Abstract
Purpose
To highlight the spectrum of pathology and patterns of gluteus minimus tendon tearing observed on MR imaging of the hip.
Methods and materials
Retrospective review of consecutive hip MRI exams with findings of gluteus minimus tendon (GMin) pathology. A total of 194 exams in 178 patients (148 female, mean age 61) were reviewed. MRI exams are assessed for GMin: tendinopathy, partial, or complete tendon tears. GMin muscular fatty atrophy, enthesopathic cortical irregularities of the greater trochanter (GT), and peri-trochanteric edema or bursal fluid collections were assessed in all cases. In all cases of complete GMin tendon tearing, position and relationship of GMin tendon were assessed relative to its normal insertion site and adjacent soft tissues.
Results
Clinical indications for MR imaging included hip pain (n = 151), and weakness or altered gait (n = 13). Insertional GMin tendinopathy was seen in 72, partial tearing in 81, and complete tendon tearing in 40 cases. Complete tendon tearing without proximal retraction was observed in 38/40 cases with soft tissue continuity visualized between distal tendon fibers and the proximal vastus lateralis muscle. Peri-trochanteric bursal fluid (n = 61), osseous irregularities of the GT (n = 118), and fatty atrophy GMin (n = 102) were statistically associated with partial or complete GMin tendon tearing (p < 0.001).
Conclusions
The spectrum of GMin tendon pathology observed on MR imaging ranges from tendinopathy to complete tears. The majority of complete distal GMin tendon tears from the greater trochanter show continuity of distal tendon fibers with the proximal vastus lateralis, distally tethering and limiting proximal tendon retraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
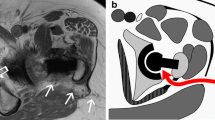
The gluteus minimus (GMin) is a functional component of the abductor gluteal complex of the hip, contributing to hip stabilization by centralizing the femoral head within the acetabulum during gait, as well as hip abduction, flexion, and internal rotation [1, 2]. The main tendon of the gluteus minimus inserts along the lateral aspect of the anterior facet of the greater trochanter of the proximal femur (Fig. 1). Pathology of the terminal tendon of the gluteus minimus includes a spectrum of conditions ranging from degenerative tendinosis or tendinopathy to partial and complete tendon tearing. Such pathology is particularly common in older female patients, and may be associated with clinical features of greater trochanteric pain syndrome (GTPS) [3,4,5,6,7,8,9,10] or as asymptomatic findings [11].
Normal gluteus minimus insertions: 35-year-old female. Coronal T1-weighted image through anterior aspect of the hip show the main tendon of the gluteus minimus (arrowheads) arising from superficial fascia of the gluteus minimus muscle (asterisk) extending distally towards its normal insertion along the anterior aspect of the greater trochanter. The capsular attachment of the gluteus minimus (arrow) can be seen extending medially to the superior ventral aspect of the hip joint capsule
Despite recognition of GMin tendon pathology as an important yet potentially under-recognized cause of hip pain and symptoms, there is a relative paucity of detailed descriptions of features and patterns of gluteus minimus tendon pathology in the literature. The purpose of our investigation was to highlight the spectrum and MR imaging patterns of gluteus minimus pathology and tearing, with correlation to anatomic features of the muscle, its primary distal tendon insertion to the anterior facet of the greater trochanter, and pathoanatomic relevance of distal tendon fiber continuity with the anterior head of the vastus lateralis.
Materials and methods
Patient selection
Institutional research ethics board approval with waiver of consent was obtained for this retrospective study.
The eligible study cohort was derived from consecutive MR imaging examinations of the hip performed at a single institution between October 2008 and January 2019 with report results including descriptive keywords of gluteus minimus tendinopathy, tendinosis, or tear. Patients with prior ipsilateral arthroplasty, fracture, or infection, as well as examinations with inadequate image quality, were excluded from study inclusion.
A total of 198 consecutive MR imaging examinations in 182 patients were identified from our institutional database including terminology of gluteus minimus tendinopathy, tendinosis, or tearing in the finalized exam report. Three examinations were excluded from study inclusion due to presence of a prior hip fracture, and one examination was excluded due to non-diagnostic/incomplete MRI acquisitions, yielding a final study cohort of 194 MRI hip examinations (“study cases”) in 178 patients (16 patients bilateral MRI hip exams). A total of 163 MRI exams were performed according to institutional routine hip protocol, and in 31 cases, hip imaging was performed according to institutional femoroacetabular impingement assessment protocol. MRI exams were of the left hip in 80 cases, and right hip in 114 cases.
