Abstract
In the framework of the preclinical validation of the hypoxic tracer [18F]EF3, a comparison was performed between uptake of [18F]EF3 and EF5 adducts detected by immunofluorescence in MCa-4, FSA, FSAII, Sa-NH and NFSA tumour-bearing mice. Mice were allowed to breath carbogen (5% CO2, 95% O2), 21% oxygen or 10% oxygen. A significant correlation (r 2=0.57; p<0.01) was found between the [18F]EF3 tumour-to-muscle ratio and the fluorescence intensity of EF5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In both experimental and human tumours, hypoxia has been shown to be an important physiological parameter influencing the malignant phenotype, tumour growth, metastatic potential and response to therapy [1]. Therefore assessment of tumour hypoxia may have profound therapeutic implications. Techniques able to determine the hypoxic fraction of human tumours in clinical conditions are thus needed.
Within this framework, we extensively studied and validated the hypoxic 2-nitroimidazole tracer [18F]EF3 in tumour-bearing mice [2]. In a nutshell, we reported a relative accumulation of [18F]EF3 in tumours compared with normal tissues [the tumour-to-muscle (T/M) ratio reaching values up to 5] when mice were breathing under normal conditions. This preferential uptake was no longer observed when animals were breathing under carbogen (5% CO2 and 95% O2) conditions, and conversely, it was increased when animals were breathing under 10% O2.
The objective of this study was to compare, on an individual tumour basis, the relative [18F]EF3 tumour uptake with the intracellular EF5 adducts as detected by immunofluorescence. This latter technique has been validated for the detection of tumour hypoxia in various animal and human tumours, and has been shown to correlate with intracellular absolute pO2 values [3, 4].
Materials and methods
Drugs and antibody
The tracer [18F]EF3 was synthesised as previously described [2, 5]. EF5 was kindly provided by the US National Cancer Institute. The EF5 adduct specific monoclonal antibody ELK3-51 conjugated to the fluorescent dye Cy-3 was kindly provided by C.J. Koch.
Animal and tumour model
Experiments were performed on male C3H HeOuJIco mice (IFFA CREDO, Brussels, Belgium). Animals were maintained in a facility approved by the Belgian Ministry of Agriculture in accordance with current regulations and standards. The experimental design was approved by the ethics committee on animal experimentation of the School of Medicine of the Université catholique de Louvain. The “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) were strictly followed. Animals were housed four to five per cage and fed ad libitum. The FSA, FSA-II, Sa-NH and NFSA fibrosarcomas and the MCa-4 mammary carcinoma syngeneic to C3Hf/Kam mice were used. These tumours were kindly provided by L. Milas. Maintenance, expansion and intramuscular generation of these tumours in vivo have been previously described [2, 6].
Experimental design
When tumours reached a diameter of 8 mm (mean±SD 8.6±0.8 mm, n=38), mice were co-injected intravenously (IV) in the tail vein with EF5 and [18F]EF3 at doses resulting in whole-body concentrations of 100 μM and 1–5 μM, respectively. For each of the five tumour strains, one or two mice were injected with the vehicle only (EF3−/EF5−) and used as control for the EF5 binding assay. After injection, mice were placed in individual cages breathing under normal oxygenation conditions (21% O2). To modify tumour hypoxia, mice were allowed to breath under 10% O2 (MCa-4 and FSA-II tumour-bearing mice; four mice each) or under carbogen (FSA-II tumour bearing mice; four mice) flowing through the cages at a constant rate of 3 l/min.
[18F]EF3 assay
Animals were sacrificed by cervico-cranial dislocation at 220 min after injection of both tracers. Blood (collected through cardiac puncture just before sacrifice of the animal), gastrocnemius muscle (contralateral to tumour-bearing limb) and tumour were harvested, weighed and counted for radioactivity. The pharmacokinetics of [18F]EF3 in blood was studied on non-tumour-bearing mice sacrificed from 5 to 330 min after marker injection. Contamination between organs when handling samples was carefully avoided. Samples were counted with a gamma counter (Cobra II Autogamma, Packard Instrument Company, Meriden, CT, USA). All data were corrected for [18F] decay, and expressed as the percentage of injected radioactivity per gram of tissue or T/M ratio.
