Abstract
[Yttrium-90-DOTA-Tyr3]-octreotide (DOTATOC) and [177Lu-DOTA-Tyr3-Thr8]-octreotide (DOTATATE) are used for peptide receptor-mediated radionuclide therapy (PRMRT) in neuroendocrine tumours. No human data comparing these two compounds are available so far. We used 111In as a surrogate for 90Y and 177Lu and examined whether one of the 111In-labelled peptides had a more favourable biodistribution in patients with neuroendocrine tumours. Special emphasis was given to kidney uptake and tumour-to-kidney ratio since kidney toxicity is usually the dose-limiting factor. Five patients with metastatic neuroendocrine tumours were injected with 222 MBq 111In-DOTATOC and 111In-DOTATATE within 2 weeks. Up to 48 h after injection, whole-body scans were performed and blood and urine samples were collected. The mean absorbed dose was calculated for tumours, kidney, liver, spleen and bone marrow. In all cases 111In-DOTATATE showed a higher uptake (%IA) in kidney and liver. The amount of 111In-DOTATOC excreted into the urine was significantly higher than for 111In-DOTATATE. The mean absorbed dose to the red marrow was nearly identical. 111In-DOTATOC showed a higher tumour-to-kidney absorbed dose ratio in seven of nine evaluated tumours. The variability of the tumour-to-kidney ratio was high and the significance level in favour of 111In-DOTATOC was P=0.065. In five patients the pharmacokinetics of 111In-DOTATOC and 111In-DOTATATE was found to be comparable. The two peptides appear to be nearly equivalent for PRMRT in neuroendocrine tumours, with minor advantages for 111In/90Y-DOTATOC; on this basis, we shall continue to use 90Y-DOTATOC for PRMRT in patients with metastatic neuroendocrine tumours.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Somatostatin receptors have been identified in high density on neuroendocrine tumours as well as on tumours of the central nervous system, the breast, the lung and the lymphatic tissue [1]. To demonstrate the presence of somatostatin receptors in vivo, scintigraphy with radiolabelled somatostatin analogues such as [111In-DTPA-d-Phe1]-octreotide (Octreoscan) has become the gold standard [2]. Peptide receptor-mediated radionuclide therapy (PRMRT) has been used for several years in the treatment of progressive, metastasised, somatostatin receptor-positive tumours. Both somatostatin analogues, [90Y-DOTA-Tyr3]-octreotide (DOTATOC) and [177Lu-DOTA-Tyr3-Thr8]-octreotide (DOTATATE), have been used for this purpose and have shown encouraging results [3–6]. Recently, Kwekkeboom et al. showed that 177Lu-DOTATATE had an up to fourfold higher tumour uptake than [111In-DTPA]-octreotide in six patients [7]. Moreover, Reubi et al. have shown that Y(III)-DOTATATE has an approximately sevenfold higher binding affinity to the somatostatin receptor subtype 2 (hsst2) compared with Y(III)-DOTATOC [8], which suggests that 111In/90Y-DOTATATE would show a higher tumour uptake in patients. However, no patient data comparing these two compounds are available so far.
Therefore, the aim of this study was to compare the pharmacokinetics of 111In-DOTATOC and 111In-DOTATATE in the same patients. Special emphasis was placed on the mean absorbed doses for tumour, kidney and bone marrow since the dose-limiting organs for PRMRT with radiolabelled somatostatin analogues are usually the kidneys and the bone marrow [3, 4, 9]. In addition, 111In-DOTATOC/111In-DOTATATE was taken as a surrogate for 90Y-DOTATOC/90Y-DOTATATE and the calculated mean absorbed doses of 90Y-DOTATOC were compared with the doses of this radiopeptide known from the literature [10–13].
Materials and methods
Patients
Five male patients (age 49–73, mean 62 years) (Table 1) with known metastatic neuroendocrine tumours were injected with 222 MBq 111In-DOTATOC and 222 MBq 111In-DOTATATE, with an interval of 2 weeks between the administrations. In three patients, 111In-DOTATATE was injected first, while in two, 111In-DOTATOC was injected first. In two patients the primary tumour was in the pancreas. In three patients the origin of the disease was unknown. None of the patients had received medication with somatostatin analogues (Octreotide s/c or LAR, Novartis Pharma; Lanreotide, Ipsen Ltd.) within the 8 weeks before the examinations. All patients had a histologically confirmed neuroendocrine tumour and had been treated with 90Y-DOTATOC before. There were at least 14 months between the last therapy and the beginning of the study (14–25 months, mean 20.25 months). Metastatic disease had been confirmed in all cases by recent morphological imaging with magnetic resonance imaging (MRI), computed tomography (CT) or ultrasonography. Based on these examinations, the tumour volumes were calculated. The study was approved by the Swiss authorities and by the local ethical committee (Ethikkommission beider Basel). All patients gave informed consent.
