Abstract
Tumour hypoxia is a well-known negative prognostic marker in almost all solid tumours. [18F]Fluoromisonidazole (FMISO)-positron emission tomography (PET) is a non-invasive method to detect tumour hypoxia. Compared to other methods of hypoxia assessment it possesses some considerable advantages: It is non-invasive, it delivers spatial information on the hypoxia distribution within the entire tumour volume, and it can be repeated during the course of radio(chemo)therapy. This chapter briefly describes different methods of hypoxia evaluation and focuses on hypoxia PET imaging, with the most commonly used tracer being FMISO. The preclinical rationale and clinical studies to use FMISO-PET for patient stratification in radiation therapy are discussed as well as possible agents or radiation-dose modifications to overcome hypoxia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Background on Tumour Cell Hypoxia
Tumour heterogeneity plays a pivotal role in several solid tumours regarding the outcome after surgery, radiotherapy and chemotherapy. Amongst the various tumour characteristics, tumour cell hypoxia is the most relevant in the field of radiation oncology. Hypoxia is generally differentiated into chronic, i.e. diffusion-limited hypoxia caused by rapid tumour growth with insufficient neovascularization and impaired oxygen supply, and acute hypoxia, i.e. acute decrease in perfusion due to functional impairment of the (neo-) vasculature. Tumour cell hypoxia is known to negatively affect patients’ outcome irrespective of the chosen therapeutic approach (surgery, radiotherapy) as shown in a landmark study for cancer of the uterine cervix (Hockel et al. 1996). It is known to promote local tumour growth, lymph node involvement and distant metastases formation. In radiotherapy, the unfavourable therapeutic effect may be caused by hampering the formation of free radicals after photon irradiation, a prerequisite for the envisaged biological effect. This phenomenon can be expressed by the oxygen enhancement ratio (OER) that compares the biological efficacy of radiotherapy under oxic versus anoxic conditions.
Several strategies to improve outcome in patients presenting with hypoxic tumours have been developed and used in clinical studies. These include the following: hyperbaric oxygen breathing, carbogen breathing or the additional application of hypoxia sensitizers to the standard treatment (Jordan and Sonveaux 2012; Janssens et al. 2012). Furthermore, the nitroimidazole derivative nimorazole has been extensively studied in several Danish studies and is now routinely used for Danish head and neck squamous cell carcinoma (HNSCC) patients. Cytotoxic agents activated under hypoxic conditions include tirapazamine (Rischin et al. 2010) or the novel agent TH-302 (Borad et al. 2014). The benefit of hypoxia modification (by various means) in HNSCC even without stratification for the hypoxic status has been shown by a large meta-analysis by Overgaard, analysing 4805 patients treated within 32 randomized clinical trials (Overgaard 2011). With modern radiation techniques facilitating highly selective dose delivery, the concept of the biological target volume, introduced by Ling et al. (Ling et al. 2000) in 2000, has gained interest. For hypoxic tumours are radiation resistant, an increase in radiation dose, either to the whole tumour volume or selectively to the hypoxic subvolumes of the tumour only (dose painting), is aimed for. The latter approach can be either performed on a (manually or semi-manually) delineated subvolume or by prescribing individual doses to every voxel (dose painting by numbers) (Thorwarth et al. 2007). Obviously, it is important to assess the level of hypoxia both globally and on tumour subvolume level before and during (chemo)radiotherapy in order to facilitate these dose-painting techniques.
This chapter briefly addresses invasive measures to quantify hypoxia, but focuses on positron emission tomography (PET) of hypoxia-related markers as they may repeatedly image the entire tumour volume, and even more importantly, this technique is non-invasive.
2 Invasive Assessment of Tumour Cell Hypoxia
The classical gold standard for determination of the oxygen partial pressure has been the Eppendorf electrode. For its easy accessibility, the longest experience for pre-therapeutic Eppendorf electrode measurements exists in carcinomas of the uterine cervix (Hockel et al. 1996; Hoogsteen et al. 2009; Yaromina et al. 2006). This technique holds some important drawbacks: it has an invasive nature, is labour-intensive and cannot be used in all solid tumours for poor accessibility. Furthermore, Eppendorf electrode measurements only analyse small portions of the tumour and are unable to distinguish between vital hypoxic and anoxic necrotic tumour subvolumes.
