Abstract
Purpose
The higher prevalence rates of depression and anxiety disorders in women compared to men have been associated with sexual dimorphisms in the serotonergic system. The present positron emission tomography (PET) study investigated the influence of sex on the major inhibitory serotonergic receptor subtype, the serotonin-1A (5-HT1A) receptor.
Methods
Sixteen healthy women and 16 healthy men were measured using PET and the highly specific radioligand [carbonyl-11C]WAY-100635. Effects of age or gonadal hormones were excluded by restricting the inclusion criteria to young adults and by controlling for menstrual cycle phase. The 5-HT1A receptor BPND was quantified using (1) the ‘gold standard’ manual delineation approach with ten regions of interest (ROIs) and (2) a newly developed delineation method using a PET template normalized to the Montreal Neurologic Institute space with 45 ROIs based on automated anatomical labeling.
Results
The 5-HT1A receptor BPND was found equally distributed in men and women applying both the manual delineation method and the automated delineation approach. Women had lower mean BPND values in every region investigated, with a borderline significant sex difference in the hypothalamus (p = 0.012, uncorrected). There was a high intersubject variability of the 5-HT1A receptor BPND within both sexes compared to the small mean differences between men and women.
Conclusions
To conclude, when measured in the follicular phase, women do not differ from men in the 5-HT1A receptor binding. To explain the higher prevalence of affective disorders in women, further studies are needed to evaluate the relationship between hormonal status and the 5-HT1A receptor expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Twice as many women compared to men suffer from major depression and anxiety disorders, which affect nearly one-fifth of the Western population and cause an immense personal, social and economic burden (see, e.g. [1]). The striking sex difference in prevalence rates appears to be independent of country and culture and cannot be entirely explained by psychosocial factors, social support or coping style [2]. Rather, it has been demonstrated that women and men differ significantly in brain structure and function (for reviews, see [3, 4]), suggesting a higher biological susceptibility to depression in females. Interestingly, a growing body of evidence suggests that the serotonergic system, which is known to be altered in affective disorders, may be sexually dimorphic. In the brain of female rodents, a higher tryptophan content and utilization rate [5], a higher serotonin synthesis and serotonin turnover [6] and overall higher serotonin levels [7] have been demonstrated. Several human studies reported a greater responsiveness to serotonergic challenges in female participants [8]. Acute tryptophan depletion, used as an experimental model for depression, was shown to affect females to a significantly larger extent than males [9]. And also, in vivo assessment of serotonergic structure and function with positron emission tomography (PET) revealed sex differences in the serotonin neurotransmission. Major methodological advancements and the development of selective radioligands allow now for a precise quantification and localisation of serotonergic receptors and the serotonin transporter [10]. The few studies that have been conducted in human subjects, however, report findings that are in part contrasting the results obtained in rodents. Using PET and the radioligand α-[11C]methyl-l-tryptophan, the serotonin synthesis rate in the brain of healthy male subjects was found to be higher than the synthesis rate in females [11]. A lower serotonin transporter binding in women was observed using the radioligand [11C]MADAM [12]. A lower 5-HT2 receptor-binding capacity in women was also reported [13]. The reason for the discrepant results is still unclear.
Several lines of evidence indicate a sexual dimorphism of the serotonin-1A (5-HT1A) receptor subtype, which is suspected to be substantially involved in the pathogenesis of depressive illness [14], anxiety disorders [15] and suicide [16]. The 5-HT1A receptor binding is of particular interest for psychiatry as it was shown to correlate with the treatment effect of SSRIs (selective serotonin reuptake inhibitors) as recently demonstrated by our group [17]. The 5-HT1A receptors serve both as somatodendritic autoreceptors on serotonergic neurons in the raphe nuclei of the brainstem and as postsynaptic heteroreceptors. The highest densities of the postsynaptic receptor are found in limbic areas (in particular in the hippocampus and the anterior cingulate cortex), while basal ganglia and the cerebellum exhibit very low densities [18]. Postsynaptically located 5-HT1A receptors influence a wide range of physiological and behavioural states by modulating cholinergic, dopaminergic, glutamatergic and GABAergic neurotransmitter release (for review, see [19]), while autoreceptor activation in the raphe nuclei reduces serotonergic cell firing and inhibits excitation and neural activation in targeted cortical areas [20].
