Abstract
Purpose
To assess the feasibility and first experience of combined 18F-FDG-PET)/dynamic contrast-enhanced (DCE) CT in evaluating breast cancer.
Methods
Nine consecutive female patients (mean age 64.2 years, range 52–74 years) with primary breast carcinoma were prospectively recruited for combined 18F-FDG PET/DCE-CT. Dynamic CT data were used to calculate a range of parameters of tumour vascularity, and tumour 18F-FDG uptake (standardized uptake value, SUVmax) was used as a metabolic indicator.
Results
One tumour did not enhance and was excluded. The mean tumour SUVmax was 7.7 (range 2.4–26.1). The mean values for tumour perfusion, perfusion normalized to cardiac output, standard perfusion value (SPV) and permeability were 41 ml/min per 100 g (19–59 ml/min per 100 g), 0.56%/100 g (0.33–1.09%/100 g), 3.6 (2.5–5.9) and 0.15/min (0.09–0.30/min), respectively. Linear regression analysis showed a positive correlation between tumour SUV and tumour perfusion normalized to cardiac output (r=0.55, p=0.045) and a marginal correlation between tumour SUV and tumour SPV (r=0.19, p=0.065). There were no significant correlations between tumour SUV and tumour perfusion (r=0.29, p=0.401) or permeability (r=0.03, p=0.682).
Conclusion
The first data from combined 18F-FDG-PET/DCE-CT in breast cancer are reported. The technique was successful in eight of nine patients. Breast tumour metabolic and vascular parameters were consistent with previous data from 15O-H2O-PET.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cause of cancer death in women [1]. However, recent guidelines for stand-alone 18F-FDG-PET generally do not recommend this modality for initial staging of breast cancer [2]. There is presently a lack of data on the use of combined 18F-FDG-PET/CT in primary carcinoma of the breast [3]. It is well recognised that combined PET/CT systems offer CT attenuation correction and anatomical localization and this has added value over PET stand-alone systems in a number of cancers [4, 5]. However, there is also potential for using functional CT imaging, which to date has been largely overlooked despite the ability to obtain perfusion data without an on-site cyclotron whilst minimizing partial volume effects when evaluating tumour perfusion with PET [6].
PET/dynamic contrast-enhanced (DCE) CT can be used to study important metabolic–flow relationships in tumours [7] as well as simultaneously staging the patient. Metabolic and flow data are important in oncology, since they can predict patient prognosis, and response to chemotherapy and radiotherapy [8–12]. Here we report a prospective study of the initial use and feasibility of combined 18F-FDG-PET/DCE-CT in patients presenting with primary early breast cancer.
Materials and methods
Patients
Nine consecutive female patients (mean age 64.2 years; range 52–74 years) presenting with primary invasive carcinoma of the breast (Table 1) were prospectively recruited for combined 18F-FDG-PET/DCE-CT from the breast outpatient clinic. All patients gave informed consent to undergo investigation and the local ethics committee approved the study. Histology was used to determine tumour grade, stage and Nottingham Prognostic Indicator (NPI) [13].
PET/CT protocol
Following a 6-h patient fast, images were acquired 1 h after injecting 200 MBq of 18F-FDG using a dedicated combined PET/64-detector CT scanner. A reduced administered activity was used to reduce patient radiation exposure for this feasibility study, and thus imaging time was doubled. A CT scan was performed (for attenuation correction) using 64 × 3.75-mm detectors, a pitch of 1.5 and a 5-mm collimation (140 kVp and 80 mA in 0.8 s). Maintaining the patient position, a whole-body 18F-FDG PET emission scan was performed covering an area identical to that covered by the CT scan. All acquisitions were carried out in 2-D mode (8 min per bed position). Transaxial emission images of 3.27 mm thickness (pixel size 3.9 mm) were reconstructed using ordered subsets expectation maximization with two iterations and 28 subsets. The axial field of view was 148.75 mm, resulting in 47 slices per bed position. Next, maintaining patient position, a DCE-CT scan was performed. Patients received 50 ml iohexol contrast medium intravenously (Omnipaque 350 mg/ml iodine; GE Healthcare, Chalfont St Giles, UK) at 4 ml/s whilst 12 CT images (one per 5 s) were acquired at 100–150 mAs, 80 KV, using 64 × 0.6 mm detectors.