Another attempt to measure tumour cell hypoxia is the immunohistochemical staining of histological samples that were obtained from the tumour or lymph node metastases. There are both exogenous markers, i.e. they need to be administered to the patient via an infusion or tablet, and endogenous markers, i.e. upregulated hypoxia-related markers. The most commonly used exogenous marker currently considered the reference standard is pimonidazole. Pimonidazole detects hypoxia below 10 mmHg (1.3 kPa) and has been used in preclinical and clinical studies (Hoogsteen et al. 2009; Yaromina et al. 2006). In the past, it required intravenous administration approximately 30–60 min before gathering the biopsy, but meanwhile an oral form exists and is FDA-approved. For logistic and financial reasons, endogenous markers of hypoxia have been pursued. These include hypoxia-inducible factor 1 alpha (HIF-1α), carbonic anhydrase-IX (CA-IX), vascular endothelial growth factor (VEGF) and the glucose transporters 1 and 3 (Glut-1 and Glut-3) (Troost et al. 2005; Ogawa et al. 2011; Goethals et al. 2006; Bussink et al. 2003). Noteworthy, the expression of these endogenous markers is influenced by a plethora of mechanisms and not exclusively driven by hypoxia. In accordance with Eppendorf electrode measurement, immunohistochemical staining of biopsies only represents a fraction of the entire tumour, is burdensome for the patients and is not ideal for repeated measurement of hypoxia.
Another attempt to quantify hypoxia is the use of serological blood biomarkers. Osteopontin is probably the best-established blood marker in the context of radiotherapy. In a HNSCC tumour cell line, hypoxia was found to upregulate osteopontin via a Ras-activated enhancer (Zhu et al. 2005). Plasma osteopontin correlated inversely with pO (Jordan and Sonveaux 2012) measured by Eppendorf electrodes in both HNSCC and NSCLC patient cohorts, and with worse clinical outcome in a variety of solid tumours (Le et al. 2003, 2006; Buijsen et al. 2014; Ostheimer et al. 2014). However, the factors influencing osteopontin levels and the additional value of combining several blood biomarkers (Osteopontin, VEGF and CA-IX) are subject of ongoing research (Ostheimer et al. 2014; Lukacova et al. 2005). A fourth strategy assessing tumour hypoxia is genetic analysis. Toustrup et al. (Toustrup et al. 2011) identified a hypoxia gene expression profile in vitro and validated the classifier in vivo in HNSCC xenograft models. In the latter model, they additionally checked whether tumour heterogeneity affected the gene profile, i.e. whether biopsies from mixed hypoxic and oxic areas yielded representative results compared to autoradiography with a hypoxia-related PET tracer. They found measurable upregulation of hypoxia genes even in tumour biopsies taken from mixed hypoxic and oxic subvolumes as identified with autoradiography PET imaging. Figure 1 gives an overview of established hypoxia detection methods.
3 PET Imaging of Hypoxia and Relevance in (Radiation) Oncology
A non-invasive method for the detection and quantification of tumour cell hypoxia is PET imaging with hypoxia specific radiotracers, amongst which imidazole derivates are most commonly used. Imaging hypoxia via PET has several advantages for radiotherapy compared to the aforementioned methods: The entire tumour volume can be assessed at several time points prior to and during treatment, and it can be used for dose-redistribution based on biological tumour characteristics, e.g. hypoxia (Hendrickson et al. 2011). Notably, [18F]Fluoro-deoxyglucose (FDG) may inherit some information on intratumoural hypoxia due to upregulation of Glut-1 by HIF-1α, but is aspecific and should, therefore, not be used for hypoxia imaging (Van Baardwijk et al. 2007).