With regard to sex differences, the presynaptic function of the 5-HT1A receptor was proposed to be decreased [21] or increased in female rodents [22]. Animal studies suggested area specific sexual dimorphisms with higher 5-HT1A receptor binding in females in some regions (e.g. the anterior cingulate cortex) while lower in other regions (e.g. the hippocampus) [23, 24]. A higher 5-HT1A receptor density in women was reported post mortem in the dorsal raphe nucleus [25] and in the prefrontal cortex [26]. Several other human post-mortem studies, however, found no gender differences in binding sites [27-29]. The few PET studies investigating sex differences in the 5-HT1A receptor in humans in vivo resulted in controversial findings. Using the radioligand [carbonyl-11C]WAY-100635, either a higher 5-HT1A receptor-binding potential (BPND, “BP non displaceable” according to the nomenclature established in the consensus paper by Innis et al. [30]) in female subjects was found [12, 31] or only age-related effects without an overall influence of sex [32].
Given the controversial results, the aim of the present study was to prove the hypothesis of sex differences in the 5-HT1A receptor-binding potential in 32 healthy volunteers (16 male and 16 female subjects) matched to age and socio-economic status. To account for the presumable influence of gonadal hormones on the receptor binding [33], all female subjects were measured within the follicular phase of the menstrual cycle. To control for a possible effect of age [32, 34], the age range of the subjects was limited to 20–35 years. Furthermore, a newly developed method for the anatomical delineation of the 5-HT1A receptor maps was introduced using a tracer-specific template, a coregistered region of interest (ROI) template and automated anatomical labelling (AAL) [35].
Materials and methods
Subjects
Thirty-six healthy subjects (18 females and 18 males) participated in the PET study approved by the Ethics Committee at the Medical University of Vienna. All subjects gave written informed consent at the screening visit and were recruited from the community via advertisements. Female and male subjects were matched for age and socio-economic status. The criteria for participation were age of 20 to 35 years and physical health as assessed by a general physical examination including neurological status, electrocardiogram and a routine laboratory screening. The narrow age range was chosen to minimize possible age effects on the 5-HT1A receptor-binding potential [34]. Exclusion criteria comprised any chronic medication or hormonal treatment including hormonal contraception within 6 months prior to the study, drug abuse, pregnancy, irregular menstrual cycles, abnormalities in the physical examination or any Axis I, DSM IV, psychiatric disorder as assessed by the MINI International Neuropsychiatric Interview obtained by an experienced psychiatrist [36]. Female participants were tested for pregnancy at the screening visit and before each PET measurement using an human choriogonadotropin urine test (ACON Laboratories, Inc., San Diego, CA, USA).
Four subjects were excluded from the final analysis either because of outliers in radiochemical variables (one female, one male) or because the time activity curves of the cerebellar regions exceeded the mean cerebellar binding by more than two standard deviations (one female, one male) [37]. The final statistical analysis included 16 female (age 24.1 ± 2.6 years, mean ± SD) and 16 male subjects (age 26.2 ± 4.2 years, mean ± SD). To control for menstrual cycle phase at PET measurement, blood samples were collected in the morning prior to the first examination, prior to PET scans and on the day of the final examination from all female participants. Plasma levels of estrogen, progesterone, testosterone, follicle stimulating hormone and luteinizing hormone (LH) were quantified by the Clinical Institute for Medical and Chemical Laboratory Diagnostics at the Medical University of Vienna (for details of standards and references, see http://www.kimcl.at). All female subjects were measured within the follicular phase, i.e. within the first 3–10 days of the menstrual cycle. The female subjects were only measured when their hormonal plasmal levels lied within the follicular reference range of 0.5–1.0 ng/ml for progesterone, 22–215 pg/ml for 17β-estradiol and 2.4–12.6 mU/ml for LH.
PET image acquisition
Subjects were measured using an ADVANCE full-ring PET scanner (General Electric Medical Systems, Milwaukee, WI, USA) at the Department of Nuclear Medicine, Medical University of Vienna. For quantification of the 5-HT1A receptor binding, the radioligand [carbonyl-11C]WAY-100635 was chosen, a highly selective and specific 5-HT1A receptor antagonist [38]. The tracer was prepared in a fully automated PET synthesizer (GE Healthcare, Uppsala, Sweden) at the Cyclotron Unit of the PET centre at the Medical University of Vienna as recently described by our group [39]. For image acquisition, the head of the subject was positioned parallel to the orbitomeatal line using a laser beam system to ensure the covering of the cerebellum in the field of view (FOV). Head movements were minimized by polyurethane moulded cushions and straps around forehead and chin. A transmission scan (5 min) was performed in two-dimensional mode for correction of tissue attenuation using a retractable 68Ge ring source. The three-dimensional image acquisition started simultaneously with the intravenous bolus injection of the radioligand [carbonyl-11C]WAY-100635, soluted in phosphate-buffered saline (pH 7.4). The mean injected activity of the radioligand was 5.65 ± 0.8 (mean ± SD) MBq/kg body weight, with a mean specific radioactivity at the time of injection of 153 ± 117 GBq/μmol, and a radiochemical purity of 97.5 ± 1.3%. Dynamic scans were collected in three-dimensional mode and comprised a series of 30 successive time frames (15 × 1 min, 15 × 5 min) resulting in a total acquisition time of 90 min. Data were reconstructed in a 128 × 128 × 35 matrix, with a slice thickness of 4.25 mm using an iterative filtered back-projection algorithm (FORE-ITER). The spatial resolution of the scanner was 4.36 mm full-width at half-maximum at the centre of the FOV.
