Abstract
Purpose
The integrin αvβ3 is expressed on sprouting endothelial cells and on various tumour cell types. Due to the restricted expression of αvβ3 in tumours, αvβ3 is considered a suitable receptor for tumour targeting. In this study the αvβ3 binding characteristics of an 111In-labelled monomeric, dimeric and tetrameric RGD analogue were compared.
Methods
A monomeric (E-c(RGDfK)), dimeric (E-[c(RGDfK)]2), and tetrameric (E{E[c(RGDfK)]2}2) RGD peptide were synthesised, conjugated with DOTA and radiolabelled with 111In. In vitro αvβ3 binding characteristics were determined in a competitive binding assay. In vivo αvβ3 targeting characteristics of the compounds were assessed in mice with SK-RC-52 xenografts.
Results
The IC50 values for DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2, and DOTA-E{E[c(RGDfK)]2}2were 120 nM, 69.9 nM and 19.6 nM, respectively. At all time points, the tumour uptake of the dimer was significantly higher as compared to that of the monomer. At 8 h p.i., tumour uptake of the tetramer (7.40±1.12%ID/g) was significantly higher than that of the monomer (2.30±0.34%ID/g), p<0.001, and the dimer (5.17±1.22%ID/g), p<0.05. At 24 h p.i., the tumour uptake was significantly higher for the tetramer (6.82±1.41%ID/g) than for the dimer (4.22±0.96%ID/g), p<0.01, and the monomer (1.90±0.29%ID/g), p<0.001.
Conclusion
Multimerisation of c(RGDfK) resulted in enhanced affinity for αvβ3 as determined in vitro. Tumour uptake of a tetrameric RGD peptide was significantly higher than that of the monomeric and dimeric analogues, presumably owing to the enhanced statistical likelihood for rebinding to αvβ3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The αvβ3 integrin is a transmembrane protein consisting of two non-covalently bound subunits, α and β. Integrin αvβ3 is preferentially expressed on proliferating endothelial cells [1], whereas it is absent on quiescent endothelial cells. For growth beyond the size of 1–2 mm in diameter, tumours require the formation of new blood vessels. Consequently, αvβ3 expression on tumour vasculature is considered as a marker of tumour-induced angiogenesis [2–4]. In addition, αvβ3 is expressed on the cell membrane of various tumour cell types, including ovarian cancer, neuroblastoma, breast cancer and melanoma. Due to this restricted expression of αvβ3 in tumours, αvβ3 is considered a suitable target for tumour targeting [5]. This integrin can bind to the arginine-glycine-aspartic acid (RGD) amino acid sequence present in extracellular matrix proteins such as vitronectin, fibrinogen and laminin [6]. Based on the RGD tripeptide sequence a series of small peptides have been designed to antagonise the function of the αvβ3 integrin [7]. Especially the cyclic peptide derivatives have a relatively high affinity for the αvβ3 integrin. A series of RGD sequence-containing peptides has been tested for their ability to bind the αvβ3 integrin. It was found that the cyclic derivative cyclic(Arg-Gly-Asp-D-Phe-Val) was a 100-fold better inhibitor of cell adhesion to vitronectin than the linear variant, having an IC50 value in the nanomolar range [8, 9].
In previous studies we showed that radiolabelled c(RGDfK) peptides specifically accumulated in αvβ3-positive ovarian cancer xenografts in athymic mice [10]. The aim of this study was to develop modified ligands for improved αvβ3 integrin targeting by preparing multimers of cyclic RGD peptides. A multimeric RGD peptide could theoretically bind multivalently and thus more avidly to the target cell. However, the distance between the RGD units of the multivalent RGD peptides is too short to allow simultaneous binding to multiple αvβ3 integrins on the cell surface. Therefore, in this manuscript we avoid use of the term “avidity”, and only use “affinity”.