Radiopharmaceuticals
Both somatostatin analogues, DOTATOC and DOTATATE, were synthesised in house according to a previously published procedure [8, 14] and radiolabelled with 111In as published previously [15]. 111In was purchased from Tyco Healthcare (Petten, The Netherlands). The labelling yield and the radiopharmaceutical purity were checked using C18 reversed-phase high-performance liquid chromatography.
Imaging
All images were acquired with a dual-head gamma camera Picker Prism 2000 XP (Philips, Eindhoven, The Netherlands). The windows were centred over both 111In photon peaks (245 and 172 keV) with a width of 20%. Parallel-hole, medium-energy general-purpose collimators were used. For both compounds, the same protocol was followed: dynamic imaging up to 20 min post injection with a field of view over the kidneys and liver from the posterior projection (80 images, 15 s per image). Whole-body scans were obtained 1, 2, 4, 24 and 48 h after injection. The acquisition time for all whole-body scans was 15 min.
Measurement of radioactivity in blood and urine
Blood samples were drawn 10, 20, 30 and 60 min and 2, 4, 24 and 48 h after injection. Urine was collected in four intervals: 0–2, 2–4, 4–24 and 24–48 h after injection. Radioactivity in blood and urine was measured with a gamma counter (Cobra II Autogamma, Packard, A Canberra Company).
Pharmacokinetics and dosimetry
Regions of interest (ROIs) were drawn manually on the whole-body scans from the anterior and posterior projections for the whole body, the kidneys, the spleen, the liver, the bladder and tumour lesions. The Odyssey XP program was used. Background regions were placed close to the ROIs for background correction. Parts of the organs showing tumour infiltration or superimposition were excluded from the evaluation of organ uptake. The geometric mean value, between anterior and posterior, was taken and corrected for attenuation and physical decay. Whole-body activity acquired 1 h after injection was defined as 100% of the injected activity (IA). The patients did not empty the bladder during this period. All data for the whole body, organs and tumour lesions were expressed in %IA. A compartment model as described previously was used to calculate the residence time from the time-activity data resulting from the scans [10]. The activity in blood was fitted by three exponential curves. The residence times were determined using these data and the respective half-lives of 111In and 90Y. Assuming no specific uptake in the red marrow, a uniform distribution of the activity, and that the red marrow clearance was the same as in blood, the dose to the red marrow was calculated with a correction factor of 1 from the residence time in blood as published previously [10].
Statistics
Paired t test was used to determine statistical significance. Differences at the 95% confidence level (P<0.05) were considered significant.
Results
Patients showed no clinical adverse reactions and no side-effects after the intravenous injection of 111In-DOTATOC or 111In-DOTATATE.
Pharmacokinetic studies
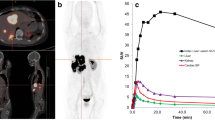
Figure 1 displays the mean plasma radioactivity (and standard deviation) expressed as %IA. The clearance of both peptides was fast. The time-activity in blood could be fitted by three exponential curves. In all patients and for both radiopeptides, the activity in blood decreased to less than 10% within the first 4 h. 111In-DOTATOC showed a somewhat slower clearance initially. After 24 and 48 h, a slightly higher amount of 111In-DOTATATE was found in the blood of all patients. The mean residence time (τ) was 1.178 h (SD±0.19 h) for 111In-DOTATOC and 1.156 h (SD±0.32 h) for 111In-DOTATATE. Therefore, the mean absorbed dose to the red marrow was not significantly different between the two compounds (Table 2). Only a small interpatient variability was found (Table 3).
Cumulative activity excreted into the urine was higher for 111In-DOTATOC in all samples and all patients (Fig. 2). For all periods (0–2, 2–4, 4–24 and 24–48 h) the difference was significant (P<0.05).
Biodistribution and dosimetry
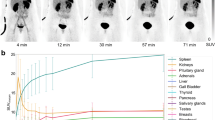
The distribution pattern of 111In-DOTATATE was initially comparable to the pattern using 111In-DOTATOC. In four of the five patients a distinct specific uptake in tumour sites was seen after approximately 2 min. Also, there was fast visualisation of the liver, kidneys and spleen. The fifth patient showed no tumour uptake with either compound due to an impressive decrease in tumour load after 90Y-DOTATOC therapy. In this patient, only two small liver metastases <1 cm were found on a recent CT scan.