Image and data analysis
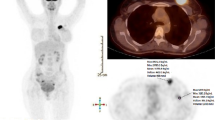
PET/CT images were displayed conventionally on a proprietary workstation (Fig. 1). FDG uptake was expressed as the maximum standardized uptake value (SUVmax).
Axial images at the level of the mid-breast, on CT (a), coregistered 18F-FDG PET (b) and fused PET/CT (c) show a 18F-FDG-avid carcinoma in the right breast. Image d, derived from the DCE-CT shows the same tumour as part of a colour parametric perfusion map. Image e shows the time enhancement curve automatically generated from the drawing of ROIs over the tumour. Image f is the accompanying derived Patlak plot. The negative HU values in the time density curve (e) are due to respiratory motion. Such values can be edited, but the limitations of the technique need to be appreciated
DCE-CT data were analysed using validated proprietary CT perfusion software (Winfun, Cambridge Computed Imaging, Bourne, UK). On the axial image displayed by the software, a region of interest (ROI) was drawn freehand around the breast tumour taking care to maintain the ROI within the boundaries of the mass, using the PET image for guidance. A further ROI was drawn freehand around the aorta taking care to maintain the ROI within the boundaries of the vessel by viewing the images on the console. Tumour perfusion values were obtained from automated processing of the enhancement–time curve data from the aortic and tumour ROIs using a previously described method [14] (Eq. 1, Fig. 1). The peak tumour enhancement value was used to calculate perfusion normalized to cardiac output (Eq. 2) and the standardized perfusion value (SPV) (Eq. 3) [15]. (Note the derivation of SPV is analogous to the derivation of SUV.) The calibration factor relating iodine concentration to measured attenuation for the PET-CT system used was determined by phantom studies as described previously [16]. Tumour vascular permeability was calculated using Patlak analysis [17].
Tumour vascular–metabolic relationships were described by the ratio of SUV to each of the four parameters reflecting tumour vascularity.
Statistical analysis
Following normality testing (Kolmogorov-Smirnov), the relationships between tumour metabolism and parameters of tumour vascularity were explored using linear regression analysis. Similarly, tumour vascular–metabolic relationships were correlated with tumour size. Metabolic–flow relationships versus histological grade, stage and NPI were examined using the Mann-Whitney U test. Statistical significance was at 5%.
Results
One of the nine breast tumours did not enhance or take up 18F-FDG and was excluded from further analysis. The mean maximum tumour diameter was 20.0 mm (range16–27 mm). The mean tumour SUVmax was 7.7 (range 2.4–26.1). The mean values for tumour perfusion and perfusion normalized to cardiac output were 41 ml/min per 100 g (19–59 ml/min per 100 g) and 0.56%/100 g (0.33–1.09%/100 g), respectively. The mean SPV was 3.6 (range=2.5–5.9). The mean tumour permeability was 0.15/min (0.09–0.30/min).
Statistical relationships
Tumour SUV was correlated with perfusion normalized to cardiac output (r=0.55; p=0.045) and there was a trend for SUV to correlate with tumour SPV (r=0.19; p=0.065). There was a negative trend between tumour perfusion and NPI (r=−0.60; p=0.115). There was no significant correlation between tumour SUV and tumour perfusion (r=0.29; p=0.401) or permeability (r=0.03; p=0.682). There was a statistically significant association between tumour grade and SUV/SPV (p=0.029), but no statistically significant associations were found between tumour vascular–metabolic relationships and tumour size or axillary nodes. There was a trend between SUV/SPV and NPI (r=0.60; p=0.117).
Discussion
The first use of combined 18F-FDG PET DCE-CT in breast cancer is described. The methodology was technically successful in all patients, although one tumour could not be characterized. Although the study population was limited, there were interesting metabolic–vascular relationships demonstrated: tumour perfusion normalized to cardiac output was significantly correlated with 18F-FDG uptake, and the relationship between tumour SPV and SUV values were of marginal significance. In view of previous studies highlighting the potential for metabolic–flow relationships to predict response of breast cancer to chemotherapy, and to assess treatment efficacy [9, 18], our findings support the possible applicability of combined 18F-FDG-PET/perfusion CT to breast cancer management.