Multivalent interactions are frequently used in nature to increase the affinity of weak ligand–receptor interactions [11, 12]. For example, the attachment of an influenza virus to its target cell occurs through multiple simultaneous interactions between haemagglutinin and sialic acid [12]. Multivalent structures for the design of drugs and research agents have become a new focus of investigation. Several research groups, including ours, have applied multivalent compounds to enhance the interaction between the ligand and the target cell [13]. Goel et al. radiolabelled divalent and tetravalent scFv’s of mAb CC49 with 99mTc and analysed the imaging potential of these novel radioimmunoconjugates. Biodistribution studies demonstrated that 99mTc-[sc(Fv)2]2 had approximately threefold higher tumour localisation than 99mTc-sv(Fv)2 [14]. Recently, a new multivalent synthetic inhibitor of epidemic keratoconjunctivitis-causing adenovirus was evaluated by Johansson et al. [11]. Their results clearly indicated that the inhibition is significantly increased with higher orders of valency. Similarly, Kok et al. and Maheshwari et al. showed increased affinity of RGD ligands with the target cell owing to multivalent interactions [15, 16].
Here we describe the synthesis of a monomeric, E-c(RGDfK), a dimeric, E-[c(RGDfK)]2, and a tetrameric, E{E[c(RGDfK)]2}2, RGD peptide. The RGD peptides were conjugated with 1,4,7,10-tetraazadodecane-N,N′,N′′,N″′-tetraacetic acid (DOTA) and radiolabelled with 111In. Subsequently, the in vitro affinity and the in vivo tumour targeting characteristics of the peptides to αvβ3-expressing tumours were determined.
Materials and methods
Synthesis of DOTA-conjugated RGD peptides
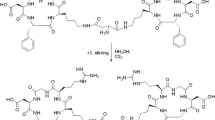
The monomeric RGD peptide was synthesised using Fmoc-based solid phase peptide synthesis, as described previously [17]. The structural formula of DOTA-E-c(RGDfK) is shown in Fig. 1a.
The synthesis of the dimeric cyclic RGD peptide E-[c(RGDfK)]2 conjugated with DOTA was described previously [18]. The structural formula of DOTA-E-[c(RGDfK)]2 is shown in Fig. 1b.
The synthesis of the tetrameric cyclic RGD peptide E{E[c(RGDfK)]2}2 conjugated with DOTA was described previously [19]. The structural formula of DOTA-E{E[c(RGDfK)]2}2 is shown in Fig. 1c.
Radiolabelling of the RGD peptides
111In-DOTA-E-c(RGDfK) was prepared by adding 14 MBq 111InCl3 (Mallinckrodt, Petten, The Netherlands) to 15 μg (13.3 nmol) DOTA-E-c(RGDfK) dissolved in 300 μl 0.5 M ammonium acetate buffer, pH 6.0, containing 0.6 mg/ml gentisic acid. DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 were radiolabelled with 111InCl3 analogously with minor modifications. DOTA-E-[c(RGDfK)]2 (28 μg, 16.4 nmol) and DOTA-E{E[c(RGDfK)]2}2 (15 μg, 4.79 nmol) were dissolved in 500 μl 0.5 M ammonium acetate buffer pH 6.0, containing 0.6 mg/ml gentisic acid. DOTA-E-[c(RGDfK)]2 was radiolabelled with 17 MBq 111InCl3 and DOTA-E{E[c(RGDfK)]2}2 was radiolabelled with 13 MBq 111InCl3. The reaction mixtures were degassed and subsequently the mixtures were heated at 100°C for 15 min. 111In-DOTA-E{E[c(RGDfK)]2}2 was further purified on a C-18 SepPak cartridge (Waters, Milford, MA, USA). After applying the sample on the methanol-activated cartridge, the cartridge was washed with 5 ml 25 mM ammonium acetate and eluted with 25% acetonitrile in 25 mM ammonium acetate. The radiochemical purity was determined by reversed-phase high-performance liquid chromatography (RP-HPLC) (HP 1100 series, Hewlett Packard, Palo Alto, CA, USA) using a C18 column (RX-C18, 4.6 mm×250 mm, Zorbax) eluted with a gradient mobile phase (8–20% B over 25 min or 8–100% B over 30 min, solvent A=25 mM ammonium acetate buffer, solvent B=acetonitrile) at 1 ml/min. The radioactivity of the eluate was monitored using an in-line radiodetector (Flo-One Beta series, Radiomatic, Meriden, CT, USA).