Study patients included 148 female and 30 males, with a mean age of 61 years (range 21–94 years, median 62 years).
MR imaging
MR imaging exams were performed using institutional routine hip or femoroacetabular impingement assessment protocols (Supplemental Table). Routine hip MRI exams were performed on a 1.5T MR imaging system with use of dedicated multi-channel surface coils (Siemens AG, Erlangen, Germany) and included turbo spin-echo; coronal T1 weighted (TR/TE = 450/10 ms, 3 mm slice thickness, no interslice gap, FOV = 36-38 cm), sagittal intermediate-weighted (TR/TE = 2500/30 ms, 3 mm slice thickness, no interslice gap, FOV = 20 cm), and axial T2-weighted fat suppressed (TR/TE = 3600/70, = 3 mm slice thickness, no interslice gap, FOV = 20–38 cm) acquisitions through the symptomatic hip and coronal T2-weighted fat suppressed imaging of the entire pelvis (TR/TE = 1800/30 ms, 5 mm slice thickness, no interslice gap, FOV = 36–38 cm). Examinations performed with institutional femoroacetabular impingement assessment protocol were performed on a 3T MR imaging platform using dedicated multi-channel surface coils (Siemens AG, Erlangen, Germany) and included turbo spin-echo, coronal intermediate-weighted fat suppressed (TR/TE = 4400/24, 2 mm slice thickness, no interslice gap, FOV = 15 cm), sagittal intermediate-weighted fat suppressed (TR/TE = 220,020, 3 mm slice thickness, no interslice gap, FOV = 15 cm) axial T1 (TR/TE = 570/15 ms, 3 mm slice thickness, no interslice gap, FOV = 15 cm), radial oblique intermediate-weighted acquisitions perpendicular to the long axis femoral neck (TR/TE = 1800/21, 4 mm slice thickness, FOV 16 cm) of the symptomatic hip, and large field-of-view axial T2 weighted fat suppressed imaging of the entire pelvis (TR/TE = 3600/62, 4 mm slice thickness, no interslice gap, FOV = 36–38 cm).
MR imaging assessment
All MR imaging examinations were reviewed by a single musculoskeletal radiologist (DAO) with 5 years of subspecialty training and experience. A randomly selected subgroup of 63 cases were reviewed independently by a second musculoskeletal radiologist (LMW) with 26 years of experience, for evaluation of inter-reader variability. Readers were blinded to patient clinical symptoms, history, and prior MR imaging results and assessments.
On each MRI exam, the distal insertional tendons of the gluteus minimus and gluteus medius were classified as normal (Fig. 2); showing features of tendinopathy manifest by increased intrasubstance T2 signal and morphologic increased or decreased tendon thickness (Supplemental Fig. 6); partial tearing with tendon irregularity and partial disruption of insertional tendon fibers (Supplemental Fig. 7); or illustrating changes of complete tendon tearing with complete discontinuity of tendon insertional fibers and fluid-like T2 signal interposed between distal tendon and its osseous insertion site (Fig. 3), with or without associated proximal tendon retraction. In all cases with complete gluteus minimus tendon tearing, continuity of the torn distal gluteus minimus tendon to adjacent soft tissue structures including the proximal origin of the vastus lateralis was evaluated (Figs. 3, 4), as was position of the distal insertional aspect of the tendon in the transverse plane, relative to its normal insertion to the anterior facet of the greater trochanter on axial imaging.