Already in 1979, J.C. Chapman from the University of Alberta, Canada, recognized the importance of hypoxic sensitizers for radiation therapy and shortly thereafter he utilized 14C-labelled misonidazole for the imaging of hypoxic areas in murine tumours (Chapman et al. 1981; Chapman 1979). He described the importance as future markers for hypoxic cells in tumours with potential clinical applicability (Garrecht and Chapman 1983). For diagnosis of ischaemia, Mathias et al. (1987) presented a first concept for radiotracers to be applied in nuclear medicine techniques based on hypoxic sensitizers.
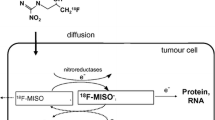
Since then, [18F]Fluoromisonidazole (FMISO) has become the hypoxia PET tracer most commonly used in a variety of solid tumours (Rajendran and Krohn 2015). Figure 2 shows the chemical structure of FMISO.
Additional imidazole tracers, including 5-[18F]fluoro-5-deoxy-d-arabinofuranosyl-2-nitroimidazole (FAZA), [18F]nitroimidazole-N-trifluoropropyl-acetamide (EF-3), [18F]nitroimidazole-N-pentafluoropropyl-acetamide (EF-5), [18F]fluoroerythronitroimidazole (FETNIM), or [18F]fluoro-nitro-H-imidazol-methyl-H-triazol-propanol (HX4), have mainly been designed to improve the slow accumulation in comparison with FMISO within the tumour, and to enhance the signal-to-background ratio (SBR). Beside them, the SPECT or PET radiotracer 5-[123/124I]iodo-5-deoxy-d-arabinofuranosyl-2-nitroimidazole (IAZA) is available (Reischl et al. 2007). Non-Imidazole tracers, e.g. [64Cu]Cu-diacetyl-bis-N-methylthiosemicarbazone (Cu-ATSM) are less frequently used and may also reflect tumour perfusion rather than being a hypoxia-selective tracer (Movahedi et al. 2012). Due to the poor availability of 64Cu a broad application is not visible. All these hypoxia specific tracers, except for Cu-ATSM, share the necessity of a relatively long interval between tracer injection and imaging and therefore are merely a surrogate of chronic instead of acute hypoxia. Off note, there are various additional imaging methods for the assessment of hypoxia, including blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) or optical spectroscopy, but discussion of these is beyond the scope of this chapter (Horsman et al. 2012; Chitneni et al. 2011).
4 Preclinical Validation Studies
Many in vivo studies focused on the correlation of different methods for hypoxia detection in order to move from invasive to non-invasive ones. One study compared FMISO uptake in rodent tumour xenograft models with robotic-guided multiple pO2 measurement via electrodes and found a high non-concordance in some individual data pairs, possibly explained by partially necrotic, i.e. anoxic, subvolumes that do not take up the tracer (Chang et al. 2009). Numerous studies have shown a reasonably good correlation between hypoxic subvolumes as detected by pimonidazole immunohistochemistry and FMISO and FAZA uptake by autoradiography and/or microPET, under varying levels of oxygenation or artificially induced clamp hypoxia (Troost et al. 2008; Busk et al. 2008, 2009, 2013; Troost et al. 2006). Figure 3 depicts examples of pimonidazole-stained and FMISO-autoradiographed xenografts under different oxygen conditions.
Pseudo-coloured grey-value pimonidazole images (top row), images after segmentation of the pimonidazole signal (middle row), and FMISO autoradiography images (bottom row) of head and neck squamous cell carcinoma (SCCNij3) and glioblastoma (E106) xenograft tumour lines for control tumours, after carbogen breathing and clamping. Figure taken with kind permission from (Troost et al. 2006)
In a study comparing the aforementioned hypoxia tracers FMISO, FAZA, HX4 and Cu-ATSM with pimonidazole and CA-IX immunohistochemistry in a head and neck xenograft tumour line, all except for Cu-ATSM showed similar distributions within the tumour (Carlin et al. 2014).