Solid-phase αvβ3 binding assay
The affinity of DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 for αvβ3 was determined using a solid-phase competitive binding assay. 111In-labelled DOTA-E-[c(RGDfK)]2 (3 MBq/μg) was prepared as described above and was used as the tracer in this assay. Microtiter 96-well vinyl assay plates (Corning B.V., Schiphol-Rijk, The Netherlands) were coated with 100 μl/well of a solution of purified human integrin αvβ3 (150 ng/ml) in Triton X-100 Formulation (Chemicon International, Temecula, CA, USA) in coating buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2 and 1 mM MnCl2) for 17 h at 4°C. The plates were washed twice with binding buffer [0.1% bovine serum albumin (BSA) in coating buffer]. The wells were blocked for 2 h with 200 μl blocking buffer (1% BSA in coating buffer). The plates were washed twice with binding buffer. Then 100 μl binding buffer containing 11.1 kBq of 111In-DOTA-E-[c(RGDfK)]2 and appropriate dilutions of non-labelled DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 in binding buffer were incubated in the wells at 37°C for 1 h. After incubation, the plates were washed three times with binding buffer. The wells were cut out and radioactivity in each well was determined in a γ-counter (1480 Wizard, Wallac, Turku, Finland). IC50 values of the RGD peptides were calculated by non-linear regression using GraphPad Prism (GraphPad Prism 4.0 Software, San Diego, CA, USA). Each data point is the average of three determinations.
Biodistribution studies
In the right flank of 6- to 8-week-old female nude BALB/c mice, 0.2 ml of a cell suspension of 15×106 cells/ml SK-RC-52 cells was injected subcutaneously (s.c.). Two weeks after inoculation of the tumour cells, mice were randomly divided into three groups (15 mice/group).To ensure that the mice in each group received equal amounts of RGD units, 0.5 μg per mouse of 111In-DOTA-E-c(RGDfK) (0.52 MBq), 111In-DOTA-E-[c(RGDfK)]2 (0.43 MBq) or 111In-DOTA-E{E[c(RGDfK)]2}2 (0.34 MBq) was administered via a tail vein. Mice were killed by CO2 asphyxiation 2, 8 and 24 h post injection (p.i.) (five mice/group). Blood, tumour and the major organs and tissues were collected, weighed and counted in a γ-counter. The percentage injected dose per gram (%ID/g) was determined for each sample.
Statistical analysis
All mean values are given ±standard deviation. Statistical analysis was performed using one-way analysis of variance. Bonferroni corrections for multiple comparisons were applied. The level of significance was set at p<0.05.
Results
Radiolabelling
RP-HPLC analysis indicated that the radiochemical purity of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 preparations used in these experiments was at least 93%. The elution profile of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 showed a single peak for each of the three compounds with an elution time of 14 min and 26 min for 111In-DOTA-E-c(RGDfK) and 111In-DOTA-E-[c(RGDfK)]2, respectively. The retention time of 111In-DOTA-E{E[c(RGDfK)]2}2 was 15 min. Note that different gradients were used for elutions.
Solid-phase αvβ3 binding assay
The affinity of DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2 for the αvβ3 integrin was determined in a competitive binding assay. The results of these assays are summarised in Fig. 2. Binding of 111In-labelled dimeric peptide, 111In-DOTA-E-[c(RGDfK)]2, to αvβ3 was competed by DOTA-E-c(RGDfK), DOTA-E-[c(RGDfK)]2 and DOTA-E{E[c(RGDfK)]2}2in a concentration-dependent manner. The IC50 values were 120 nM for DOTA-E-c(RGDfK), 69.9 nM for DOTA-E-[c(RGDfK)]2 and 19.6 nM for DOTA-E{E[c(RGDfK)]2}2.