a–d Normal insertion gluteus minimus tendon: 47-year-old male. Axial (a–c) fat suppressed T2-weighted and sagittal fat suppressed intermediate-weighted (d) images through the greater trochanter show normal low signal intensity gluteus minimus tendon (white arrowheads) extending distally to insert along the lateral ridge of the anterior facet greater trochanter (arrow). Insertion of the gluteus medius tendon (black arrowheads in a and d) can be seen to the superior-posterior facet of the greater trochanter. Anatomic proximity of distal insertion normal gluteus minimus tendon and proximal origin of the vastus lateralis (VL) can be seen (c and d)
a–d Complete insertional tear gluteus minimus: 68-year-old female with 9-month history lateral hip pain. Axial (a, b) fat suppressed T2-weighted, sagittal (c) fat suppressed intermediate-weighted and coronal (d) fat suppressed T2-weighted images through greater trochanter and right hip show tearing of the distal gluteus minimus tendon (white arrows) from anterior facet greater tuberosity (white arrowheads). The stripped gluteus minimus tendon is seen displaced anteriorly and medially, with distal fibers contiguous with proximal origin vastus lateralis (VL, in b, c, and d). Normal insertion posterior tendon gluteus medius (black arrowheads in a and c)
a–c Complete tear gluteus minimus tendon: 62-year-old female with history of systemic lupus erythematosus and clinical complaints of left hip pain. Axial (a–c) fat suppressed T2-weighted images through greater trochanter show anterior and medial displacement of the distal gluteus minimus tendon (white arrows) torn from its insertion to the anterior facet greater tuberosity (white arrowheads). The torn tendon is thickened with heterogeneous intrasubstance increased signal, and can be followed distally where distal fibers blend with fascia and proximal fibers of the vastus lateralis (VL). A small associated trochanteric bursa (asterisk in a) can be seen adjacent to the lateral margin of the greater trochanter
Muscular fatty atrophic changes of the anterior, middle, or posterior thirds of the gluteus minimus and gluteus medius muscles were assessed on T1-weighted imaging in the transverse plane at the level of the anterior inferior iliac spine. The degree of fatty atrophy in each third (anterior, middle, posterior) was graded according to the Goutallier classification system initially developed for assessment of fatty infiltration of the rotator cuff musculature [12]. Using this classification system, grade 0 is indicative of normal muscle bulk, grade 1 mild fatty infiltration, 2 increased fatty infiltration involving less than 50% of the muscle, 3 fatty infiltration involving 50% of muscle (equivalent fat and muscle), and grade 4 greater than 50% fatty infiltration.
Insertional osseous irregularity and enthesopathic change was assessed at the anatomic insertion of the distal gluteus minimus tendon to the anterior facet of the greater trochanter, with osseous irregularity defined as osseous cortical irregularities protruding 2 mm or more from the underlying cortex of the anterior facet of the greater trochanter [13].
All examinations were additionally assessed for presence or absence of greater trochanteric fascial edema, defined as poorly circumscribed fascial increased T2 signal changes, and the presence or absence of a greater trochanteric bursa, defined as a well-delineated fluid collection on T2-weighted images measuring greater than 3 mm in minimum axial dimension subjacent to the greater trochanter [11, 14].
Statistical analysis
Statistical analysis was performed using SPSS software (IBM SPSS, Version 26.0.01 Armonk, NY; IBM Corp). Inter-rater agreement between the radiologist readers was assessed using inter-class correlation Cohen’s kappa analysis, assuming a two-way random model, with a 95% confidence level of p < 0.05 [15]. Sample size determination for estimating inter-reader correlation was based on sample size requirements sufficient to keep the lower boundary of the 95% confidence interval within a pre-determined confidence width (0.15), assuming substantial agreement (kappa 0.79) between two independent readers [16]. The level of agreement was defined based on the kappa coefficient (k) as described by Landis and Koch [15]: 0.01–0.20 slight, 0.21–0.4 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect.
Statistical relationships between gluteus minimus tendon tears and gluteus medius tendon tearing, gluteus minimus muscular fatty atrophy, trochanteric bursal fluid, and enthesopathic cortical irregularity along the anterior facet of the greater trochanter were evaluated using binomial logistic regression, with a significance level of p < 0.05.
Results
Indications for MR imaging examination across the 194 study cases, based on MRI referral histories provided, included investigation of symptoms of pain in 151 cases, and weakness or alternations in gait in only 13 cases. No specific symptoms were provided in the referral history of 30 cases. Clinical referral details indicated suspected intra-articular hip pathology in 112 cases, suspected extra-articular hip pathology in 69 cases, and was not specified in 13 cases. In only 43 cases was a specific differential diagnosis of abductor (gluteus medius or minimus) tendon pathology, greater trochanteris bursitis, or greater trochanteric pain syndrome provided in the MR imaging referral history.