EF5 binding assay
After radioactivity determination (on average between 10 and 15 min), tumours were coated with Tissue-Tek OCT Compound (Sakura Finetek Inc., Torrance, USA), plunged into 100% ethanol and frozen in liquid nitrogen for 30 s. Tumours were then dry stored at −80°C until processing for EF5 immunofluorescence. Frozen tumours were cut in 8-μm-thick sections using a HM 500 M cryostat (Microm Laborgeräte GmbH, Walldorf, Germany). An average of five to six sections were obtained per tumour. Sections were then processed for immunofluorescence staining as previously described [4, 7]. For each tumour, one section was incubated with the vehicle only and used as negative control of tissue background fluorescence. Tumour sections from control mice (EF3−/EF5−) were stained and used to assess the non-specific binding of the antibody.
The acquisition and analysis of the fluorescence images have been detailed elsewhere [4]. The main modification was the use of a lower magnification (Zeiss Neofluar 25×objective), an additional 1.25× lens and a camera adapter resulting in images covering an area of 760 μm×560 μm. For each EF5+ tumour, an average (±SD) of 91 (±20) randomly selected microscopic fields were digitised amongst five Ab+ sections. In control EF5−/EF3− tumours, 61 (±13) fields were selected from an average of 4.1 (±0.9) Ab+ sections. It has been shown (unpublished data) that for 8-mm-diameter tumours, averaging the fluorescence intensity (FI) on at least 90 randomly selected 760×560 μm2 fields is adequate to take into account the intra-tumour variability. Hypoxia was quantified by the raw FI measured in the whole tumour area, corrected for non-specific antibody binding and for day-to-day and lifetime mercury arc bulb variations by subtracting the FI of control samples processed on the same day [4].
Autoradiography
FSA-II-bearing animals were sacrificed by lethal injection of ketamine and xylazine at 220 min after injection of both tracers and then plunged into liquid nitrogen for a few minutes. Animals were cut in 2- to 3-mm-thick coronal slices using an ELU EBS 3601 electrical saw. Slices were placed in a BAS cassette 2025 with an imaging plate BAS-IP MS202+ (Fuji) for 30 min at −20°c. The imaging plate was scanned at a 50-μm resolution using a Fujifilm FLA-5100 phosphorimager (LASER at 635 nm, IP mode, Image Reader FLA-500 software). Images were analysed using the AIDA Image Analyzer v4.04 software (Fuji). Thereafter, slices were processed for EF5 immunofluorescence detection and scanned at a 10-μm resolution with the Fujifilm FLA-5100 phosphorimager (LASER at 532 nm, Fuji).
Statistical analysis
The coefficient of correlation r 2 was calculated for plots between two different assays, e.g. FI versus radioactivity. A Student’s t test on the mean value was used between two samples processed by the same assay. Statistical significance was set at a p value of ≤0.05.
Results and discussion
As a tracer dose of [18F]EF3 was used, it was important to check that the 20- to 100-fold excess amount of EF5 did not modify the pharmacokinetics or the biodistribution of the tracer. The pharmacokinetics of [18F]EF3 was not modified by the co-injection of EF5 (Fig. 1). The blood half-time calculated using a mono-exponential decay reached 87.8 min (95% CI 79.4–97.7 min) without EF5 and 110.8 min (95% CI 103.7–119.0 min) with EF5 (p<0.05). This significant difference largely derived from the difference in the data points at 5 min, which resulted from sub-optimal IV injection of the tracers. Indeed, when these two data points were excluded from the fits, blood half-times of [18F]EF3 reached 96.6 min (95% CI 85.7–110.5 min) and 101.1 min (95% CI 95.1–108.0 min) with and without co-injection of EF5, respectively. Similarly, in tumours and muscle, the tracer uptake was not significantly different in the presence or absence of EF5 (Table 1). These data illustrate that in our animal model and under the experimental conditions used, the in vivo metabolism of the two nitroimidazole compounds was non-saturable.
