Abstract
The current technique of choice for perfusion imaging is gated single-photon emission computed tomography (SPECT), which allows the simultaneous assessment of perfusion and left ventricular (LV) function. We examined the relationships of infarct size and severity with LV ejection fraction (EF) and volumes in 215 myocardial infarction patients treated with primary percutaneous coronary intervention within 6 h of symptom onset. Patients were studied with resting gated SPECT 1 month later. Infarct size was expressed as LV percent, and infarct severity as the lowest activity ratio within the defect. LVEF, end-diastolic (ED) and end-systolic (ES) volume indexes (Vi) were calculated with commercial software. There was a significant correlation between infarct size and LVEF (r=−0.68, P<0.00001), EDVi (r=0.53, P<0.00001), and ESVi (r=0.62, P<0.00001). Slightly lower correlations were demonstrated using infarct severity. LVEF and volumes were related to infarct location. A significantly higher correlation was observed between infarct size and LVEF in anterior than in non-anterior infarctions (r=−0.75 vs −0.60, P<0.05). In multivariate analysis, infarct size and infarct location were significant predictors of LVEF (R 2=0.50) and ESV (R 2=0.40). Infarct size and infarct severity were significant predictors of EDVi (R 2=0.29). Infarct size (and severity) and LVEF (and volumes) derived from a single gated SPECT study correlate closely. Infarct location influences this relationship, with anterior infarctions showing a lower LVEF than inferior or lateral ones of the same extent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In patients with recent myocardial infarction, resting left ventricular (LV) ejection fraction (EF) is known to be important for prognostic stratification [1, 2, 3]. Also, the final extent of the infarct scar has been demonstrated to have major prognostic implications [3, 4]. Several studies have shown the value of infarct size measured using 99mTc-sestamibi single-photon emission computed tomography (SPECT), its relationship with LV function and volumes and the importance of the mismatch between these parameters [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Furthermore, 99mTc-sestamibi SPECT allows the assessment of infarct severity, and this parameter, which is related to the residual viability within the infarct area, has been shown to be important in predicting functional recovery [14, 15, 16, 17].
The measurement of LV function and volumes to be compared with infarct size has in the past been performed using a separate imaging modality, most frequently radionuclide angiocardiography. Nowadays, gated SPECT is becoming the standard modality for perfusion scintigraphy [18]. This method allows the assessment of LV function and the measurement of volumes during a perfusion scan, and has been reported to be accurate and reproducible [19, 20, 21, 22, 23, 24]. To our knowledge, however, a comparison of infarct size and LVEF assessed using gated SPECT has not previously been reported. Moreover, scanty data are available on the relationship between infarct severity and LV function and volumes, particularly in patients submitted to early reperfusion therapy [17, 25, 26]. The aim of the present study was to evaluate the relationships of infarct size and severity with LVEF and LV volumes, all obtained from a single gated SPECT study, and to examine which factors influence these relationships in patients submitted to successful primary percutaneous coronary intervention for acute myocardial infarction.
Materials and methods
Patient population and study protocol
Between January 2001 and January 2003, 254 patients admitted to our Cardiology Department because of acute myocardial infarction within 6 h of symptom onset and submitted to successful primary percutaneous coronary intervention with stenting of the infarct-related vessel, were referred to our Nuclear Medicine laboratory for the assessment of infarct size at 1 month. The diagnosis of acute myocardial infarction required the presence of typical chest pain lasting more than 30 min together with >0.1 mV ST segment elevation in at least two contiguous electrocardiographic leads. A total of 24 patients with a history of prior infarction before the index infarction were excluded. Of 230 eligible patients, 14 were excluded because arrhythmia due to atrial fibrillation precluded gating of SPECT, and in one patient the acquisition of gated SPECT failed for technical reasons. Thus, the final study cohort included 215 patients (176 men and 39 women, mean age 63±13 years, range 23–90).
Coronary angiography and mechanical revascularisation
Selective coronary angiography was performed in multiple projections before mechanical reperfusion. Immediately after diagnostic angiography, percutaneous coronary intervention with stenting of the infarct-related vessel was performed using standard material. Successful primary percutaneous coronary intervention was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 3 coronary flow in the treated vessel with a residual stenosis <20% [27]. All patients underwent control angiography at 1 month after index infarction to exclude the occurrence of restenosis of the infarct-related artery.