Combining metabolic data from 18F-FDG PET with tumour vascularity measurements using CT obviates the need for an on-site cyclotron. Additionally, the spatial resolution of CT can reduce the impact of partial volume effects on perfusion values obtained by PET whilst avoiding spill-over of counts from vascular structures [6]. However, it has been argued that 15O-H20 PET is the ideal method for examining tumour flow [19]. In early breast cancer this approach has yet to be used, but there are data combining PET with 15O-H20 in more advanced breast cancer [9, 18, 20]. In two studies [18, 20], breast tumour perfusion values obtained with 15O-H20 (32 and 52 ml/min per 100 g) were in keeping with our findings (41 ml/min per 100 g). One of these studies also showed a significant correlation between FDG metabolic rate and perfusion (r=0.70, p<0.001) [18]. Our results did not show a correlation between FDG uptake and perfusion. However, we expressed 18F-FDG uptake as SUV rather than metabolic rate. Nevertheless, we did find a significant correlation with SUV when perfusion was normalized to cardiac output. Given that a change in cardiac output will alter tumour perfusion regardless of the density of tumour vessels, perfusion values normalized to cardiac output will more directly reflect the status of the tumour vasculature.
Breast cancer is a heterogeneous disease with a wide range of recognized prognostic factors. It was interesting that combining 18F-FDG PET with DCE-CT was helpful in predicting the histological grade of tumour. More complicated prognostic data are obtained by combining multiple risk factors in the NPI [13]. Since our population was limited in size it would have been challenging to show a statistically significant correlation between the NPI and combined 18F-FDG PET and DCE-CT data. However, there was a trend between SUV/SPV and NPI. Interestingly, despite 8 mm being within the spatial resolution of CT the tumour that did not enhance had the lowest NPI. This tumour would be regarded as at the limit of detectability on PET. If larger series confirm the prognostic significance of combined 18F-FDG-PET/DCE-CT parameters, it may ultimately be possible to propose a clinical role for the combined technique in the identification of patients with higher risk breast cancer who may benefit from more aggressive chemotherapy.
Although, this present study is the first to look at combined PET/CT perfusion, one group has performed (stand-alone) CT perfusion on breast tumours [21]. Whilst the exclusion rate was higher than our initial findings, their results were encouraging and the presented perfusion data (mean perfusion 31 ml/min per 100 g) were similar to our own (41 ml/min per 100 g), which suggests good reproducibility [21]. The only other DCE-CT data on breast cancer were applied to axillary lymph node assessment [22]. This technique showed promise in differentiating metastatic from inflammatory axillary lymph nodes in patients with breast carcinoma. An alternative and common approach to imaging vascular properties in breast cancer has been the use of DCE MRI [8]. This has the advantage that both structure and function can be assessed without ionizing radiation. However, two imaging systems are required to compare MR measurements of tumour vascularity with PET measurements of tumour metabolism as hybrid PET/MRI machines are currently only in development. Nonetheless there have been small investigations into the metabolic–flow relationships in breast cancer tumours using MRI and 18F-FDG PET [12, 23] and these have shown a positive relationship between metabolism and flow.
Presently there is only one published prospective study on the use of 18F-FDG-PET/CT in primary breast cancer, and that study excluded stage I disease [24]. PET/CT led to the restaging of 18% of the patients and to a change in the management of 13%. Therefore there may be a role for 18F-FDG-PET/CT in the management of patients with primary breast cancer, and even the new guidelines recognize that more evidence is required [2]. From our small cohort, an axillary metastasis was seen in one patient and in another a pleural fibroma was identified. The limited sensitivity of 18F-FDG PET in detecting axillary metastases has been shown [3]. Interestingly, in the prospective study discussed above [14], previously undetected axillary lymph node metastases were found in three patients.
Conclusion
We present the first data from combined PET/DCE-CT in patients with breast cancer. Despite a limited number of patients, the technique was successful in all but one patient. There was a positive relationship between some parameters of tumour metabolism and vascularity in keeping with other studies using PET 15O-H20 [18] and MRI [12]. The breast tumour derived CT perfusion values in this study were consistent with other data obtained by CT [21] and 15O-H20 PET [18, 20]. As such the multimodality technique of combined 18F-FDG-PET/DCE-CT is technically feasible and has potential to contribute to the management of patients with breast cancer.
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137–50.
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 2008;49:480–508.
Tatsumi M, Cohade C, Mourtzikos KA, Fishman EK, Wahl RL. Initial experience with FDG-PET/CT in the evaluation of breast cancer. Eur J Nucl Med Mol Imaging 2006;33:254–62.