Pharmacokinetics of [18F]EF3 in blood with (◯) or without (●) co-injection of an excess amount of EF5. Data points represent the mean±1 SD from four to eight different mice. At each time point, the difference between the two experimental conditions did not reach the level of significance, except at 5 min after injection
Using the EF5 adducts to assess cellular hypoxia, the ranking of the five tumour strains, i.e. SA-NH (very hypoxic)>MCa-4>NFSA>FSA>FSA-II (less hypoxic), was similar to previous measurements performed in our laboratory after a 3-h EF5 incubation (P. Mahy, unpublished data; [8]). Using the [18F]EF3 assay, the ranking of the T/M ratio for the five tumour strains were similar, i.e. MCa-4 (very hypoxic)>SA-NH>NFSA≈FSA>FSA-II (less hypoxic). On an individual tumour basis, there was a significant correlation between the relative tumour uptake of [18F]EF3 (expressed as the T/M ratio) and the FI of the EF5 adducts (r 2=0.57, p<0.01, n=38) (Fig. 2). A similar correlation was also observed when the [18F]EF3 uptake was expressed by the tumour-to-blood ratio (data not shown) (r 2=0.53, p<0.01, n=30). When the data obtained from tumour-bearing mice breathing carbogen or 10% O2 were excluded from the plot, the correlation between [18F]EF3 uptake and EF5 FI still held (data not shown) (r 2=0.30; p<0.01). Last, at the microscopic level, there was a good match between the distribution of the FI and the [18F]EF3 uptake (Fig. 3).
Correlation between the FI of EF5 adducts and the T/M ratio of [18F]EF3 on an individual tumour basis (r 2=0.57; p<0.01). Symbols represent MCa-4 (◆), FSA (○), FSA-II (■), Sa-NH (●) and NFSA (△) tumours under 21%O2, FSA-II (+) under carbogen breathing, and MCa-4 (◇) and FSA-II (□) under 10% O2 breathing
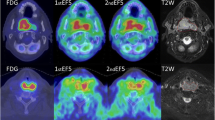
Coronal view of a 10-mm-diameter FSA-II tumour-bearing mouse sacrificed at 220 min after co-injection of [18F]EF3 and EF5. From left to right, coronal frozen section through the mid-abdomen and the tumour implanted in the right leg, autoradiography of the same section, and close-up views of the tumour (top: autoradiography; middle: EF5 immunofluorescence; bottom: haematoxylin and eosin staining on an 8-μm adjacent section). The radioactive and the immunofluorescent signal were quantitated on the upper and lower halves of the section (yellow ROIs). On autoradiography, the positive hypoxic area was calculated at 35% and 65% for the upper half and the lower half, respectively. On the IF section, corresponding values reached 34% and 64%, respectively
As can be observed in Fig. 2, increasing hypoxia in a relatively well-oxygenated tumour such as FSA-II resulted in a marked increase in the T/M ratio, which increased from 1.7±0.4 (ambient air) to 2.7±1.0 (10% O2) (p<0.05). Carbogen breathing did not have a dramatic effect; the T/M ratio falling only slightly to 1.2±0.2 (p<0.05). These data confirmed a previous set of experiments where a significant increase in the T/M ratio of [18F]EF3 was observed under similar experimental conditions (2.8±0.3 under ambient air versus 4.8±0.6 under 10% O2; p<0.01) [2]. Similarly, in the same individual tumours, EF5 FI increased from 0.4±0.9 (mean±sem) under ambient air conditions to 3.2±1.3 under 10% O2 breathing (p<0.05). Under carbogen breathing, mean FI did not vary significantly (0.7±0.4, p=NS) (Fig. 2). This absence of difference between ambient air and carbogen can be explained by the rather low sensitivity of the camera coupled to the fluorescent microscope. Conversely, 10% O2 breathing did not have a significant effect on an intrinsically more hypoxic tumour such as MCa-4: the TMR increased only from 3.5±0.7 (ambient air) to 3.9±0.6 (10% O2) (p=NS). Surprisingly, under similar experimental conditions, EF5 FI increased from 1.9±0.7 (ambient air) to 6.5±0.8 (10% O2) (p<0.0001).
We do not have a clear explanation for the difference between the two assays in the MCa-4 tumour type. In preliminary experiments it was observed that the hypoxic conditions (10% O2) modified the renal excretion of the unbound [18F]EF3 in both FSA-II and MCa-4 tumour-bearing mice, in turn increasing the background radioactivity throughout the body (unpublished data). This observation may explain the dismal increase in tumour-to-muscle ratio in MCa-4-bearing mice under 10% O2 breathing. Such a phenomenon did not occur with the immunofluorescence assay, where the possible increase in the background level of unbound marker has no influence because of the extensive rinsing of the sections. Whatever the explanation, this finding illustrates the difficulty of using experimental models and settings departing from physiological conditions.