Comparing the different tracers per se, modelling as well as preclinical studies have suggested a superior SBR of FAZA compared to FMISO, however, data are conflicting (Busk et al. 2013; Busk et al. 2009). Peeters et al. (2015) recently compared FMISO, FAZA and HX4 in rats bearing syngeneic rhabdomyosarcoma R1 tumours. As expected and probably due to its high lipophilicity, the maximum SBR for FMISO was not reached until 6 h, as compared to 2 h for FAZA and 3 h for HX4. Remarkably, whereas all three tracers were able to monitor artificially induced hypoxia, only FMISO was able to successfully depict hypoxia-modifying treatment with nicotinamide and carbogen. Finally, the spatial reproducibility of FMISO in two consecutive scans obtained within a 48-h interval was best. The low conformity of FAZA shown in this study is somehow surprising for another study showed a good reproducibility of FAZA hypoxic volumes, even after fractionated radiotherapy (Busk et al. 2013). In other publications, FMISO and HX4 were both able to detect decreased levels of hypoxia after carbogen breathing (Troost et al. 2006; Dubois et al. 2011), and FAZA uptake decreased after pure oxygen breathing (Piert et al. 2005).
Data on Cu-ATSM are conflicting. Cu-ATSM has neither shown a good correlation with pimonidazole and CA-IX staining in solid tumour models, nor with FAZA uptake (Yuan et al. 2006; McCall et al. 2012). A correlation with FMISO uptake and oxygen probe measurement existed when applying an exceptional long time period between tracer injection and acquisition (O’Donoghue et al. 2005).
When investigating the value of hypoxia imaging, pre-therapeutic FAZA-PET was found to predict the therapeutic efficacy of adding nimorazole to radiotherapy in a preclinical sarcoma, but not in a glioma model (Bol et al. 2015). In rhabdomyosarcoma and NSCLC tumour models, Peeters et al. (2015) showed an association between the HX4-PET-derived hypoxic volume and tumour growth delay after treatment, and a benefit of the novel hypoxia specific cytotoxic agent TH-302 when combined with single-dose radiotherapy. In a recent study in HNSCC xenografts, Schütze et al. (2014) asserted the role of the FMISO-derived hypoxic volume and possible implications for subsequent dose escalation. Tumours with hypoxic volumes below the median had a significantly better local control rate after single-dose radiotherapy than those with hypoxic volumes above median. Interestingly, an increase of the single dose by another 10 Gy lead to similar increases of local control for both subgroups. Hence, tumour hypoxia as depicted by FMISO-PET is probably not barely a shift on the dose-effect curve for tumour control probability. If this had been the case, the same dose increase would have led to a much lower gain in tumour control in the hypoxic tumour subgroup. Rather, other effects most probably also play a role. The observations by Schütze et al. justify an attempt to moderately escalate the dose in hypoxic tumours to substantially increase the tumour control probability.
Taken together the preclinical data support the strong role of preclinical FMISO-PET-imaging as it correlates well with other established methods of hypoxia detection, it has a high reproducibility, and it reflects relevant therapy-induced changes.
5 Clinical Studies
After primary or (neo)adjuvant (chemo)radiotherapy, hypoxia in particular affects treatment outcome in tumours with unsatisfactory local control rates, e.g. glioblastomas, locally advanced NSCLC and locally advanced HNSCC (Mortensen et al. 2012; Rischin et al. 2006). In a recent review article, the prognostic value of PET-measured hypoxia, in particular FMISO-PET, for local tumour control has been highlighted (Rajendran and Krohn 2015). Moreover, a large meta-analysis underlined the positive effect of hypoxia modification on local tumour control and treatment outcome (Overgaard 2007). In order to select patients benefitting from treatment modification and to prevent those patients not benefitting from suffering undesired treatment-related side effects, methods to stratify patients are mandatory.