MR image acquisition
High-resolution T1-weighted images were acquired from all subjects using a 3-tesla whole-body MEDSPEC S300 MR-scanner (Bruker BioSpin, Ettlingen, Germany) and a magnetization-prepared rapid gradient-echo sequence (128 slices, 256 × 256 matrix, slice thickness 1.56 mm, voxel size 0.78 × 0.86 mm). The structural MR images were coregistered to summed PET images (PETADD) for definition of ROIs.
Data analysis
For quantification of the 5-HT1A receptor BPND, we applied the Simplified Reference Tissue Model (SRTM) [40–42] as implemented in PMOD 2.9 (PMOD Technologies Ltd., Zurich, Switzerland, http://www.pmod.com) [43] using the cerebellum as reference region. Furthermore, in a second approach, the 5-HT1A receptor BPND was quantified by applying the non-invasive Logan Plot method [44]. Cerebellar time activity curves were normalized to its peak to identify and exclude subjects with high specific binding in the reference region [37]. Two complementary approaches for the delineation of ROIs were applied, a manual delineation method with 10 ROIs (including the cerebellum as reference region) and an ROI-template-based, automated method with 45 ROIs (including the cerebellum as reference region), which was used to exclude possible bias caused by manual delineation.
Manual delineation of ROIs
For the manual delineation method, the individual MR images were coregistered to individual summed PET images (PETADD) of 30 dynamic time frames using Statistical Parametric Mapping software (SPM2, http://www.fil.ion.ucl.ac.uk/spm/) [45, 46]. Nine regions of interest known for their high 5-HT1A receptor density [18] were delineated on the coregistered MR images according to the standardised anatomical criteria established by Bremner et al. [47] and described by our group [15, 17]. Delineation was done by one investigator (C.S.) who was blind to gender of the subjects. The regions of interest (given in Fig. 3) included the anterior and posterior cingulate cortices, insula, hippocampus, hypothalamus, amygdala, the medial orbitofrontal cortex, the retrosplenial cortex and the cerebellum as region of reference [48]. The raphe nuclei were defined on the PETADD image by fixing a circular volume of interest (0.08 cm3) over the highest binding signal in the dorsal midbrain area.
Automated delineation of regions of interest
For the automated delineation method, we used an ROI-template normalized to the 5-HT1A distribution map in the stereotactic space of the MNI/ICBM brain (Montreal Neurologic Institute/International Consortium for Brain Mapping) and PMOD 2.9 [45]. Individual dynamic PET data were normalized to this standardised 5-HT1A distribution map that corresponded to the ROI template. All 45 ROIs of this approach were based on the anatomical AAL atlas implemented in the SPM2 software [35]. The ROIs are given in Table 1. Figures 1 and 2 show representative ROIs overlayed on the 5-HT1A receptor-binding potential map. Time activity curves of the 45 regions were used for quantification in PMOD 2.9.