Syed R, Bomanji JB, Nagabhushan N, Hughes S, Kayani I, Groves A, et al. Impact of combined (18)F-FDG PET/CT in head and neck tumours. Br J Cancer 2005;92:1046–50.
Lardinois D, Weder W, Hany TF, Kamel EM, Korom S, Seifert B, et al. Staging of non-small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 2003;348:2500–7.
Bacharach SL, Libutti SK, Carrasquillo JA. Measuring tumor blood flow with H(2)(15)O: practical considerations. Nucl Med Biol 2000;27:671–6.
Miles KA, Griffiths MR, Keith CR. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging 2006;33:22–8.
Hayes C, Padhani AR, Leach MO. Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR Biomed 2002;15:154–63.
Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Schubert EK, Tseng J, et al. Changes in blood flow and metabolism in locally advanced breast cancer treated with neoadjuvant chemotherapy. J Nucl Med 2003;44:1806–14.
Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999. EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773–82.
Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000;18:1676–88.
Semple SI, Gilbert FJ, Redpath TW, Staff RT, Ahearn TS, Welch AE. The relationship between vascular and metabolic characteristics of primary breast tumours. Eur Radiol 2004;14:2038–45.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
Miles KA. Measurement of tissue perfusion by dynamic computed tomography. Br J Radiol 1991;64:409–12.
Miles KA, Griffiths MR, Fuentes MA. Standardised perfusion value: a universal contrast enhancement scale that correlates with FDG-PET in lung cancer. Radiology 2001;220:548–53.
Miles KA, Young H, Chica SL, Esser PD. Quantitative contrast-enhanced computed tomography: is there a need for system calibration? Eur Radiol 2007;17:919–26.
Miles KA, Kelley BB. CT measurements of capillary permeability within nodal masses: a potential technique for assessing the activity of lymphoma. Br J Radiol 1997;70:74–9.
Mankoff D, Dunnwald LK, Gralow JR, Georgiana EK, Charlop A, Lawton TJ, et al. Blood flow and metabolism in locally advanced breast cancer: relationship to response to therapy. J Nucl Med 2002;43:500–9.
Anderson H, Price P. Clinical measurement of blood flow in tumours using positron emission tomography: a review. Nucl Med Commun 2002;23:131–8.
Hentschel M, Paulus T, Mix M, Moser E, Nitzsche EU, Brink I. Analysis of blood flow and glucose metabolism in mammary carcinomas and normal breast: a H2(15)O PET and 18F-FDG PET study. Nucl Med Commun 2007;28:789–97.
Hirasawa H, Tsushima Y, Hirasawa S, Takei H, Taketomi-Takahasi A, Takano A, et al. Perfusion CT of breast carcinoma: arterial perfusion of nonscirrhous carcinoma was higher than that of scirrhous carcinoma. Acad Radiol 2007;14:547–52.
Liu Y, Bellomi M, Gatti G, Ping X. Accuracy of computed tomography perfusion in assessing metastatic involvement of enlarged axillary lymph nodes in patients with breast cancer. Breast Cancer Res 2007;9:R40.
Brix G, Henze M, Knopp MV, Lucht R, Doll J, Junkermann H, et al. Comparison of pharmacokinetic MRI and [18F]fluorodeoxyglucose PET in the diagnosis of breast cancer: initial experience. Eur Radiol 2001;11:2058–70.
Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys 2008;71:695–704.
Acknowledgments
The Breast Cancer Campaign (UK charity) funded this work. This work was undertaken at UCLH UCL, which received a proportion of the funding from the NIHR Biomedical Research Centres funding scheme of the UK Department of Health. Gordon Wishart receives some funding from the Cambridge Biomedical Research Centre. None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this article. We acknowledge the expertise of Professor Brian Hutton, Chair of Nuclear Medicine Physics, for his advice on normalizing SUV. We are grateful to Alison Hallett and other staff of the Cambridge Breast Unit for their help with this project, and thank the patients for volunteering for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Groves, A.M., Wishart, G.C., Shastry, M. et al. Metabolic–flow relationships in primary breast cancer: feasibility of combined PET/dynamic contrast-enhanced CT. Eur J Nucl Med Mol Imaging 36, 416–421 (2009). https://doi.org/10.1007/s00259-008-0948-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0948-1