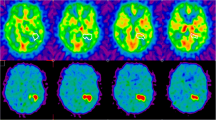
Stratification may be based on relatively simple parameters including tumour necrosis, on plasma markers, or a hypoxia gene set (Eustace et al. 2013). However, for aforementioned reasons, non-invasive imaging is of particular interest. Several recent studies have shown the prognostic value of hypoxia PET imaging regarding outcome after (chemo)radiotherapy in various solid tumours (Mortensen et al. 2012; Servagi-Vernat et al. 2014; Zips et al. 2012; Bollineni et al. 2014; Zegers et al. 2013). Zips et al. (2012) highlighted the prognostic value of FMISO-PET imaging obtained after one and two weeks of (chemo)radiotherapy in a cohort of 25 HNSCC patients. Conversely, FMISO-PET imaging obtained before treatment did not correlate with outcome. Figure 4 shows two patients of this study with sequential FMISO scans. In a small study cohort, Dirix et al. (2009) investigated FDG- and FMISO-PET, T1- and T2-weighted MRI, diffusion-weighted-(DWI-)MRI and dynamic contrast-enhanced (DCE-)MRI at various time points before and during primary radiotherapy in 15 HNSCC patients. Apart from the prognostic value of DWI- and DCE-MRI for locoregional recurrence, the prognostic value of FMISO-PET was underlined. A Danish study also found FAZA-PET imaging to stratify patients into groups according to loco-regional control (Mortensen et al. 2012). FMISO-PET imaging was part of a phase II clinical study on hypoxia-modification in patients with advanced HNSCC. In a substudy on 45 patients, FMISO-PET imaging prior to chemoradiotherapy was found to be a predictive marker selecting patients benefitting from the addition of tirapazamine (Rischin et al. 2006). In the cohort treated with chemoradiotherapy, 8 of 13 patients with tumour cell hypoxia as depicted by FMISO-PET experienced local relapse, whereas in the experimental arm, only 1 of 19 patients with hypoxia developed local recurrence.
Although older publications have shown conflicting results, recent studies have reported a good spatial reproducibility of repeated FMISO-PET-based hypoxic subvolumes obtained prior to initiation of (chemo)radiotherapy (Okamoto et al. 2013). Regarding reproducibility during treatment, Bittner et al. (2013) analysed the size, location and overlap of FMISO-PET positive subvolumes in 16 HNSCC patients. In patients with persistent hypoxia after 2 weeks of chemoradiotherapy, the FMISO-positive subvolumes mostly remained geographically stable. Summarizing these data, they prompt the scientific community to integrate hypoxia PET imaging into radiation treatment planning for hypoxia-directed dose escalation strategies.
At present, a German mono-institutional randomized phase II clinical trial investigates the clinical feasibility of FMISO-PET-based dose escalation in HNSCC (Welz et al. 2014). Patients in the standard arm undergo a conventional total dose of 70 Gy (chemo)radiotherapy, whereas in the experimental arm, patients receive 10 % dose escalation, i.e. 77 Gy, to the FMISO-PET-defined hypoxic subvolume. The planned interim analysis for the first 20 patients recruited into this study showed that dose escalation in this order of magnitude is well tolerated. Additionally, results of this study have thus far confirmed a prognostic model relating the dynamic FMISO-PET data to the individual tumour control probability established in an earlier study (Thorwarth et al. 2014).
References
Bittner M-I, Wiedenmann N, Bucher S, Hentschel M, Mix M, Weber WA et al (2013) Exploratory geographical analysis of hypoxic subvolumes using (18)F-MISO-PET imaging in patients with head and neck cancer in the course of primary chemoradiotherapy. Radiother Oncol 108(3):511–516
Bol A, Labar D, Cao-Pham TT, Jordan B, Grégoire V et al (2015) Predictive value of (18)F-FAZA PET imaging for guiding the association of radiotherapy with nimorazole: a preclinical study. Radiother Oncol 114(2):189–194