We found higher mean absorbed doses to the kidneys and liver for 111In-DOTATATE. The calculated difference for 90Y, when taking 111In as a surrogate, was significant in the liver (P<0.05) but not in the kidneys (P=0.135). The dose to the spleen showed a high interpatient variability. Although in three patients the mean absorbed dose to the spleen for 111In-DOTATATE was higher (Table 2), the difference did not reach significance (P=0.205). The calculated absorbed doses for 90Y-DOTATOC (taking 111In-DOTATOC as the surrogate) for the various organs are comparable with the doses known from the literature [10–12]. Our values, along with literature data, are shown in Table 3.
Overall, nine metastases could be evaluated in scintigraphic images and correlated with a lesion on CT, MRI or sonography. In five of the nine lesions, a somewhat higher mean absorbed dose (mGy/MBq) was found for 111In-DOTATOC; however, high variability between the lesions was observed (Table 4).
Since most often the dose-limiting organ in therapy with radiopeptides is the kidneys, the absorbed dose ratio of lesion to kidneys will determine the therapeutic window. Due to the high variability in mean absorbed doses in the tumours, we found a high variability in the dose ratios as well. However, in seven out of nine lesions, 111In-DOTATOC showed a higher ratio and the difference in mean values almost reached significance (P=0.065) (Table 4).
In two patients, whole-body scans 4, 24 and 48 h after injection showed better visualisation of liver metastases with 111In-DOTATOC. In the other three patients, the scans were visually identical. The better demarcation was due to the lower uptake in the normal liver. Findings in one of the patients with better demarcation of the liver metastases with 111In-DOTATOC are shown in Fig. 3.
Discussion
In this study, both radiopeptides, 111In-DOTATOC and 111In-DOTATATE, showed the expected high specific uptake in somatostatin receptor-positive tissue. Visually, the results obtained with the two compounds were comparable, although better visualisation of some liver metastases was found with 111In-DOTATOC. The dosimetric analyses showed small differences between the radiopeptides, but a significantly higher mean absorbed dose to the liver was found for 111In-DOTATATE, and a favourable tumour-to-kidney ratio (P=0.065) was calculated for 111In-DOTATOC. These findings were unexpected since data from animal models have shown a more favourable biodistribution for DOTATATE-derived radiopeptides [16]. Because the total administered therapeutic dose with radiolabelled somatostatin analogues is determined by tumour-to-kidney mean absorbed dose ratios (and/or tumour-to-red marrow absorbed dose ratios), these ratios are the most important parameters for therapeutic success. Slightly better results were found for 111In-DOTATOC in three of the four patients with well-defined uptake in metastases.
In this study, we used 111In as a surrogate for 90Y, as we assume high similarity between the tracers although differences in pharmacological parameters have been shown if DOTATOC is labelled with 67Ga/68Ga instead of 111In [15]. In addition, de Jong et al. [17] showed differences in the biodistribution of 111In-DOTATOC and 90Y-DOTATOC in rats bearing the CA 20948 tumour. On the other hand, Froidevaux et al. [18] showed an essentially similar performance of 111In-DOTATOC and 90Y-DOTATOC when using the AR4-2J bearing mouse model.
The mean absorbed doses calculated for 90Y-DOTATOC are comparable with the absorbed doses published in the literature so far [9–13] (Table 3), confirming the accuracy of our methodology. Although the absorbed doses calculated for the red marrow were higher than the doses reported by Cremonesi et al. [10] and Förster et al. [11], they are comparable with the doses published by Krenning et al. [12] (Table 3).
A high interpatient absorbed tumour dose variability was found, which is not unexpected as receptor densities vary markedly among patients and tumours. This fact is well known from the literature [10–13].
A better tumour-to-kidney absorbed dose ratio can be achieved by co-infusion of amino acids, especially lysine and arginine [13, 19–21]. It is unclear whether the results obtained in comparing 111In-DOTATOC and 111In-DOTATATE would be the same if the measurements were to be performed with co-infusion of amino acids. The difference in charge (positive overall charge of 111In-DOTATOC and neutral charge of 111In-DOTATATE) might lead to different results with regard to the kidney absorbed dose after amino acid co-infusion.
The accuracy of the absolute values obtained by organ dosimetry using gamma-scintigraphy may still be limited owing to many potential sources of error. However, since the main aim of this study was to compare two compounds in the same patients with the same methods, this would not have affected the reliability of the findings.
We could not confirm the assumption, based on animal experiments, that 90Y-DOTATATE may have more favourable characteristics for PRMRT compared with 90Y-DOTATOC. Therefore, we will continue treatment with 90Y-DOTATOC.