Gated SPECT
Gated SPECT acquisition began 60 min after 99mTc-sestamibi injection (740 MBq), using a double-head camera (Picker Irix, Philips Medical System, Andover, MA) equipped with high-resolution collimators, a 180° rotation arc, 34 projections, 60 s/projection, 8 frames/heart cycle and 64×64 matrices. The studies were reconstructed using filtered back-projection without attenuation or scatter correction and realigned along the heart axis. Perfusion defects were quantified as percentage of LV wall, with the defect threshold set at 60% of peak uptake [6]. Infarct severity was defined as the lowest ratio of minimal to maximal counts in the short axis slices examined for infarct size evaluation [14, 15, 16]. The measurement of LV end-diastolic (EDV) and end-systolic volume (ESV) and LVEF was performed by an automated and validated method [19]. Volumetric data were corrected for body surface area, and indicated as indexes by EDVi and ESVi.
Statistical analysis
Results are expressed as mean value ± standard deviation. The correlation between continuous variables was calculated using the Pearson’s correlation coefficient. The comparisons between groups were performed by one-way analysis of variance with the Tukey post-hoc test. The interactions between infarct size, infarct severity, infarct location, infarct-related vessel, LVEF and LV volumes were analysed with multiple regression analysis. A P value <0.05 was considered statistically significant.
Results
According to admission electrocardiographic data, 87 patients had an anterior, 104 an inferior and 24 a lateral myocardial infarction. In the admission coronary angiogram, the infarct-related artery was the left anterior descending in 87 patients, the right coronary artery in 95 and the left circumflex in 33. The infarct-related artery was the sole diseased vessel in 122 patients; 67 patients had two-vessel coronary artery disease and 26 three-vessel disease. Peak creatine kinase was 2,460±2,079 U/l.
The mean interval between index infarction and follow up was 36±8 days. At the time of follow-up, all patients were alive and asymptomatic and none presented restenosis of the infarct-related artery. A perfusion defect was visually detected in 180 patients, and a measurable defect was detected in 188. The location of the detectable infarctions was in agreement with the admission data in all patients. The infarct size measured according to gated SPECT was 16.9%±13.1%. The infarct severity in the 188 patients with measurable defects was 0.40±0.13. The LVEF measured by gated SPECT was 45.7%±11.6%. The EDVi was 61±21 ml, and the ESVi, 35±19 ml.
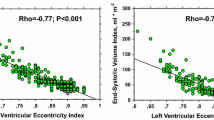
There was a significant correlation between peak creatine kinase and infarct size [r=0.61, P<0.00001, standard error of estimate (SEE)=10.4] and between peak creatine kinase and infarct severity [r=−0.46, P<0.00001, SEE 0.21]. The infarct size was significantly correlated to LVEF (r=−0.68, P<0.00001, SEE=8.5%), EDVi (r=0.53, P<0.00001, SEE=11 ml) and ESVi (r=0.62, P<0.00001, SEE=10 ml) (Fig. 1). Slightly lower, but still highly significant correlations were registered between infarct severity and LVEF (r=0.58, P<0.00001, SEE=9.4%), EDVi (r=−0.49, P<0.00001, SEE=19 ml) and ESVi (r=−0.54, P<0.00001, SEE=16 ml).
In univariate analysis, neither the infarct size nor the infarct severity was correlated to the infarct location or to the infarct-related vessel. Conversely, LVEF and LV volumes were significantly related to both parameters (Table 1). In particular, anterior infarctions had a significantly (P<0.005) lower LVEF (41.6%±12.3%) than inferior (48%±10%) and lateral infarctions (50.4%±11.2%) (Fig. 2). A similar result was obtained when the infarctions were divided according to the infarct-related vessel, with nine inferior infarctions being reclassified because they were related to disease of a dominant left circumflex artery.
When the relationship between infarct size or infarct severity and LVEF or volumes was examined separately in the anterior versus the non-anterior (inferior or lateral) infarctions, the correlation coefficients were higher for the anterior infarctions, and the difference was significant for the correlation between infarct size and LVEF (r=−0.75 for the anterior vs r=−0.60 for the non-anterior infarctions, P<0.05) (Table 2, Fig. 3).
In multivariate analysis, both infarct size and infarct location were selected as significant predictors of LVEF, with R 2=0.50. Infarct size and infarct severity were significant predictors of EDVi, with R 2=0.29. Finally, infarct size and infarct location were both significant predictors of ESVi, with R 2=0.40 (Table 3).