Bollineni VR, Koole MJB, Pruim J, Brouwer CL, Wiegman EM, Groen HJM et al (2014) Dynamics of tumor hypoxia assessed by 18F-FAZA PET/CT in head and neck and lung cancer patients during chemoradiation: possible implications for radiotherapy treatment planning strategies. Radiother Oncol 113(2):198–203
Borad MJ, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL et al (2014) Randomized phase II trial of gemcitabine plus TH-302 versus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol
Buijsen J, van Stiphout RG, Menheere PPCA, Lammering G, Lambin P (2014) Blood biomarkers are helpful in the prediction of response to chemoradiation in rectal cancer: a prospective, hypothesis driven study on patients with locally advanced rectal cancer. Radiother Oncol 111(2):237–242
Busk M, Horsman MR, Jakobsen S, Keiding S, van der Kogel AJ, Bussink J et al (2008) Imaging hypoxia in xenografted and murine tumors with 18F-fluoroazomycin arabinoside: a comparative study involving microPET, autoradiography, PO2-polarography, and fluorescence microscopy. Int J Radiat Oncol Biol Phys 70(4):1202–1212
Busk M, Horsman MR, Jakobsen S, Hansen KV, Bussink J, van der Kogel A et al (2009) Can hypoxia-PET map hypoxic cell density heterogeneity accurately in an animal tumor model at a clinically obtainable image contrast? Radiother Oncol 92(3):429–436
Busk M, Mortensen LS, Nordsmark M, Overgaard J, Jakobsen S, Hansen KV et al (2013) PET hypoxia imaging with FAZA: reproducibility at baseline and during fractionated radiotherapy in tumour-bearing mice. Eur J Nucl Med Mol Imaging 40(2):186–197
Bussink J, Kaanders JHAM, van der Kogel AJ (2003) Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol 67(1):3–15
Carlin S, Zhang H, Reese M, Ramos NN, Chen Q, Ricketts S-A (2014) A comparison of the imaging characteristics and microregional distribution of 4 hypoxia PET tracers. J Nucl Med 55(3):515–521
Chang J, Wen B, Kazanzides P, Zanzonico P, Finn RD, Fichtinger G et al (2009) A robotic system for 18F-FMISO PET-guided intratumoral pO2 measurements. Med Phys 36(11):5301–5309
Chapman JD (1979) Hypoxic sensitizers–implications for radiation therapy. N Engl J Med 301(26):1429–1432
Chapman JD, Franko AJ, Sharplin J (1981) A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer 43(4):546–550
Chitneni SK, Palmer GM, Zalutsky MR, Dewhirst MW (2011) Molecular imaging of hypoxia. J Nucl Med 52(2):165–168
Dirix P, Vandecaveye V, De Keyzer F, Stroobants S, Hermans R, Nuyts S (2009) Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with (18)F-FDG PET, (18)F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med 50(7):1020–1027
Dubois LJ, Lieuwes NG, Janssen MHM, Peeters WJM, Windhorst AD, Walsh JC et al (2011) Preclinical evaluation and validation of [18F]HX4, a promising hypoxia marker for PET imaging. Proc Natl Acad Sci USA 108(35):14620–14625
Eustace A, Irlam JJ, Taylor J, Denley H, Agrawal S, Choudhury A et al (2013) Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol 108(1):40–47
Garrecht BM, Chapman JD (1983) The labelling of EMT-6 tumours in BALB/C mice with 14C-misonidazole. Br J Radiol 56(670):745–753
Goethals L, Debucquoy A, Perneel C, Geboes K, Ectors N, De Schutter H et al (2006) Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys 65(1):246–254
Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K, Rajendran J (2011) Hypoxia imaging with [F-18] FMISO-PET in head and neck cancer: potential for guiding intensity modulated radiation therapy in overcoming hypoxia-induced treatment resistance. Radiother Oncol 101(3):369–375
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56(19):4509–4515
Hoogsteen IJ, Lok J, Marres HAM, Takes RP, Rijken PFJW, van der Kogel AJ et al (2009) Hypoxia in larynx carcinomas assessed by pimonidazole binding and the value of CA-IX and vascularity as surrogate markers of hypoxia. Eur J Cancer 45(16):2906–2914
Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J (2012) Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol 9(12):674–687
Janssens GO, Rademakers SE, Terhaard CH, Doornaert PA, Bijl HP, van den Ende P et al (2012) Accelerated radiotherapy with carbogen and nicotinamide for laryngeal cancer: results of a phase III randomized trial. J Clin Oncol 30(15):1777–1783