PET template for automated delineation of ROIs. The 45 delineated ROIs are based on the AAL atlas implemented in the SPM2 software (toolbox) [35]. The figure shows representative ROIs on two axial slices (a/b vs. c/d) overlayed on a serotonin-1A receptor (5-HT1A) receptor distribution map normalized to the MNI space either in black/white (a, c) or in colour (b, d). Low 5-HT1A receptor BPND values are dark blue and high BPND values are dark red (see colour bar). The numbering of the regions corresponds to the regions of interest given in Table 1
A three-dimensional view on the PET template for automated delineation of ROIs. a Dorsolateral view on the ROI template. The numbering of the regions corresponds to the regions of interest given in Table 1. b Sagittal and an axial views of the serotonin-1A receptor (5-HT1A) receptor distribution map. The labelling indicates the localisation of regions of interest used for the manual delineation method. ACC Anterior cingulate cortex, PCC posterior cingulate cortex, HIP hippocampus, INS insula. c Three-dimensional sagittal view on the ROI template. CER Cerebellum (region of interest), DRN dorsal raphe nuclei (used as an ROI for the quantification of the presynaptic 5-HT1A receptor BPND). d Three-dimensional sagittal view on the ROI template projected on an axial slice of the 5-HT1A receptor distribution map. The numbering of the regions corresponds to the regions of interest given in Table 1
Statistical analysis
Statistical analyses of the regional mean 5-HT1A receptor BPND were done using the software SPSS 12.0.1 (SPSS Inc., Chicago, IL, USA). The threshold of significance was set at p < 0.05, all tests were two-tailed. To control for normal distribution and equality of co-variance, the Kolmogorov–Smirnov test and the Levene’s test were performed, respectively. Independent-samples t tests were used to test for sex differences in age, radiochemical variables and the normalized regional tracer delivery (R 1). Two-tailed Pearson product-moment correlation coefficients were calculated to test for a possible effect of age or radiochemical variables on the 5-HT1A receptor BPND (i.e. injected activity, radiochemical purity, weight of WAY-100634, weight of unlabelled WAY-100635 and specific activity of the radioligand). Variables without influence on the 5-HT1A receptor-binding potential were dropped from further analysis. To evaluate a mean effect of sex on the 5-HT1A receptor BPND, a two-way analysis of variance (ANOVA) was conducted using sex as between-subject factor, region as within-subject factor, subjects as random factor and the interaction term sex by region. For an additional, exploratory analysis, independent-sample t tests, with sex as independent variable, were conducted in each region of interest. Bonferroni adjustment for multiple testing was used to correct for type I error. Both delineation methods were statistically analysed in the described way.
Results
The regional 5-HT1A receptor BPND values separated for men and women are given in Fig. 3 for the manual delineation method and in Table 1 for the ROI-template-based approach. The regional distribution of the 5-HT1A receptor BPND was in accordance with published in vivo and post-mortem studies showing the highest 5-HT1A receptor expression in limbic areas as in the hippocampus and the anterior cingulate cortex [48]. Female and male participants did not differ significantly by age, radiochemical variables, regional tracer delivery (R 1) or binding in the reference region (p > 0.05). There was no effect of age or radiochemical variables on the 5-HT1A receptor BPND (Pearson correlation, p > 0.05).
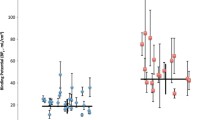
The serotonin-1A receptor (5-HT1A) binding potential (BPND) in men and women using a manual delineation approach in nine regions of interest and the cerebellum as region of reference (black bars men, white bars women). An independent-samples ANOVA revealed no significant sex differences in the mean 5-HT1A receptor BPND between men and women (p > 0.05). The asterisk (*) indicates a lower 5-HT1A receptor BPND in women (p = 0.012) in the hypothalamus that did not withstand the Bonferroni correction for multiple testing
Results of the manual delineation method
Using the manual delineation method, the mean 5-HT1A receptor BPND over all regions of interest was 3.38 ± 0.07 (mean ± SE) for men and 3.11 ± 0.07 (mean ± SE) for women. The two-way ANOVA did not confirm the hypothesis of a main effect for sex (F 1,30 = 1.3, p = 0.278). However, a slightly lower mean 5-HT1A receptor BPND was observed in all regions of interest in females (Fig. 3). Also, no significant interaction was found between sex and region. Post hoc independent-samples t tests done in nine manually drawn regions of interest (anterior and posterior cingulate cortex, amygdala, hippocampus, hypothalamus, insula, orbitofrontal cortex, retrosplenial cortex and raphe nuclei) did not reveal any significant sex differences except for a trend in the hypothalamus (t 30 = 2.7; p = 0.012) that did not withstand the Bonferroni correction (adjusted significance level of p < 0.0056). The results do not support the hypothesis of sex differences in the 5-HT1A receptor BPND or in the 5-HT1A receptor distribution.
Results of the automated delineation method
Using the automated delineation method, the mean 5-HT1A receptor BPND over all regions of interest was 3.54 ± 0.05 (mean ± SE) for men and 3.32 ± 0.05 (mean ± SE) for women. The two-way ANOVA did not reveal any significant effect for sex (F 1,30 = 0.8, p = 0.378), and there was no significant interaction between sex and region. Post hoc independent-samples t tests done in the 45 AAL regions of interest did not reveal any significant sex differences (the hypothalamus, however, is not included in the AAL regions). Therefore, the results of the automated method do not support the hypothesis of sex differences in the 5-HT1A receptor BPND or in the 5-HT1A receptor distribution. A second analysis in the AAL regions applying the non-invasive Logan plot yielded similar results to the SRTM (results not shown).