Within the framework of the preclinical validation of [18F]EF3, an important issue is how this tracer compares with other labelled hypoxic tracers, the most widely used being [18F]FMISO [9]. It is beyond the scope of this study to extensively discuss the merits of the various labelled hypoxic tracers. Comprehensive comparison between [18F]EF3 and [18F]FMISO has been recently performed in a mouse tumour model under various experimental conditions. Data will be reported elsewhere, but it appeared that although the two tracers were able to quantify tumour hypoxia, [18F]EF3 was more specific for hypoxia than [18F]FMISO. A possible explanation was the non-hypoxia-related metabolism of the latter tracer.
In conclusion, the present experiments confirm our previous data and validate the use of [18F]EF3 to detect tumour hypoxia. Despite intrinsic lower signal-to-background ratios compared with immunofluorescence of EF5 adducts, the use of [18F]EF3 with a PET camera has the advantage of being a non-invasive, repeatable procedure that assesses the whole tumour. In addition, it allows registration with other imaging modalities or other PET tracers assessing various aspects of the tumour microenvironment. Based on these encouraging results, a phase I study with [18F]EF3 has been launched in patients with head and neck squamous cell carcinoma, and another study comparing [18F]EF3, [18F]FMISO and pimonidazole in patients is in preparation.
References
Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst 2001;93:266–276
Mahy P, De Bast M, Levêque P, Gillart J, Labar D, Marchand J, Grégoire V. Preclinical validation of the hypoxic tracer 2-(2-Nitroimidazol-1-yl)-N-(3,3,3-[18F]trifluoropropyl)-acetamide, [18F]EF3. Eur J Nuc Med Mol Imaging 2004;31:1263–1272
Koch CJ. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol 2002;352:3–31
Mahy P, De Bast M, Gallez B, Gueulette J, Koch CJ, Scalliet P, Grégoire V. In vivo colocalisation of 2-nitroimidazole EF5 fluorescence intensity and electron paramagnetic resonance oximetry in mouse tumors. Radiother Oncol 2003;67:53–61
Josse O, Labar D, Georges B, Grégoire V, Marchand-Brynaert J. Synthesis of [18F]-labeled EF3 [2-(2-nitroimidazol-1-yl)-N-(3,3,3-trifluoropropyl)-acetamide], a marker for PET detection of hypoxia. Bioorg Med Chem 2001;9:665–675
Milas L, Hunter N, Mason K, Withers HR. Immunological resistance to pulmonary metastases in C3Hf/Bu mice bearing syngeneic fibrosarcoma of different sizes. Cancer Res 1974;34:61–71
Evans SM, Joiner B, Jenkins WT, Laughlin KM, Lord EM, Koch CJ. Identification of hypoxia in cells and tissues of epigastric 9L rat glioma using EF5 [2-(2-nitro-1H-imidazol-1yl)-N-(2,2,3,3,3-pentafluoropropyl)acetamide]. Br J Cancer 1995;72:875–882
Mahy P, Reychler H, Gueulette J, Scalliet P, Grégoire V. Immunofluorescence analysis of hypoxia in squamous cell carcinoma and fibrosarcoma murine tumors using a pentafluorinated nitroimidazole compound (EF5). J Cranio-Maxillofac Surg 1998;26:111
Rasey JS, Grunbaum Z, Magee S, Nelson NJ, Olive PL, Durand RE, Krohn KA. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res 1987;111:292–304
Acknowledgements
This work was supported by research grants from the “Fonds de la Recherche Scientifique Médicale” (grants # 3.4571.95, 3.4610.99 and 3.4560.00) of Belgium and by grants from the “Fonds Joseph Maisin”, Brussels, Belgium.
The authors wish to thank C. Koch (Radiation Oncology Dept., School of Medicine, University of Pennsylvania, Philadelphia, USA) and the US National Cancer Institute for providing EF5 and antibody free of charge.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahy, P., De Bast, M., Gillart, J. et al. Detection of tumour hypoxia: comparison between EF5 adducts and [18F]EF3 uptake on an individual mouse tumour basis. Eur J Nucl Med Mol Imaging 33, 553–556 (2006). https://doi.org/10.1007/s00259-005-0049-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0049-3