Interobserver agreement in assessment of the status of the gluteus minimus tendon (normal, tendinosis, partial tear, complete tear) was substantial (k = 0.790). Almost perfect interobserver agreement was observed in the assessment of gluteus minimus fatty atrophy (k = 0.838), with substantial to almost perfect interobserver agreement in the MRI evaluation of the presence of absence of greater trochanteric bursa (k = 0.695) and enthesopathic cortical irregularities anterior facet greater trochanter (k = 0.860).
Overall, the distal insertional aspect of the gluteus minimus tendon was assessed as normal in 1 case, illustrating tendinopathy in 72 cases, partial tearing in 81 cases and complete tendon tearing in 40 hips (Table 1). In only 2 cases with complete distal tendon tearing was proximal retraction of the disrupted gluteus minimus tendon noted, with no proximal retraction noted in the other 38 cases with complete tendon tears. In 33 of the 38 cases with complete distal tendon tearing from the anterior facet of the greater trochanter without proximal tendon retraction, axial MR imaging at the level of the anterior facet demonstrated anterior and medial displacement of the torn tendon from its normal insertion to the anterior lateral margin of the facet, with the other 5 cases showing anterior displacement without medial or lateral displacement in the transverse plane. The 38 non-retracted full thickness gluteus minimus tendon tears demonstrated soft tissue continuity of torn distal gluteus minimus tendon fibers with fascia and superior insertional fibers of the anterior superior origin of the vastus lateralis (Figs. 3, 4).
In our study cohort of 194 hip MRI exams, gluteus medius insertional tendons were assessed as follows: normal or illustrating tendinopathy in 104 cases; partial tendon tearing in 40; and complete gluteus medius tendon tears in 2 cases. Peri-trochanteric soft tissue fascial edema was seen in 85 cases, and a discrete peri-trochanteric bursal fluid collection was present in 61 cases. Osseous cortical irregularities involving the anterior facet of the greater trochanter were observed in 118 cases, including 37.5% (27/72) of cases with MR findings of tendinopathy, 66.7% (54/81) of cases with partial tears, and in 92.5% (37/40) of cases with complete tendon tears. Statistically significant associations were observed between gluteus minimus tendon tears (partial and complete) and corresponding tears of the gluteus medius (p < 0.001), peri-trochanteric bursal fluid collections (p < 0.001), and osseous irregularities of the anterior facet of the greater trochanter (p < 0.001).
Fatty atrophy of the gluteus minimus muscle was observed in a total of 102 of the study cases (102/194, 53%), with a significant association demonstrated between findings of gluteus minimus muscle fatty atrophy and gluteus minimus tendon partial and complete tendon tears (p < 0.001). Of the cases with fatty atrophy, grade 1 changes were seen in 45 cases (45/102, 44.1%), grade 2 changes in 32 (32/102, 31.4%), grade 3 changes in 17 (17/102, 16.7%), and grade 4 changes in 8 (8/102, 7.8%) cases. There was an increasing proportion of cases with higher grade atrophy with progression from tendinopathy, partial tear, and complete tearing of the gluteus minimus tendon (Table 1), as well as with increasing patient age (Fig. 5). When present, fatty atrophic changes involved the anterior and middle thirds of the gluteus minimus in all cases, with 37 (37/102, 36.3%) of cases with fatty atrophy additionally illustrating involvement of the posterior third of the muscle.
Discussion
Pathology of the gluteus minimus tendon observed in our study cohort included tendinopathy, as well as partial and complete tendon tearing. Tears of the gluteus minimus tendon showed statistically significant associations with concurrent findings of gluteus medius tendon pathology, peri-trochanteric brusal fluid collections, osseous enthesopathic cortical irregularities of the anterior facet of the greater trochanter, and fatty atrophic changes of the gluteus minimus muscle. The vast majority of cases with complete gluteus minimus tendon tears from the greater trochanter illustrated tendon fiber continuity with the proximal femoral origin of the vastus lateralis distally tethering and limiting proximal tendon retraction.