Discussion
The main finding of this in vivo study is the lack of a sex-specific 5-HT1A receptor binding. This in vivo investigation using PET and the highly specific radioligand [carbonyl-11C]WAY-100635 did not confirm the hypothesis of sex differences in the 5-HT1A receptor BPND suggested in some previous PET studies [12, 31]. Our results were obtained independently using two delineation methods for regions of interest and the non-invasive SRTM [41] and Logan Plot method [44]. The absence of sex effects in the 5-HT1A receptor BPND is in line with several human post-mortem [27–29] and in vivo studies [32, 49]. Interestingly, the mean observed 5-HT1A receptor BPND tended to be lower in females in all regions of interest (see Fig. 3), which is in contrast to three previous studies performed using the same radioligand in healthy subjects [12, 31, 50]. One of these studies reported a higher 5-HT1A receptor BPND in females using a clearly broader age range compared to our study. The sex difference was found only when using arterial input function but not when using the non-invasive SRTM [31]. A second, subsequent study also found a higher 5-HT1A receptor BPND in females using both an arterial input function and the SRTM for comparison [50]. Limits of this study were a small sample size (six females and eight males) and a broad age range in males (25–65 years), while the age range for females was quite narrow (47–52 years). Both groups did not control for the possible effects of gonadal steroids [51]. A third study, however, found a significantly higher 5-HT1A receptor BPND using SRTM in females in a demographically comparable sample to ours [12]. Given the similar study design (control for hormonal status and young age of the participants), it remains unclear why the suggested sex difference in the 5-HT1A receptor binding was not replicated in the present investigation. Even more surprising, the mean 5-HT1A receptor BPND in the present study was lower (though not significantly) in females compared to males in every region examined, which is the opposite of the findings of Jovanovic et al. [12].
Several issues and limitations need to be considered in the interpretation of these results. First, the disaccording results may reflect a small effect size of sex, which would conflict with the inherent limitations of PET studies. These include the particularly high intersubject variability compared to the broad overlapping range between the male and female 5-HT1A receptor BPND [37] and the small sample size (because of ethical considerations and high costs of PET measurements). Second, given the significant correlations between the 5-HT1A receptor BPND and personality traits as aggression [31] or anxiety [52], recruiting by advertisement and random inclusion of subjects in the lower or higher range in personality scales might bias the study sample. Methodological bias, however, is unlikely as the present results have been obtained using two independent approaches for definition of brain regions after a careful control for sex differences in binding of the reference region. In addition, two independent approaches for quantification have been applied, the SRTM and the Logan non-invasive approach that yielded comparable results in the ANOVA (data derived from the Logan approach not shown). By using an automated delineation, for the first time, a comprehensive distribution map of the 5-HT1A receptor BPND in 45 regions of interest has been obtained in males and females.
Another contributing factor for diverging results may be the sex-specific effect on age-dependent increase or decrease in the 5-HT1A receptor binding. Several post-mortem studies using the 5-HT1A receptor agonist [3H]8-OH-DPAT as radioligand did not report any significant sex differences in receptor binding or distribution, while an age-dependent decline of binding sites was found in men but not in women [27–29]. In women, the 5-HT1A receptor binding in the occipital cortex even seemed to increase with age [27]. A similar trend was also observed in recent human PET studies using the radioligand [carbonyl-11C]WAY-100635. Here, a significant inverse correlation of the 5-HT1A receptor BPND with age was found in men [34, 49] but not in women [32]. Therefore, comparing the 5-HT1A receptor expression in inhomogeneous groups of subjects with regard to age may yield deceptive results. The 5-HT1A receptor BPND may be higher in females when comparing men and women in older age but lower in young adulthood. The results of the present study would be in line with the hypothesis since the female subjects included in the present study showing a trend towards a lower mean 5-HT1A receptor BPND were particularly young (24.1 ± 2.6 years, mean age ± SD).