Discussion
The present results confirm that there is a significant relationship between infarct size and both LVEF and LV volumes assessed on the basis of a single gated SPECT perfusion study performed at 1 month after first myocardial infarction treated using primary percutaneous coronary intervention. Furthermore, it appears that, together with the infarct size, the infarct location (or the infarct-related artery) also influences LVEF and volumes, with lower LVEF values in anterior than in inferior or lateral infarctions of the same extent, and larger EDVi and ESVi in anterior than in inferior or lateral infarctions. As regards infarct severity, this study demonstrates a significant relationship with LVEF and LV volumes. Infarct severity offers additional information over infarct size for predicting the EDVi.
The relationship between perfusion infarct size and LVEF has previously been demonstrated using separate imaging modalities, mainly equilibrium or first-pass radionuclide angiocardiography [3, 7, 8, 12]. However, since gated SPECT is the current state-of-the-art technique for myocardial perfusion imaging, it is difficult to justify either the administration of an additional amount of radioactive tracer or the acquisition of a separate study to obtain the LVEF. On the other hand, some differences between gated SPECT measurements and those obtained using other techniques, mainly because of the limited time framing currently used, have been reported [19]. Therefore, it is interesting that the present study demonstrated a very close linear relation between infarct size and LVEF in the setting of gated SPECT, and a SEE that compares well with that reported using first-pass angiocardiography in a larger patient population [12]. Moreover, a major advantage of gated SPECT over both gated blood pool and first-pass angiocardiography is that it can easily provide a quite reliable estimate of LV volumes. It was possible to demonstrate a linear relation between infarct size and both ED and ES volumes, and the relationship appears well comparable to that registered in larger patient populations by other authors [3, 8].
Unlike the relationship between infarct size and LVEF, that between infarct severity and LVEF and volumes has not been extensively examined. According to our data, there is a significant relationship between infarct severity and LVEF and volumes, although the correlation coefficients always appeared lower than the corresponding values obtained using the infarct size.
An additional result of this study was to identify in univariate analysis a significant influence of infarct location (or of the infarct-related artery) on LVEF and LV volumes, but not on infarct size or infarct severity. In multivariate analysis both infarct size and infarct location were included in the model as significant predictors of LVEF and of ESVi, while infarct size was selected together with infarct severity as a significant predictor of EDVi. Also the relationship between infarct size and LVEF was influenced by the infarct location, with a steeper slope and a significantly higher correlation coefficient in the anterior infarctions. The same trend towards a higher correlation coefficient in anterior infarctions, although without statistical significance, was registered for the relationships between infarct severity and LVEF and volumes. Previous data obtained in the setting of acute reperfusion therapy with primary angioplasty had demonstrated a larger extent of risk area and of infarct size measured at hospital discharge in anterior versus non-anterior infarctions [14]. We have no explanations for the disagreement between those data and our results, although the shorter time interval between symptom onset and vessel reperfusion in our series could have affected more favourably the final infarct size of the potentially more severe anterior infarctions than that of the inferior or lateral ones [28]. In their population studied within 16 days of index infarction, Burns et al. did not find a significant influence of infarct location on the relation between infarct size and LVEF or ESVi [3]. Conversely, in an echocardiographic study, McClements et al. observed a lower LVEF for similar infarct size, assessed as extent of regional wall motion, in the antero-apical than in the other infarctions [29]. Also our results suggest that for any extent of infarct (and for any degree of infarct severity), the LVEF is lower, and the EDVi and ESVi are larger, if the infarction is anterior than if it is inferior or lateral. From the clinical point of view, this supports the most aggressive therapeutic approach in anterior infarctions, because an important functional impairment has to be expected even in the presence of a relatively limited infarct extent.
Another interesting finding of this study is that in multivariate analysis, infarct severity was selected with infarct size as a significant predictor of EDVi. Since infarct severity is an expression of infarct transmurality and of viability within the infarcted area, this finding is in agreement with other observations about the influence of viability on LV remodelling [30].
The results of the present study must be evaluated with caution because of its limitations. We have already referred to the possible underestimation of LVEF because of the poor temporal resolution with the time framing (8 frames/cycle) most frequently used for gated SPECT [19]. In spite of the good correlation with infarct size demonstrated in our series, further comparative studies using better temporal resolution, for instance with 16 frames/cycle, would be desirable. We assessed the infarct size at 1 month after index infarction instead of at hospital discharge, as in the majority of previously published studies. On the other hand, this circumstance should be more effective in preventing interference of myocardial stunning with the extent of perfusion defects [31]. Moreover, the execution of a coronary angiographic control before gated SPECT allowed the exclusion of infarct-related vessel restenosis. Finally, because we studied a patient population submitted to a very aggressive revascularisation protocol, including early direct percutaneous coronary intervention, caution should be exercised when extending our data to infarct patients submitted to other types of reperfusion therapy.