The gluteus minimus arises as a fan-shaped muscle from the external surface of the ilium with a broad-based origin roughly parallel to the iliac crest, spanning between the inferior margin of the anterior superior iliac spine and extending posteriorly to the anterior margin of the greater sciatic notch [1, 17]. The gluteus minimus is covered laterally almost completely by the overlying gluteus medius. Muscle fibers of the gluteus minimus converge as they extend distally, with posterior muscle fibers running in a posterior-to-anterior direction and fibers of the anterior aspect of the muscle running in a superior-inferior orientation. The main tendon of the gluteus minimus arises from the superficial muscle fascia and extends distally in a coronal oblique orientation over the anterior aspect of the greater trochanter. A second variable short muscular or capsular insertion of the gluteus minimus arises from deep fibers of the gluteus minimus distally (Fig. 1) to insert to the ventral superior aspect of the hip joint capsule [1, 2, 17]. The main tendon of the gluteus minimus inserts along a superior-to-inferiorly oriented crescentic or “L”-shaped insertional footprint to the lateral aspect of the anterior facet of the greater trochanter [1, 18] (Fig. 2).
Analogous to the supraspinatus and infraspinatus tendon insertions to the humeral head in the shoulder, the gluteus minimus and gluteus medius tendon insertions to the greater trochanter have been referred to as the “rotator cuff” of the hip [14, 17, 19, 20]. Biomechanically, the gluteus minimus functions synergistically with the anterior gluteus medius to facilitate normal abduction, flexion, and internal rotation of the hip, as well as stabilization of the femoral head within the acetabular fossa. Pathology of the distal gluteus minimus tendon includes a spectrum of conditions with progression from microtraumatic degenerative changes of tendinosis or tendinopathy, to partial tendon tears and eventual complete tendon tearing. Such pathology of the gluteus minimus tendon, which may be seen in asymptomatic patients, is also recognized as an important potential etiology of abductor weakness, or anterior lateral hip pain in the clinical setting of greater trochanteric pain syndrome [3,4,5,6,7,8,9,10,11]. The true incidence of gluteus minimus pathology is unclear; however, similar to the demographics of our study cohort, prior investigations have shown an increased prevalence of pathology in middle-to-elderly age patients, and a female-to-male ratio of 4 to 1 [3, 6, 11, 19,20,21].
Tearing of the gluteus minimus tendon is believed to arise secondary to age-related degenerative tendinopathy as a result of repetitive tensile shearing and compressive stress upon its distal insertional fibers to the greater trochanter [7, 22, 23]. As with pathology of the rotator cuff of the shoulder [24, 25], disproportionate mechanical loads upon deep insertional tendon fibers lead to histopathologic findings of tendinopathy and partial thickness tearing concentrated along the undersurface of the gluteus minimus tendon, with ongoing shearing and compressive stresses leading to tear progression from deep partial undersurface tears to complete tendon tearing from the anterior facet of the greater trochanter.
Tendon disruption with myotendinous retraction are classic diagnostic MR imaging features of complete tendon tears. However, gluteus minimus tendon retraction in the setting of complete tendon tearing has been anecdotally noted to be a rare finding in musculoskeletal imaging clinical practice [3]. In our cohort, only 2 of the 40 cases with complete tendon tearing illustrated proximal tendon retraction. In the other 38 cases with non-retracted complete distal tendon tears, we observed mild anterior and anterior-medial displacement of the stripped gluteus minimus tendon from its normal insertion along the lateral ridge of the anterior facet of the greater trochanter (Fig. 3), and continuity of distal insertional tendon fibers with the proximal femoral origin of the vastus lateralis (Fig. 4).
The vastus lateralis anatomically originates from the anterior cortex of the femur, with its proximal fibers arising from the region of the vastus tubercle subjacent to the inferior margin of anterior facet of the greater trochanter immediately adjacent to the distal insertion of the gluteus minimus tendon (Fig. 2) [18, 26]. Anatomic aponeurotic fibrotendinous interconnection of terminal tendon fibers of the gluteus minimus and anterior gluteus medius, blending with superficial proximal fibers of the vastus lateralis, is widely described in the surgical literature, and serves as an important and well-recognized anatomic consideration in trochanteric osteotomy and transgluteal operative surgical approaches to the hip. [18, 26,27,28]. In contrast, there is a paucity of the description of this relationship in the radiology literature. This fibrotendinous aponeurotic continuity of tissues can be recognized on MR imaging in the setting of complete tears of the gluteus minimus tendon from the greater trochanter distally tethering complete tendon tears and explaining the infrequent observation of proximal tendon retraction with complete insertional gluteus minimus tendon tears. Distal fibrotendinous continuity with the vastus lateralis and lack of proximal tendon retraction may also provide background pathoanatomic explanation for observations of gluteus medius and minimus tendon “elongation” on MRI. Cvitanic and coauthors [8], proposed tendon elongation as a correlative secondary MRI finding of complete hip abductor tendon tears, in which the apparent superior-inferior length of the abductor tendons appear elongated as a result of muscular atrophy, in the absence of proximal tendon retraction.