Biodistribution studies
The results of the biodistribution studies of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 in athymic mice with s.c. SK-RC-52 tumours are summarised in Table 1 and Fig. 3. 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 all cleared rapidly from the blood. At 8 h p.i., tumour uptake of the tetramer (7.40±1.12%ID/g) was significantly higher than that of the monomer (2.30±0.34%ID/g), p<0.001, and the dimer (5.17±1.22%ID/g), p<0.05. Furthermore, uptake of the dimer in the tumour was significantly higher than that of the monomer, p<0.01 (Fig. 3a). At 24 h p.i., uptake in the tumour was significantly higher for the tetramer (6.82±1.41%ID/g) than for the dimer (4.22±0.96%ID/g), p<0.01, and the monomer (1.90±0.29%ID/g), p<0.001 (Fig. 3b). Co-injection of an excess unlabelled DOTA-E-[c(RGDfK)]2 (50 μg) along with 0.5 μg of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 or 111In-DOTA-E{E[c(RGDfK)]2}2 resulted in a significant decrease in radioactivity concentration in the tumour, indicating that uptake of the major fraction of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2in the tumour was αvβ3 mediated. Uptake in non-target organs like lung, spleen and intestine was also reduced in the presence of an excess of unlabelled RGD peptide, indicating that the uptake in these tissues was also at least partly αvβ3 mediated.
a Biodistribution of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 at 8 h p.i. in athymic mice with s.c. SK-RC-52 tumours (five mice/group). b Biodistribution of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2 at 24 h p.i. in athymic mice with s.c. SK-RC-52 tumours (five mice/group)
Discussion
In this study the potential advantage of using multimeric RGD peptides for targeting of the αvβ3 integrin receptor was investigated. The binding affinity of DOTA-E{E[c(RGDfK)]2}2 (IC50=19.6 nM), as determined in a solid phase competitive binding assay, was about four times higher than that of DOTA-E-[c(RGDfK)]2 (IC50=69.9 nM) and about six times higher than that of DOTA-E-c(RGDfK) (IC50=120 nM). Enhanced affinity of αvβ3-expressing cells for multiple RGD sequences on a protein backbone as compared with the monomeric RGD peptides was demonstrated by Kok et al. [15]. They postulated that the enhanced affinity was due to cooperative binding of multiple RGD units. Recently, Thumshirn et al. [20] synthesised multimeric RGD peptides by oxime ligation. These multimeric RGD peptides showed retained affinity for the αvβ3 integrin. Their tetrameric RGD compound with a Hegas spacer showed enhanced affinity as compared to the monomeric and the dimeric analogue. However, the tetrameric RGD compound with an aminohexanoic acid spacer showed a lower affinity than the dimeric analogue [20], indicating clearly the importance of the proper choice of spacer unit.
In athymic mice with s.c. SK-RC-52 tumours, all three RGD peptides of this study showed specific uptake in the tumour: in the presence of an excess of unlabelled DOTA-E-[c(RGDfK)]2, the specificity of the tumour targeting of the monomeric, dimeric and tetrameric RGD peptide was clearly demonstrated. The tumour uptake of 111In-DOTA-E{E[c(RGDfK)]2}2 beyond 2 h p.i. was significantly higher than that of 111In-DOTA-E-[c(RGDfK)]2. Similarly, the tumour uptake of 111In-DOTA-E-[c(RGDfK)]2 was significantly higher than that of 111In-DOTA-E-c(RGDfK) at all time points (Fig. 4). Thus, the higher the binding affinity for αvβ3, the higher the accumulation of the compound in αvβ3-expressing tumours. It appears that the enhanced uptake in the tumour of the tetrameric RGD peptide is mainly determined by early uptake in the tumour, while retention in the tumour at later time points of the monomer, dimer and tetramer was similar.
Tumour uptake of 111In-DOTA-E-c(RGDfK), 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTAE{E[c(RGDfK)]2}2 at 2, 8 and 24 h after injection in athymic mice with s.c. SK-RC-52 tumours. Resultsare expressed as mean %ID/g±SD. Values were analysed using one-way analysis of variance; *p<0.05, **p<0.01, # p<0.05, ## p<0.01. The p values refer to differences in tumour uptake between111In-DOTA-E-c(RGDfK) and 111In-DOTA-E-[c(RGDfK)]2 or between 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E{E[c(RGDfK)]2}2
Recently, Wu and co-workers tested 64Cu-DOTA-E{E[c(RGDfK)]2}2 for positron emission tomography imaging of αvβ3 expression in athymic nude mice with s.c. U87MG glioma xenografts [19]. 64Cu-DOTA-E{E[c(RGDfK)]2}2 accumulated rapidly and efficiently in the tumour (9.93±1.05%ID/g at 30 min p.i.). At 2 h p.i., the uptake of this 64Cu-labelled compound in the tumour was 7.61±0.68%ID/g, slowly decreasing to 4.56±0.51%ID/g at 24 h p.i. Although our compound was labelled with another radionuclide and tested in another animal model, the uptake in the tumour during 24 h after injection was very similar. At 2 h p.i., the uptake in the tumour of 111In-DOTA-E{E[c(RGDfK)]2}2 was 7.32±2.45%ID/g and it decreased to 6.82±1.41%ID/g at 24 h p.i.