In conclusion, our data confirm that infarct size (and severity) and LVEF (and volumes) measured using the data of a single perfusion gated SPECT study are closely correlated. Infarct location influences the relationship between infarct size and LV functional parameters (LVEF and ESVi), with lower LVEF in anterior than in inferior or lateral infarctions of the same extent. Infarct severity and infarct size are significant predictors of EDVi.
References
Multicenter Post-infarction Research Group. Risk stratification and survival after myocardial infarction. N Engl J Med 1983; 309:331–336.
Touboul P, Andre-Fouet X, Leizorovicz A, Itti R, Lopez M, Sayegh Y, Milon H, Kirkorian G. Risk stratification after myocardial infarction. A reappraisal in the era of thrombolysis. The Groupe d’Etude du Pronostic de l’Infarctus du Myocarde (GREPI). Eur Heart J 1997; 18:99–107.
Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, Anderson JL, Yusuf S, CORE Study Investigators. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002; 39:30–36.
Miller TD, Christian TF, Hopfenspirger MR, Hodge DO, Gersh BJ, Gibbons RJ. Infarct size after acute myocardial infarction measured by quantitative tomographic99mTc sestamibi imaging predicts subsequent mortality. Circulation 1995; 92:334–341.
Gibbons RJ, Verani MS, Behrenbeck T, Pellikka PA, O’Connor MK, Mahmarian JJ, Chesebro JH, Wackers FJ. Feasibility of tomographic99mTc-hexakis-2-methoxy-2-methylpropyl-isonitrile imaging for the assessment of myocardial area at risk and the effect of treatment in acute myocardial infarction. Circulation 1989; 80:1277–1286.
O’Connor MK, Hammel T, Gibbons RJ. In vitro validation of a simple tomographic technique for estimation of percentage myocardium at risk using methoxyisobutyl isonitrile technetium 99m (sestamibi). Eur J Nucl Med 1990; 17:69–76.
Christian TF, Behrenbeck T, Pellikka PA, Huber KC, Chesebro JH, Gibbons RJ. Mismatch of left ventricular function and infarct size demonstrated by technetium-99m isonitrile imaging after reperfusion therapy for acute myocardial infarction: identification of myocardial stunning and hyperkinesia. J Am Coll Cardiol 1990; 16:1632–1638.
Christian TF, Behrenbeck T, Gersh BJ, Gibbons RJ. Relation of left ventricular volume and function over one year after acute myocardial infarction to infarct size determined by technetium-99m-sestamibi. Am J Cardiol 1991; 68:21–26.
Chareonthaitawee P, Christian TF, Hirose K, Gibbons RJ, Rumberger JA. Relation of initial infarct size to extent of left ventricular remodeling in the year after acute myocardial infarction. J Am Coll Cardiol 1995; 25:567–573.
O’Connor MK, Gibbons RJ, Juni JE, O’Keefe J, Ali A. Quantitative myocardial SPECT for infarct sizing: feasibility of a multicenter trial evaluated using a cardiac phantom. J Nucl Med 1995; 36:1130–1136.
Miller TD, Hodge DO, Sutton JM, Grines CL, O’Keefe JH, DeWood MA, Okada RD, Fletcher WO Jr, Gibbons RJ. Usefulness of technetium-99m sestamibi infarct size in predicting posthospital mortality following acute myocardial infarction. Am J Cardiol 1998; 81:1491–1493.
Chareonthaitawee P, Christian TF, Miller TD, Hodge DO, Gibbons RJ. Correlation of resting first-pass left ventricular ejection fraction and resting myocardial infarct size. Am J Cardiol 1998; 81:1281–1285.
Gibbons RJ, Miller TD, Christian TF. Infarct size measured by single-photon emission computed tomographic imaging with99mTc-sestamibi: a measure of the efficacy of therapy in acute myocardial infarction. Circulation 2000; 101:101–108.
Christian TF, Schwartz RS, Gibbons RJ. Determinants of infarct size in reperfusion therapy for acute myocardial infarction. Circulation 1992; 86:81–90.