Furthermore, the sex-dependent age effect on the 5-HT1A receptor binding might be associated with lifetime changes in the production of gonadal steroids in men and women. An abundant amount of literature demonstrates the influence of steroid hormones on the 5-HT1A receptor expression. Long-term administration of estrogen was demonstrated to increase the activity of the tryptophan hydroxylase [53] while down regulating the pre- and postsynaptic 5-HT1A receptor binding [33, 54]. The effect was particularly pronounced in the raphe nuclei and in the hippocampus, i.e. in regions with a lower 5-HT1A receptor-binding potential in female rodents [24]. A phase effect of the menstrual cycle on the 5-HT1A receptor BPND was demonstrated in preclinical research [55] and indicated in a recent PET study, which, however, lacked statistical power [56]. A correlation of the 5-HT1A receptor binding with progesterone plasma levels has been observed by our group [unpublished results]. Since we observed a slightly lower 5-HT1A receptor BPND in women measured in the early follicular phase vs. in women measured in the late follicular phase, this might be indicative of a down regulation of the receptor by high progesterone levels at the end of the menstrual cycle, with a recovery of the 5-HT1A receptor BPND later in the cycle. However, as long as the temporal relation of changes in the 5-HT1A receptor BPND with regard to steroid hormone plasma levels is not known, these assumptions remain highly speculative.
A heightened serotonin neurotransmission caused by the long-term down regulation of somatodendritic 5-HT1A receptors by higher estrogen and progesterone levels in females would be in line with animal studies that demonstrated a higher serotonin synthesis and turnover [6] and overall higher 5-HT levels [7] in the brain of female rodents. According to this hypothesis, a slightly lower overall 5-HT1A receptor expression in females, as indicated in the present study, would lead to a reduced serotonergic inhibition on postsynaptic mainly glutamatergic neurons [19] and increased serotonin turnover [57]. This would be compatible with a greater serotonergic responsiveness in females. Indirectly, the higher prevalence rate of mood disorders in women that becomes apparent only with the onset of puberty while diminishing after the menopause might be an evidence for the effect of ovarian steroids on the serotonin neurotransmission [58]. Therefore, the restriction of female participants to women with regular menstrual cycle duration and the conductance of PET measurements in a restricted phase of the menstrual cycle appear as important prerequisites for revealing an either higher or lower 5-HT1A receptor BPND in women compared to men.
Finally, it must be considered that the hypothesis of sex differences in the human serotonergic system may be simply false. Indeed, the present study is the fourth example of differing results with regard to sex effects on the serotonergic system. The 5-HT synthesis has been reported both lower in females [11, 59] and lower in males [60]. The 5-HTT binding potential was reported to be both higher in females [61] or higher in males [12]. In a large study sample of 88 subjects, Praschak-Rieder et al. found no sex differences in 5-HTT binding [62]. Another study with 52 healthy subjects did not reveal any sex differences in the 5-HT2A receptor binding [63], which was suggested before by Biver et al. [13]. Therefore, sex differences in the incidence of affective disorders might be not adequately explained by sex differences in serotonergic receptor or transporter densities.
In summary, our results do not confirm the hypothesis of sex differences in the 5-HT1A receptor BPND in 16 healthy young women when compared to 16 healthy young men. This was demonstrated using two independent delineation methods (manual vs. normalized ROI-template-based) and two non-invasive quantification approaches (SRTM vs. Logan). The automated delineation method was shown to be reliable in comparison with the manual delineation with the additional benefit of a high number of regions of interest and a lower variability in regional volumes. A slightly, though not significantly, lower mean 5-HT1A receptor BPND in female subjects was observed in all regions investigated, which is in contrast with some previous studies. Given the high intersubject variability of the 5-HT1A receptor BPND within both sexes and the usually low sample size in PET studies, the inclusion criteria of the study sample might significantly influence results on sexual dimorphism. Therefore, sex differences in 5-HT1A receptor binding remain a matter of debate.
References
Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, et al. Prevalence of mental disorders in Europe: results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand 2004;420(Suppl 1):21–7.
Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry 1998;55:405–13.
Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 2006;7:477–84.
Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007;62:847–55.
Carlsson M, Svensson K, Eriksson E, Carlsson A. Rat-brain serotonin—biochemical and functional evidence for a sex difference. J Neural Transm 1985;63:297–313.
Haleem DJ, Kennett GA, Curzon G. Hippocampal 5-hydroxytryptamine synthesis is greater in female rats than in males and more decreased by the 5-HT1A agonist 8-OH-DPAT. J Neural Transm 1990;V79:93–101.
Dickinson SL, Curzon G. 5-Hydroxytryptamine-mediated behaviour in male and female rats. Neuropharmacology 1986;25:771–76.
McBride PA, Tierney H, DeMeo M, Chen J-S, Mann JJ. Effects of age and gender on CNS serotonergic responsivity in normal adults. Biol Psychiatry 1990;27:1143–55.