One may argue that the enhanced tumour uptake of 111In-DOTA-E{E[c(RGDfK)]2}2 might be due to the higher molecular weight of the tetramer, resulting in a longer blood circulation time. However, not only the uptake in the tumour but also the tumour-to-blood ratio of 111In-DOTA-E{E[c(RGDfK)]2}2 (402±96, 24 h p.i.) was significantly higher than that of 111In-DOTA-E-[c(RGDfK)]2 (287±30) and 111In-DOTA-E-c(RGDfK) (223±39) (p<0.05 and p<0.01, respectively) (Fig. 5).
While the potential benefits of multivalency of targeting vehicles are universally accepted, the cause of the enhanced affinity is not yet clear [21]. Multivalent compounds could have enhanced affinity due to subsite binding, statistical rebinding or receptor clustering [12, 22]. Subsite binding refers to binding of a ligand to sites other than the binding site. It is not likely that this mechanism is responsible for the enhanced uptake of RGD multimers in αvβ3-expressing tumours because subsites have not been identified for these peptides.
Cells can form a cluster of many monovalent receptors on a small area of the cell surface [22] and multivalent ligands that can span the required distance between binding sites could then bind multiple receptors simultaneously. However, it is unlikely that the multivalent RGD peptides used in this study could bind multiple αvβ3 integrins simultaneously because the distance between the RGD units is very short. Therefore, statistical rebinding might be the most likely explanation for the enhanced affinity of the multimeric RGD peptides. The receptor binding of one RGD unit will significantly enhance the local concentration of the other RGD unit in the vicinity of the receptor. This could lead to a higher “on rate” of receptor binding and/or a lower “off rate” of the RGD multimer [19].
The three RGD peptides showed a remarkable difference in uptake in the kidneys. The uptake of 111In-DOTA-E{E[c(RGDfK)]2}2 is at all time points significantly higher than that of 111In-DOTA-E-[c(RGDfK)]2 and 111In-DOTA-E-c(RGDfK). Uptake in the kidney was not αvβ3 mediated; thus the enhanced αvβ3 affinity cannot explain the enhanced kidney uptake. Most likely, the difference in charge between the three peptides could cause the difference in renal uptake. It has been shown that positively charged peptides are more efficiently reabsorbed by the proximal renal tubular cell than neutral peptides [23]. Owing to the presence of more guanidine groups, the tetrameric RGD peptide is more positively charged than the dimeric and monomeric RGD peptide.
In conclusion, the present study shows that multimeric RGD peptides have enhanced affinity for the αvβ3 integrin, most likely due to statistical rebinding of these RGD peptides. The tetrameric RGD peptide demonstrated improved tumour targeting compared with the dimeric RGD peptide. Analogously, the dimeric RGD peptide exhibited improved tumour targeting compared with the monomeric RGD peptide. The tetrameric RGD peptide is the favourable ligand for αvβ3 targeting in vivo. The uptake of this compound in the kidneys and intestines is relatively high and could hamper imaging of tumours in the abdomen.