References
Reubi JC, Laissue JA. Multiple actions of somatostatin in neoplastic disease. Trends Pharmacol Sci 1995;16:110–5
Krenning EP, Kwekkeboom DJ, Bakker WH et al. Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716–31
Waldherr C, Pless M, Maecke H, Schumacher T, Crazzolara A, Nitzsche E, Haldemann A, Mueller-Brand J. Tumor response and clincical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med 2002;43:610–6
Kwekkeboom D, Bakker W, Kam BLR, Teunissen J, Kooij P, Herder W, Feelders R, Eijck C, Jong M, Srinivasan A, Erion J, Krenning E. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA0, Tyr3]octreotate. Eur J Nucl Med 2003;30:417–22
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, Maecke H, Mueller J. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26:1439–47
Paganelli G, Bodei L, Handkiewicz Junak D, Rocca P, Papi S, Lopera Sierra M, Gatti M, Chinol M, Bartolomei M, Fiorenza M, Grana C. 90Y-DOTA-d-Phe1-Tyr3-octreotide in therapy of neuroendocrine malignancies. Biopolymers 2002;66:393–8
Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, Schmidt MA, Bugaj JL, de Jong M, Krenning EP. [177Lu-DOTA0Tyr3]octreotate: comparison with [111In-DTPA0]octreotide in patients. Eur J Nucl Med 2001;28:1319–25
Reubi J, Schaer J, Waser B, Wenger S, Heppeler A, Schmitt J, Maecke H. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273–82
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, Caracciolo M, Maecke H, Chinol M, Paganelli G. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging 2003;30:207–16
Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin M, Orsi F, Maecke H, Jermann E, Robertson C, Fiorenza M, Tosi G, Paganelli G. Biokinetics and dosimetry in patients administered with 111In-DOTA-Tyr3-octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 1999;26:877–86
Förster GJ, Engelbach M, Brockmann J, Reber H, Buchholz H-G, Maecke HR, Rösch F, Herzog H, Bartenstein P. Preliminary data on biodistribution and dosimetry for therapy planning of somatostatin receptor positive tumours: comparison of 86Y-DOTATOC and 111In-DTPA-octreotide. Eur J Nucl Med 2001;28:1743–50
Krenning EP, de Jong M, Jamar F, Valkema R, Kwekkeboom DJ, Kvols LK, Smith C, Pauwels E. Somatostatin receptor-targeted radiotherapy of tumors: preclinical and clinical findings. In: Lamberts S, Dogliotti L, eds. The expanding role of octreotide I: advances in oncology. Bristol: BioScientifica; 2002:211–23
Jamar F, Barone R, Mathieu I, Walrand S, Labar D, Carlier P, De Camps J, Schran H, Chen T, Smith MC, Bouterfa H, Valkema R, Krenning EP, Kvols LK, Pauwels S. 86Y-DOTA0-d-Phe1-Tyr3-octreotide (SMT 487)—a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging 2003;30:510–8
Wild D, Schmitt JS, Ginj M, Maecke HR, Bernard BF, Krenning E, De Jong M, Wenger S, Reubi JC. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 2003;30:1338–47
Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, Hennig M. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 1999;5:1016–23
Erion J, Schmidt M, Wilhelm R, Achilefu S, Srinivasan A. Biodistribution and radiotherapy studies using samarium-153 and lutetium-177 DTPA conjugates of Y3-Octreotate. J Nucl Med 1999;40(Suppl):223
de Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, Visser TJ, Jermann E, Béhé M, Powell P, Maecke HR. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0,d-Phe1,Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997;24:368–71
Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, Eisenwiener K, Beglinger C, Maecke HR. Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer 2002;98:930–7
Bernard BF, Krenning EP, Breeman WA, Rolleman EJ, Bakker WH, Visser TJ, Macke H, de Jong M. d-Lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med 1997;38:1929–33
Behr TM, Goldenberg DM, Becker W. Reducing the renal uptake of radiolabeled antibody fragments and peptides for diagnosis and therapy: present status, future prospects and limitations. Eur J Nucl Med 1998;25:201–12
Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging 2003;30:9–15
Acknowledgements
The authors wish to thank all supporting personnel of the Division of Radiological Chemistry, especially P. Powell, and the Institute of Nuclear Medicine, especially I. Gutierrez, for their expert help and effort. This work was supported by the Swiss National Foundation (project 3100 AO-100390) and was performed within the COST B12 action. We also wish to thank Drs. M. Konijnenberg (Tyco Healthcare, Petten, The Netherlands) and H. Roser and Prof. J. Roth (Division of Medical Physics, University Hospital Basel) for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forrer, F., Uusijärvi, H., Waldherr, C. et al. A comparison of 111In-DOTATOC and 111In-DOTATATE: biodistribution and dosimetry in the same patients with metastatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 31, 1257–1262 (2004). https://doi.org/10.1007/s00259-004-1553-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1553-6