Sambeth A, Blokland A, Harmer CJ, Kilkens TOC, Nathan PJ, Porter RJ, et al. Sex differences in the effect of acute tryptophan depletion on declarative episodic memory: a pooled analysis of nine studies. Neurosci Biobehav Rev 2007;31:516–29.
Kasper S, Tauscher J, Willeit M, Stamenkovic M, Neumeister A, Kufferle B, et al. Receptor and transporter imaging studies in schizophrenia, depression, bulimia and Tourette’s disorder—implications for psychopharmacology. World J Biol Psychiatry 2002;3:133–46.
Nishizawa S, Benkelfat C, Young SN, Leyton M, Mzengeza S, de Montigny C, et al. Differences between males and females in rates of serotonin synthesis in human brain. Proc Natl Acad Sci U S A 1997;94:5308–13.
Jovanovic H, Lundberg J, Karlsson P, Cerin A, Saijo T, Varrone A, et al. Sex differences in the serotonin 1A receptor and serotonin transporter binding in the human brain measured by PET. Neuroimage 2008;39:1408–19.
Biver F, Lotstra F, Monclus M, Wikler D, Damhaut P, Mendlewicz J, et al. Sex difference in 5HT2 receptor in the living human brain. Neurosci Lett 1996;204:25–8.
Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry 1999;46:1375–87.
Lanzenberger RR, Mitterhauser M, Spindelegger C, Wadsak W, Klein N, Mien L-K, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry 2007;61:1081–9.
Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology 2001;25:892–903.
Spindelegger C, Lanzenberger R, Wadsak W, Mien L-K, Stein P, Mitterhauser M, et al. Influence of escitalopram treatment on 5-HT1A receptor binding in limbic regions in patients with anxiety disorders. Mol Psychiatry 2008; in press.
Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res 1997;745:96–108.
Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 2007;59:360–417.
Hajos Mih l, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology 2003;45:72–81.
Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part I: effects of gender and pregnancy. Neuropharmacology 2002;43:1119–28.
Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav 2003;80:203–10.
Li Q, Wichems C, Heils A, Lesch KP, Murphy DL. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J Neurosci 2000;20:7888–95.
Schiller L, Jahkel M, Oehler J. The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Res 2006;1103:76–87.
Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res 2007;42:433–42.
Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Res 1995;688:121–33.
Palego L, Marazziti D, Rossi A, Giannaccini G, Naccarato AG, Lucacchini A, et al. Apparent absence of aging and gender effects on serotonin 1A receptors in human neocortex and hippocampus. Brain Res 1997;758:26–32.
Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. J Neural Transm Gen Sect 1991;85:181–94.
Dillon KA, Gross-Isseroff R, Israeli M, Biegon A. Autoradiographic analysis of serotonin 5-HT1A receptor binding in the human brain postmortem: effects of age and alcohol. Brain Res 1991;554:56–64.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007;27:1533–9.
Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res 2002;954:173–82.
Meltzer CC, Drevets WC, Price JC, Mathis CA, Lopresti B, Greer PJ, et al. Gender-specific aging effects on the serotonin 1A receptor. Brain Res 2001;895:9–17.
Pecins-Thompson M, Bethea CL. Ovarian steroid regulation of serotonin-1A autoreceptor messenger RNA expression in the dorsal raphe of rhesus macaques. Neuroscience 1999;89:267–77.
Tauscher J, Verhoeff NP, Christensen BK, Hussey D, Meyer JH, Kecojevic A, et al. Serotonin 5-HT1A receptor binding potential declines with age as measured by [11C]WAY-100635 and PET. Neuropsychopharmacology 2001;24:522–30.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl 20):22–33.
Rabiner EA, Messa C, Sargent PA, Husted-Kjaer K, Montgomery A, Lawrence AD, et al. A database of [(11)C]WAY-100635 binding to 5-HT(1A) receptors in normal male volunteers: normative data and relationship to methodological, demographic, physiological, and behavioral variables. Neuroimage 2002;15:620–32.
Pike VW, McCarron JA, Lammertsma AA, Osman S, Hume SP, Sargent PA, et al. Exquisite delineation of 5-HT1A receptors in human brain with PET and [carbonyl-11 C]WAY-100635. Eur J Pharmacol 1996;301:R5–7.
Wadsak W, Mien L-K, Ettlinger DE, Lanzenberger R, Haeusler D, Dudczak R, et al. Simple and fully automated preparation of [carbonyl-11C]WAY-100635. Radiochimica acta 2007;95:417–22.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996;4:153–8.
Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, et al. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage 1998;8:426–40.
Wu Y, Carson RE. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 2002;22:1440–52.
Mikolajczyk K, Szabatin M, Rudnicki P, Grodzki M, Burger C. A JAVA environment for medical image data analysis: initial application for brain PET quantitation. Med Inform (Lond) 1998;23:207–14.
Logan J, Fowler JS, Volkow ND, Ding YS, Wang GJ, Alexoff DL. A strategy for removing the bias in the graphical analysis method. J Cereb Blood Flow Metab 2001;21:307–20.
Meyer JH, Gunn RN, Myers R, Grasby PM. Assessment of spatial normalization of PET ligand images using ligand-specific templates. Neuroimage 1999;9:545–53.
Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage 1995;2:45–53.
Bremner JD, Bronen RA, De Erasquin G, Vermetten E, Staib LH, Ng CK, et al. Development and reliability of a method for using magnetic resonance imaging for the definition of regions of interest for positron emission tomography. Clin Positron Imaging 1998;1:145–59.
Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int 1997;30:565–74.
Moller M, Jakobsen S, Gjedde A. Parametric and regional maps of free serotonin 5HT1A receptor sites in human brain as function of age in healthy humans. Neuropsychopharmacology 2007;32:1707–14.
Bhagwagar Z, Montgomery AJ, Grasby PM, Cowen PJ. Lack of effect of a single dose of hydrocortisone on serotonin1A receptors in recovered depressed patients measured by positron emission tomography with [11C]WAY-100635. Biol Psychiatry 2003;54:890–95.
Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002;23:41–100.
Tauscher J, Bagby RM, Javanmard M, Christensen BK, Kasper S, Kapur S. Inverse relationship between serotonin 5-HT(1A) receptor binding and anxiety: a [(11)C]WAY-100635 PET investigation in healthy volunteers. Am J Psychiatry 2001;158:1326–8.
Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci 1996;16:7021–9.
Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology 2002;27:12–24.
Flugge G, Pfender D, Rudolph S, Jarry H, Fuchs E. 5HT(1A)-receptor binding in the brain of cyclic and ovariectomized female rats. J Neuroendocrinology 1999;11:243–49.
Jovanovic H, Cerin A, Karlsson P, Lundberg J, Halldin C, Nordstr”m A-L. A PET study of 5-HT1A receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res 2006;148:185–93.
Drossopoulou G, Antoniou K, Kitraki E, Papathanasiou G, Papalexi E, Dalla C, et al. Sex differences in behavioral, neurochemical and neuroendocrine effects induced by the forced swim test in rats. Neuroscience 2004;126:849–57.
Bebbington P. The origins of sex differences in depressive disorder: bridging the gap. Int Rev Psychiatry 1996;8:295–332.
Sakai Y, Nishikawa M, Leyton M, Benkelfat C, Young SN, Diksic M. Cortical trapping of [alpha]-[11C]methyl-l-tryptophan, an index of serotonin synthesis, is lower in females than males. Neuroimage 2006;33:815–24.
Chugani DC, Muzik O, Chakraborty P, Mangner T, Chugani HT. Human brain serotonin synthesis capacity measured in vivo with alpha-[C-11]methyl-l-tryptophan. Synapse 1998;28:33–43.
Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, et al. Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 2001;41:275–84.
Praschak-Rieder N, Willeit M, Wilson AA, Houle S, Meyer AS. Seasonal variation in human brain serotonin transporter binding. Arch Gen Psychiatry 2008; in press.
Adams KH, Pinborg LH, Svarer C, Hasselbalch SG, Holm S, Haugbol S, et al. A database of [(18)F]-altanserin binding to 5-HT(2A) receptors in normal volunteers: normative data and relationship to physiological and demographic variables. Neuroimage 2004;21:1105–13.
Acknowledgements
This research was supported by grants from the Austrian National Bank (OENB P11468) and the Medical Science Fund of the City of Vienna (BMF P2515) to R. Lanzenberger and a grant from the Austrian Science Fund (FWF P16549). The authors thank Veronica Witte, Andreas Hahn, Alexander Holik, Alexander Becherer and Christian Bieglmayer for their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stein, P., Savli, M., Wadsak, W. et al. The serotonin-1A receptor distribution in healthy men and women measured by PET and [carbonyl-11C]WAY-100635. Eur J Nucl Med Mol Imaging 35, 2159–2168 (2008). https://doi.org/10.1007/s00259-008-0850-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0850-x