References
Brooks PC. Role of integrins in angiogenesis. Eur J Cancer 1996;32A:2423–2429
Fiedler W, Graeven U, Ergun S, Verago S, Kilic N, Stockschlader M, et al. Vascular endothelial growth factor, a possible paracrine growth factor in human acute myeloid leukemia. Blood 1997;89:1870–1875
Foss HD, Araujo I, Demel G, Klotzbach H, Hummel M, Stein H. Expression of vascular endothelial growth factor in lymphomas and Castelman’s disease. J Pathol 1997;183:44–50
PerezAtayde AR, Sallan SE, Tedrow U, Conners S, Allred E, Folkman J. Spectrum of tumor angiogenesis in the bone marrow of children with acute lymphoblastic leukemia. Am J Pathol 1997;150:815–821
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995;1:27–31
Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligands binding to integrins. J Biol Chem 2000;275:21785–21788
Haubner R, Finsinger D, Kessler H. Stereoisomeric peptide libraries and peptidomimetics for designing selective inhibitors of the αvβ3 integrin for a new cancer therapy. Angew Chem Int Ed Engl 1997;36:1374–1389
Aumailley M, Gurrath M, Muller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett 1991;291:50–54
Gurrath M, Muller G, Kessler H, Aumailley M, Timpl R. Conformation/activity studies of rationally designed potent anti-adhesive RDG peptides. Eur J Biochem 1992;210:911–921
Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, et al. Tumor targeting with radiolabeled alpha-v-beta-3 integrin binding peptides in a nude mouse model. Cancer Res 2002;62:6146–6151
Johansson SMC, Arnberg N, Elofsson M, Wadell G, Kihlberg J. Multivalent HSA conjugates of 3′-sialyllactose are potent inhibitors of adenoviral cell attachment and infection. Chembiochem 2005;6:358–364
Mammen M, Choi SK, Whitesides GM. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed 1998;37:2754–2794
Joosten JAF, Loimaranta V, Appeldoorn CCM, Haataja S, Ait El Maate F, Liskamp RMJ, et al. Inhibition of Streptococcus suis adhesion by dendritic galabiose compounds at low nanomolar concentration. J Med Chem 2004;47:6499–6508
Goel A, Baranowska-Kortylewicz J, Hinrichs SH, Wisecarver J, Pavlinkova G, Augustine S, et al. 99mTc-labeled divalent and tetravalent CC49 single-chain Fv’s: novel imaging agents for rapid in vivo localization of human colon carcinoma. J Nucl Med 2001;42:1519–1527
Kok RJ, Schraa AJ, Bos EJ, Moorlag HE, Ásgeirsdóttir SA, Everts M, et al. Preparation and functional evaluation of RGD-modified proteins as αvβ3 integrin directed therapeutics. Bioconjugate Chem 2002;13:128–135
Maheshwari G, Brown G, Lauffenburger DA, Wells A, Griffith LG. Cell adhesion and motility depend on nanoscale RGD clustering. J Cell Sci 2000;113:1677–1686
Dijkgraaf I, Boerman OC, Frielink C, Kruijtzer JAW, Liskamp RMJ, Oyen WJ, et al. Synthesis and preclinical evaluation of new αvβ3-integrin binding peptidomimetics for tumor targeting. Eur J Nucl Med Mol Imaging 2004;31 (Suppl 2):S281
Liu S, Cheung E, Ziegler M, Rajopadhye M, Edwards DS. 90Y and 177Lu labeling of a DOTA-conjugated vitronectin receptor antagonist useful for tumor therapy. Bioconjugate Chem 2001;12:559–568
Wu Y, Zhang X, Xiong Z, Cheng Z, Fisher DR, Liu S, et al. MicroPET imaging of glioma integrin αvβ3 expression using 64Cu-labeled tetrameric RGD peptide. J Nucl Med 2005;46:1707–1718
Thumshirn G, Hersel U, Goodman SL, Kessler H. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chem Eur J 2003;9:2717–2725
Vrasidas I, André S, Valentini P, Böck C, Lensch M, Kaltner H, et al. Rigified multivalent lactose molecules and their interactions with mammalian galectins: a route to selective inhibitors. Org Biomol Chem 2003;1:803–810
Kiessling LL, Pohl NL. Strength in numbers: non-natural polyvalent carbohydrate derivatives. Chem Biol 1996;3:71–77
Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med 1998;25:201–212
Acknowledgements
We thank Gerry Grutters and Hennie Eikholt for technical assistance during animal experiments. All animal experiments were approved by the local animal welfare committee in accordance with the Dutch legislation and carried out in accordance with their guidelines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dijkgraaf, I., Kruijtzer, J.A.W., Liu, S. et al. Improved targeting of the αvβ3 integrin by multimerisation of RGD peptides. Eur J Nucl Med Mol Imaging 34, 267–273 (2007). https://doi.org/10.1007/s00259-006-0180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0180-9