Tears of the abductor tendons likened to tears of the rotator cuff of the shoulder were described by Bunker et al. [19] as rounded or oval defects of the abductor tendons typically originating within the gluteus minimus, with progressive extension posteriorly along the lateral insertional fibers of the gluteus medius. A predominance of gluteus minimus tearing was similarly observed in a recent cadaveric population study where gluteus minimus tears were more commonly observed and where all complete tears of the gluteus medius were associated with concurrent tearing of the gluteus minimus [23]. Other surgical as well as MR and ultrasound imaging investigations have in contrast shown a predominance of gluteus medius tendon tearing in patients with abductor tendon pathology, with a significant association between gluteus minimus tearing and concomitants gluteus medius pathology [3,4,5,6,7, 20].
Findings of our investigation concur with prior studies illustrating positive associations between abductor tendon pathology and peri-trochanteric bursal fluid collections [11] as well as trochanteric cortical osseous irregularities [13], with bursal fluid collections and osseous degenerative enthesopathic cortical irregularities of the anterior facet of the greater trochanter observed in 72% and 92.5% of our study cases with complete gluteus minimus tendon tearing, respectively.
As with rotator cuff muscle atrophy, fatty atrophy of the gluteus minimus and medius has been described in conjunction with abductor dysfunction and tendon tearing. Fatty atrophy of the gluteus medius and minimus musculature has been shown to be associated with increasing age and increasing severity of insertional tendon degeneration and tearing [3, 17, 29]. Differential patterns of atrophy in the anterior/mid vs posterior thirds of the gluteus minimus muscle and potential implications of atrophy of the posterior third of the muscle in symptomatic patients have also been highlighted in non-operative and hip arthroplasty patients [3, 30]. We found a strong association of gluteus minimus atrophy with tendon tears, with increasing relative prevalence and grade of fatty atrophy in cases of complete tendon tears as well as in conjunction with increasing age groups of study patients, and increased proportion of cases with atrophy involving the posterior third of the muscle, observed in cases of complete tendon tears.
Clinical management of hip abductor dysfunction is controversial. This is partially due to the overlap in clinical presentation of abductor pathology and other pathologies involving the hip and para-articular soft tissues as well as the spectrum of clinical symptoms patients with abductor tendon pathology may present with ranging from asymptomatic to pain and gait disturbances. Evidence-based recommendations in the management of gluteus minimus pathology is additionally complicated by the relative paucity of studies describing or investigating the management of gluteus minimus tears uniquely or in association with concomitant gluteus medius tendon pathology.
Even though, in general, initial symptomatic management of recognized hip abductor tendon pathology is usual, identification of gluteus minimus and medius tendon pathology is important in ensuring appropriate treatment. Conservative treatment options include activity modification, physical therapy, non-steroidal anti-inflammatory medications, and local injections of corticosteroid or anesthetic into the trochanteric bursa. In the setting of failed response to conservative measures, direct open or endoscopic surgical repair has been described for small non-retracted tendon tears, and surgical reconstruction with allograft or tendon transfer procedures have been described for surgical management of larger full thickness abductor tendon tears [22]. Unfortunately, outcomes of such procedures are often variable, with heterogeneous patient populations and a limited number of high quality studies elucidating optimal clinical management [31].
