weather [7].
It has been postulated that cold stimuli modulate sympathetic stimulation of BAT through adrenergic receptors which increases glucose uptake. Similarly, FDG, a glucose analogue, accumulates in BAT that has been stimulated by the cold [7, 8]. Two approaches have been adopted to decrease FDG uptake by BAT. Pharmacologic disruption of BAT sympathetic innervation with patient premedication has been shown to decrease FDG uptake [9–12]. Alternatively the patient’s ambient environmental temperature prior to imaging may be controlled [13–15]. In a small study, Christensen et al. studied ten patients (age 15–45 years) with a history of hypermetabolic BAT on PET [14]. The patients were randomized to one of three groups at the time of a repeat FDG-PET scan. Three patients were covered with warm blankets between tracer injection and imaging. Four patients were kept in a warm environment for 48 hours prior to the PET scan and during the uptake interval. Three patients were kept in a warm environment for 48 hours before the PET scan and during the uptake interval and were also given 5 mg of diazepam orally at the time of FDG administration. Nine patients (90%) showed complete or near-complete resolution of hypermetabolic BAT on PET. Another study conducted by Garcia et al., included ten patients, mostly adults, with a history of hypermetabolic BAT on PET [13]. They found a decrease of FDG uptake by BAT if patients were kept in a temperature-controlled room for between 15 minutes and 2 hours before FDG injection as well as during the uptake phase after tracer administration.
Our study provides new data on the benefits of ambient warming for a paediatric population who, by their age, were at increased risk of tracer uptake in BAT [5]. We included a larger number of patients than in previously reported studies [13, 14]. We also outline a simple and standardized protocol that provides an efficient method to suppress uptake by BAT (Fig. 3).
Our study demonstrates a significantly decreased incidence of uptake by BAT in patients who were scanned following preparation in a temperature-controlled room prior to and during FDG uptake. These results emphasize the utility of ambient temperature control, an easily tolerated intervention to reduce tracer uptake by BAT particularly in patients where a pharmacologic approach is undesirable.
There are limitations to this study. First, this study was not a randomized clinical controlled trial. The potential for selection bias was minimized by including all patients imaged in the two time frames except for those that received sedation. We included two groups of patients imaged when outdoor ambient temperatures were comparable. All of the patients included in the study were given the same instructions and the only parameter that was altered between the two groups was exposure to the warmed injection room prior to and following tracer administration. It was assumed that the patients were exposed to similar outdoor temperatures prior to arrival in the department. Second, we did not evaluate the benefit of warming blankets compared to the effect of ambient temperature control. Further research into the most effective method of patient warming could be beneficial. Finally, we chose to maintain the temperature of the injection room at 24°C based on results suggested in the literature [13, 14], as well as practical considerations for the patients and the department. There were two patients prepared in the warmed injection room who showed FDG uptake by BAT. Both of these patients were scanned when the outdoor temperature was below freezing (0°C, 32°F). It is possible that increasing the temperature of the injection room or the time spent in the warmed room prior to scanning may have reduced tracer accumulation in BAT. For example, when outdoor temperatures are below freezing perhaps patients should be warmed for a longer period of time prior to tracer administration. Further research into the development of a patient warming protocol optimized for the environmental temperature may prove beneficial.
When available, PET-CT is often effective in distinguishing pathologic FDG uptake from that by BAT. However, extensive uptake by BAT can compromise the interpretation of the PET scan even if CT is available. Therefore, it is always prudent to minimize uptake by BAT, and, therefore, we consider our approach useful even when PET-CT is available.
Conclusion
Controlling the room temperature at a constant 24°C (75°F) half an hour prior to and during the uptake period significantly decreases FDG uptake by BAT in children. One method to ensure that a constant temperature is maintained prior to imaging includes installing an individualized thermostatic control in the injection room and monitoring room temperature with a thermometer. Since physiologic tracer accumulation may be a source of false-positive scans or may obscure adjacent metabolically active disease, altering the patient preparation protocol to include warming prior to imaging could prove beneficial. This is likely to be particularly important during the winter season when the likelihood of metabolically active BAT is higher.
References
Clarke JR, Brglevska S, Lau EW, Ramdave S, Hicks RJ. Atypical brown fat distribution in young males demonstrated on PET/CT. Clin Nucl Med 2007;32:679–82.
Yeung HW, Grewal RK, Gonen M, Schöder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 2003;44:1789–96.
Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med Mol Imaging 2002;29:1393–98.
Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J Nucl Med 2003;44:170–76.
Wehrli NE, Bural G, Houseni M, Alkhawaldeh K, Alavi A, Torigian DA. Determination of age-related changes in structure and function of skin, adipose tissue, and skeletal muscle with computed tomography, magnetic resonance imaging, and positron emission tomography. Semin Nucl Med 2007;37:195–205.
Cohade C, Mourtzikos K, Wahl R. “USA-Fat”: prevalence is related to ambient outdoor temperature – evaluation with 18F-FDG PET/CT. J Nucl Med 2003;44:1267–70.
Kim S, Krynyckyi BR, Machac J, Kim CK. Temporal relation between temperature change and FDG uptake in brown adipose tissue. Eur J Nucl Med Mol Imaging 2008;35:984–89.
Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277–359.
Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol 2005;35:984–90.
Parysow O, Mollerach AM, Jager V, Racioppi S, San Roman J, Gerbaudo VH. Low-dose oral propranolol could reduce brown adipose tissue F-18 FDG uptake in patients undergoing PET scans. Clin Nucl Med 2007;32:351–57.
Söderlund V, Larsson SA, Jacobsson H. Reduction of FDG uptake in brown adipose tissue in clinical patients by a single dose of propranolol. Eur J Nucl Med Mol Imaging 2007;34:1018–22.
Tatsumi M, Engles JM, Ishimori T, Nicely O, Cohade C, Wahl RL. Intense (18)F-FDG uptake in brown adipose fat can be reduced pharmacologically. J Nucl Med 2004;45:1189–93.
Garcia CA, Van Nostrand D, Atkins F, Acio E, Butler C, Esposito G, et al. Reduction of brown fat 2-deoxy-2-[F-18]fluoro-D-glucose uptake by controlling environmental temperature prior to positron emission tomography scan. Mol Imaging Biol 2006;8:24–9.
Christensen CR, Clark PB, Morton KA. Reversal of hypermetabolic brown adipose tissue in F-18 FDG PET imaging. Clin Nucl Med 2006;31:193–96.
Garcia CA, Van Nostrand D, Majd M, Atkins F, Acio E, Sheikh A, et al. Benzodiazepine-resistant "brown fat" pattern in positron emission tomography: two case reports of resolution with temperature control. Mol Imaging Biol 2004;6:368–72.
Gessner K. Conradi Gesneri medici Tigurini Historiae Animalium: Lib 1 - De Quadrupedibus Viviparis (1551).
Cannon B, Nedergaard J. Developmental biology: Neither fat nor flesh. Nature 2008;454:947–48.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zukotynski, K.A., Fahey, F.H., Laffin, S. et al. Constant ambient temperature of 24°C significantly reduces FDG uptake by brown adipose tissue in children scanned during the winter. Eur J Nucl Med Mol Imaging 36, 602–606 (2009). https://doi.org/10.1007/s00259-008-0983-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0983-y