Christian TF, O’Connor MK, Schwartz RS, Gibbons RJ, Ritman EL. Technetium-99m MIBI to assess coronary collateral flow during acute myocardial infarction in two closed-chest animal models. J Nucl Med 1997; 38:1840–1846.
Christian TF, Berger PB, O’Connor MK, Hodge DO, Gibbons RJ. Threshold values for preserved viability with a noninvasive measurement of collateral blood flow during acute myocardial infarction treated by direct coronary angioplasty. Circulation 1999; 100:2392–2395.
Sciagrà R, Sestini S, Bolognese L, Cerisano G, Buonamici P, Pupi A. Comparison of dobutamine echocardiography and99mTc-sestamibi tomography for prediction of left ventricular ejection fraction outcome after acute myocardial infarction treated with successful primary coronary angioplasty. J Nucl Med 2002; 43:8–14.
ASNC Executive Council. American Society of Nuclear Cardiology position statement on electrocardiographic gating of myocardial perfusion SPECT scintigrams. J Nucl Cardiol 1999; 6:470–471.
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, Van Train KF, Berman DS. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995; 36:2138–2147.
Williams KA, Taillon LA. Left ventricular function in patients with coronary artery disease assessed by gated tomographic myocardial perfusion images. Comparison with assessment by contrast ventriculography and first-pass radionuclide angiography. J Am Coll Cardiol 1996; 27:173–181.
Cwajg E, Cwajg J, He ZX, Hwang WS, Keng F, Nagueh SF, Verani MS. Gated myocardial perfusion tomography for the assessment of left ventricular function and volumes: comparison with echocardiography. J Nucl Med 1999; 40:1857–1865.
Nichols K, Lefkowitz D, Faber T, Folks R, Cooke D, Garcia EV, Yao SS, DePuey EG, Rozanski A. Echocardiographic validation of gated SPECT ventricular function measurements. J Nucl Med 2000; 41:1308–1314.
Vaduganathan P, He ZX, Vick W, Mahmarian JJ, Verani MS. Evaluation of left ventricular wall motion, volumes and ejection fraction by gated myocardial tomography with technetium 99m-labeled tetrofosmin: a comparison with cine magnetic resonance imaging. J Nucl Cardiol 1999; 6:3–10.
Mansoor MR, Heller GV. Gated SPECT imaging. Semin Nucl Med 1999; 29:271–278.
Galli M, Marcassa C, Bolli R, Giannuzzi P, Temporelli PL, Imparato A, Silva Orrego PL, Giubbini R, Giordano A, Tavazzi L. Spontaneous delayed recovery of perfusion and contraction after the first 5 weeks after anterior infarction. Evidence for the presence of hibernating myocardium in the infarcted area. Circulation 1994; 90:1386–1397.
Kang X, Berman DS, Van Train KF, Amanullah AM, Areeda J, Friedman JD, Kiat H, Germano G. Clinical validation of automatic quantitative defect size in rest technetium-99m-sestamibi myocardial perfusion SPECT. J Nucl Med 1997; 38:1441–1446.
The TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase 1 findings. N Engl J Med 1985; 312:932–936.
Bates ER. Revisiting reperfusion therapy in inferior myocardial infarction. J Am Coll Cardiol 1997; 30:334–342.
McClements BM, Weyman AE, Newell JB, Picard MH. Echocardiographic determinants of left ventricular ejection fraction after acute myocardial infarction. Am Heart J 2000; 140:284–290.
Bolognese L, Cerisano G, Buonamici P, Santini A, Santoro GM, Antoniucci D, Fazzini PF. Influence of infarct-zone viability on left ventricular remodeling after acute myocardial infarction. Circulation 1997; 96:3353–3359.
Sinusas AJ, Shi Q, Vitols PJ, Fetterman RC, Maniawski P, Zaret BL, Wackers FJ. Impact of regional ventricular function, geometry, and dobutamine stress on quantitative99mTc-sestamibi defect size. Circulation 1993; 88:2224–2234.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sciagrà, R., Imperiale, A., Antoniucci, D. et al. Relationship of infarct size and severity versus left ventricular ejection fraction and volumes obtained from 99mTc-sestamibi gated single-photon emission computed tomography in patients treated with primary percutaneous coronary intervention. Eur J Nucl Med Mol Imaging 31, 969–974 (2004). https://doi.org/10.1007/s00259-004-1482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1482-4