Although we were able to confirm good to excellent interobserver agreement in MRI evaluation of gluteus minimus pathology and associated features, an acknowledged limitation of our investigation is the lack of surgical correlation in confirmation of the accuracy of MR imaging in the diagnosis of gluteus minimus pathology. Few prior studies have evaluated the accuracy of MR imaging in diagnosis of gluteus minimus tearing, with limited surgical correlative results across heterogeneous small size study subgroups. The purpose of our investigation was to retrospectively evaluate patterns of gluteus minimus pathology observed on dedicated MR imaging of the hip and as such designed our study around the review of a preselected cohort with clinical report findings of gluteus minimus pathology. This preselection bias limits assessment of the prevalence of gluteus minimus tearing in symptomatic patients by exclusion of patients with normal MRI appearance of the gluteus minimus tendon and may have excluded cases with subtle MRI pathology not explicitly described in clinical report text. Similarly, evaluation of dedicated unilateral hip MRI exams, as opposed to bilateral hip examinations, limited our ability to assess the prevalence of concurrent pathology of the contralateral hip.
In summary, the spectrum of gluteus minimus tendon pathology observed on MR imaging ranges from tendinopathy to complete tears. MRI findings of gluteus minimus tendon tearing are associated with concurrent findings of gluteus medius pathology, bursal fluid collections, enthesopathic irregularities of the anterior facet greater trochanter, and increasing relative incidence and grade of gluteus minimus muscular fatty atrophy. The majority of complete distal gluteus minimus tendon tears from the greater trochanter show continuity of torn distal tendon fibers with the proximal head of the vastus lateralis, without features of proximal tendon retraction on dedicated MR imaging examinations of the hip.
References
Beck M, Sledge JB, Gautier E, Dora CF, Ganz R. The anatomy and function of the gluteus minimus muscle. J Bone Joint Surg (Br). 2000;82-B:358–63.
Walters J, Solomons M, Davies J. Gluteus minimus: observations on its insertion. J Anat. 2001;198:239–42.
Chi AS, Long SS, Zoga AC, Read PJ, Deely DM, Parker L, et al. Prevalence and pattern of gluteus medius and minimus tendon pathology and muscle atrophy in older individuals using MRI. Skelet Radiol. 2015;44:1727–33.
Kingzett-Taylor A, Tirman PFJ, Feller J, McGann W, Prieto V, Wischer T, et al. Tendinosis and tears of gluteus medius and minimus muscles as cause of hip pain: MR imaging findings. AJR. 1999;173:1123–6.
Lequesne M, Djian P, Vuillemin V, Mathieu P. Prospective study of refractory greater trochanter pain syndrome. MRI findings of gluteal tendon tears seen at surgery. Clinical and MRI results of tendon repair. Joint Bone Spine. 2008;75:458–64.
Connell DA, Bass D, Sykes CJ, Young D, Edwards E. Sonographic evaluation of gluteus medius and minimus tendinopathy. Eur Radiol. 2003;13:1339–47.
Hoffman DF, Sellon JL, Moore BJ, Smith J. Sonoanatomy and pathology of the gluteus minimus tendon. J Ultrasound Med. 2020;39:647–57.
Cvitanic O, Henzie G, Skezas N, Lyons J, Minter J. MRI diagnosis of tears of the hip abductor tendons (gluteus medius and gluteus minimus). AJR. 2004;182:137–43.
Hoffman A, Pfirrmann CWA. The hip abductors at MR imaging. Eur J Radiol. 2012;81:3755–62.
Muller M, Tohtz S, Winkler T, Dewey M, Springer I, Perka C. MRI findings of gluteus minimus muscle damage in primary total hip arthroplasty and the influence on clinical outcome. Arch Orthop Trauma Surg. 2010;130:927–35.
Blankenbaker DG, Ullrick SR, Davis KW, De Semt AA, Haaland B, Fine JP. Correlation of MRI findings with clinical findings of trochanteric pain syndrome. Skelet Radiol. 2008;37:903–9.
Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop. 1994;304:78–83.
Steinert L, Zanetti M, Hodler J, Pfirrmann CWA, Dora C, Saupe N. Are radiographic trochanteric surface irregularities associated with adbuctor tendon abnormalities? Radiology. 2010;257(3):754–63.
Pfirrmann CWA, Chung CB, Theumann NH, Trudell DJ, Resnick D. Greater trochanter of the hip: attachment of the abductor mechanism and a complex of three bursae – MR imaging and MR bursography in cadavers and MR imaging in asymptomatic volunteers. Radiology. 2001;221:469–77.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Arifin WN. A web-based sample size calculator for reliability studies. Educ Med J. 2018;10(3):67–76.