Jordan BF, Sonveaux P (2012) Targeting tumor perfusion and oxygenation to improve the outcome of anticancer therapy. Front Pharmacol 3:94
Le Q-T, Sutphin PD, Raychaudhuri S, Yu SCT, Terris DJ, Lin HS et al (2003) Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res 9(1):59–67
Le Q-T, Chen E, Salim A, Cao H, Kong CS, Whyte R et al (2006) An evaluation of umor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res 12(5):1507–1514
Ling CC, Humm J, Larson S, Amols H, Fuks Z, Leibel S et al (2000) Towards multidimensional radiotherapy (MD-CRT): biological imaging and biological conformality. Int J Radiat Oncol Biol Phys 47(3):551–560
Lukacova S, Khalil AA, Overgaard J, Alsner J, Horsman MR (2005) Relationship between radiobiological hypoxia in a C3H mouse mammary carcinoma and osteopontin levels in mouse serum. Int J Radiat Biol 81(12):937–944
Mathias CJ, Welch MJ, Kilbourn MR, Jerabek PA, Patrick TB, Raichle ME et al (1987) Radiolabeled hypoxic cell sensitizers: tracers for assessment of ischemia. Life Sci 41(2):199–206
McCall KC, Humm JL, Bartlett R, Reese M, Carlin S (2012) Copper-64-diacetyl-bis(N(4)-methylthiosemicarbazone) pharmacokinetics in FaDu xenograft tumors and correlation with microscopic markers of hypoxia. Int J Radiat Oncol Biol Phys 84(3):e393–e399
Mortensen LS, Johansen J, Kallehauge J, Primdahl H, Busk M, Lassen P et al (2012) FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol 105(1):14–20
Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K et al (2012) Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res 72(16):4165–4177
O’Donoghue JA, Zanzonico P, Pugachev A, Wen B, Smith-Jones P, Cai S et al (2005) Assessment of regional tumor hypoxia using 18F-fluoromisonidazole and 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) positron emission tomography: Comparative study featuring microPET imaging, PO2 probe measurement, autoradiography, and fluorescent microscopy in the R3327-AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys 61(5):1493–1502
Ogawa K, Chiba I, Morioka T, Shimoji H, Tamaki W, Takamatsu R et al (2011) Clinical significance of HIF-1alpha expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Anticancer Res 31(6):2351–2359
Okamoto S, Shiga T, Yasuda K, Ito YM, Magota K, Kasai K et al (2013) High reproducibility of tumor hypoxia evaluated by 18F-fluoromisonidazole PET for head and neck cancer. J Nucl Med 54(2):201–207
Ostheimer C, Bache M, Güttler A, Reese T, Vordermark D (2014a) Prognostic information of serial plasma osteopontin measurement in radiotherapy of non-small-cell lung cancer. BMC Cancer 14:858
Ostheimer C, Bache M, Güttler A, Kotzsch M, Vordermark D (2014b) A pilot study on potential plasma hypoxia markers in the radiotherapy of non-small cell lung cancer. Osteopontin, carbonic anhydrase IX and vascular endothelial growth factor. Strahlenther Onkol 190(3):276–282
Overgaard J (2007) Hypoxic radiosensitization: adored and ignored. J Clin Oncol 25(26):4066–4074
Overgaard J (2011) Hypoxic modification of radiotherapy in squamous cell carcinoma of the head and neck—a systematic review and meta-analysis. Radiother Oncol 100(1):22–32
Peeters SGJA, Zegers CML, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GAMS et al (2015a) A comparative study of the hypoxia PET tracers [18F]HX4, [18F]FAZA, and [18F]FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys 91(2):351–359
Peeters SGJA, Zegers CML, Biemans R, Lieuwes NG, van Stiphout RGPM, Yaromina A et al (2015b) TH-302 in combination with radiotherapy enhances the therapeutic outcome and is associated with pretreatment [18F]HX4 hypoxia PET imaging. Clin Cancer Res
Piert M, Machulla H-J, Picchio M, Reischl G, Ziegler S, Kumar P et al (2005) Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med 46(1):106–113
Rajendran JG, Krohn KA (2015) F-18 fluoromisonidazole for imaging tumor hypoxia: imaging the microenvironment for personalized cancer therapy. Semin Nucl Med 45(2):151–162
Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D et al (2007) Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA–first small animal PET results. J Pharm Pharm Sci 10(2):203–211
Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S et al (2006) Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 24(13):2098–2104
Rischin D, Peters LJ, O’Sullivan B, Giralt J, Fisher R, Yuen K et al (2010) Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol 28(18):2989–2995
Schütze C, Bergmann R, Brüchner K, Mosch B, Yaromina A, Zips D et al (2014) Effect of [(18)F]FMISO stratified dose-escalation on local control in FaDu hSCC in nude mice. Radiother Oncol 111(1):81–87
Servagi-Vernat S, Differding S, Hanin F-X, Labar D, Bol A, Lee JA et al (2014) A prospective clinical study of 18F-FAZA PET-CT hypoxia imaging in head and neck squamous cell carcinoma before and during radiation therapy. Eur J Nucl Med Mol Imaging 41(8):1544–1552
Thorwarth D, Eschmann S-M, Paulsen F, Alber M (2007) Hypoxia dose painting by numbers: a planning study. Int J Radiat Oncol Biol Phys 68(1):291–300
Thorwarth D, Monnich D, Wack L et al (2014) Validation of a hypoxia TCP model and dose painting in HNC: Planned interim analysis of a phase II trial. Radiother Oncol 111(Suppl 1):134
Toustrup K, Sørensen BS, Nordsmark M, Busk M, Wiuf C, Alsner J et al (2011) Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res 71(17):5923–5931
Troost EGC, Bussink J, Kaanders JHAM, van Eerd J, Peters JPW, Rijken PFJW et al (2005) Comparison of different methods of CAIX quantification in relation to hypoxia in three human head and neck tumor lines. Radiother Oncol 76(2):194–199
Troost EGC, Laverman P, Kaanders JHAM, Philippens M, Lok J, Oyen WJG et al (2006) Imaging hypoxia after oxygenation-modification: comparing [18F]FMISO autoradiography with pimonidazole immunohistochemistry in human xenograft tumors. Radiother Oncol 80(2):157–164
Troost EGC, Laverman P, Philippens MEP, Lok J, van der Kogel AJ, Oyen WJG et al (2008) Correlation of [18F]FMISO autoradiography and pimonidazole [corrected] immunohistochemistry in human head and neck carcinoma xenografts. Eur J Nucl Med Mol Imaging 35(10):1803–1811
Van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C et al (2007) The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer 43(9):1392–1398
Welz S, Pfannenberg C, Reimold M et al (2014) Hypoxia dose-escalation with chemo-radiation in head and neck cancer: planned interim analysis of a randomized study. Radiother Oncol 111(Suppl 1):155–156
Yaromina A, Zips D, Thames HD, Eicheler W, Krause M, Rosner A et al (2006) Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: the need for a multivariate approach in biomarker studies. Radiother Oncol 81(2):122–129
Yuan H, Schroeder T, Bowsher JE, Hedlund LW, Wong T, Dewhirst MW (2006) Intertumoral differences in hypoxia selectivity of the PET imaging agent 64Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone). J Nucl Med 47(6):989–998
Zegers CML, van Elmpt W, Wierts R, Reymen B, Sharifi H, Öllers MC et al (2013) Hypoxia imaging with [18F]HX4 PET in NSCLC patients: defining optimal imaging parameters. Radiother Oncol 109(1):58–64
Zhu Y, Denhardt DT, Cao H, Sutphin PD, Koong AC, Giaccia AJ et al (2005) Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene 24(43):6555–6563
Zips D, Zöphel K, Abolmaali N, Perrin R, Abramyuk A, Haase R et al (2012) Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 105(1):21–28
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Zschaeck, S., Steinbach, J., Troost, E.G. (2016). FMISO as a Biomarker for Clinical Radiation Oncology. In: Baumann, M., Krause, M., Cordes, N. (eds) Molecular Radio-Oncology. Recent Results in Cancer Research, vol 198. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-49651-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-662-49651-0_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-49649-7
Online ISBN: 978-3-662-49651-0
eBook Packages: MedicineMedicine (R0)