Flack NAMS, Nicholson HD, Woodley SJ. The anatomy of the hip abductor muscles. Clin Anat. 2014;27:241–53.
Philippon MJ, Michalski MP, Campbell KJ, Goldsmith MT, Devitt BM, Wijdicks CA, et al. Surgically relevant bony and soft tissue anatomy of the proximal femur. Orthop J Sports Med. 2014;2(6):2325967114535188.
Bunker TD, Esler CN, Leach WJ. Rotator-cuff tear of the hip. J Bone Joint Surg (Br). 1997;79:618–20.
Kagan A. Rotator cuff tears of the hip. Clin Orthop Relat Res. 1999;368:135–40.
Howell GE, Biggs RE, Bourne RB. Prevalence of abductor mechanism tears of the hips in patients with osteoarthritis. J Arthroplast. 2001;16:121–3.
Zhu MF, Musson DS, Cornish J, Young SW, Munro JT. Hip abductor tendon tears: where are we now? Hip Int. 2020;30(5):500–12.
Zhu MF, Smith B, Krishna S, Musson DS, Riordan PR, McGlashan SR, et al. The pathological features of hip abductor tendon tears - a cadaveric study. BMC Musculoskelet Disord. 2020;21(1):778.
Seitz AL, McClure PW, Finucane S, Boardman ND, Michener LA. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech. 2011;26:1–12.
Huang C, Wang VM, Pawluk RJ, Bucchieri JS, Levine WN, Bigliani LU, et al. Inhomogeneous mechanical behavior of the human supraspinatus tendon under uniaxial loading. J Orthop Res. 2005;23:924–30.
Fulkerson JP, Crelin ES, Keggi KJ. Anatomy and osteotomy of the greater trochanter. Arch Surg. 1979;114:19–21.
Nazarian S, Tisserand P, Brunet C, Muller ME. Anatomic basis of the transgluteal approach to the hip. Surg Radiol Anat. 1987;9:27–35.
Dunn T, Heller CA, McCarthy SW, Dos Remedios C. Anatomical study of the “trochanteric bursa”. Clin Anat. 2003;16(3):233–40.
Takano Y, Kobayashi H, Yuri T, Yoshida S, Naito A, Kiyoshige Y. Fat infiltration in the gluteus minimus muscle in older adults. Clin Interv Aging. 2018;13:1011–7.
Pfirrmann CWA, Notzli HP, Dora C, Hodler J, Zanetti M. Abductor tendons and muscles assessed at MR imaging after total hip arthroplasty in asymptomatic and symptomatic patients. Radiology. 2005;235:969–76.
Kenanidis E, Lund B, Christofilopoulos P. A roadmap to develop clinical guidelines for open surgery of acute and chronic tears of hip abductor tendons. Knee Surg Sports Traumatol Arthrosc. 2020. https://doi.org/10.1007/s00167-020-06320-x.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional research ethics board approval with waived consent was obtained for this retrospective study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental Figure I, A
Tendinopathy gluteus minimus tendon insertion: 56 year old female. Axial (A, B) and coronal (C) T2-weighted fat suppressed through the left hip and greater trochanter show mild thickening and mild increased intrasubstance signal intensity gluteus minimus tendon (white arrows) close to its insertion to anterior facet greater trochanter (open white arrow in B). A small amount of fluid seen surround the gluteus minimus tendon at the level of the greater trochanter consistent with gluteus minimus bursitis (white arrowheads) (PDF 4.79 mb)
Supplemental Figure I, B
Tendinopathy gluteus minimus tendon insertion: 56 year old female. Axial (A, B) and coronal (C) T2-weighted fat suppressed through the left hip and greater trochanter show mild thickening and mild increased intrasubstance signal intensity gluteus minimus tendon (white arrows) close to its insertion to anterior facet greater trochanter (open white arrow in B). A small amount of fluid seen surround the gluteus minimus tendon at the level of the greater trochanter consistent with gluteus minimus bursitis (white arrowheads) (PNG 5.20 mb)
Rights and permissions
About this article
Cite this article
White, L.M., Oar, D.A., Naraghi, A.M. et al. Gluteus minimus tendon: MR imaging features and patterns of tendon tearing. Skeletal Radiol 50, 2013–2021 (2021). https://doi.org/10.1007/s00256-021-03745-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-021-03745-4