Abstract
Introduction
MRI is often used to determine the presence of residual disease following unplanned excisions (UPE) of soft tissue sarcomas (STS). We sought to identify MRI features associated with histologic evidence of residual disease after TBE.
Materials and methods
This was an IRB-approved retrospective review of 27 patients with R1-type UPE of STS over a 32-month period, with subsequent MRI and TBE. MRI studies were retrospectively evaluated to determine depth of tissue involvement, presence of nodular enhancement, and maximum length of soft tissue edema normalized to extremity size. MRI findings were correlated with histology from unplanned excision and TBE.
Results
Among the 21 subjects, there were 13 males and 8 females, mean age 58. Eighteen of 21 STS were grade 2 or 3. Deep compartments were involved in 5/21 cases. Original margins were positive in 17/21 UPE, with inadequate margin assessment in the remaining 4 cases. Residual tumor was present at TBE in 11/21 cases; it was found in 4/6 cases with nodular enhancement and 7/15 cases without nodular enhancement (sensitivity = 0.36; specificity = 0.80; PPV = 0.67; NPV = 0.53). Increased extent of soft tissue edema increased the likelihood of residual tumor at TBE (OR = 35.0; 95% CI = 1.6 to 752.7; p = 0.023).
Conclusion
Nodular enhancement is neither sensitive nor specific in predicting residual microscopic tumor in TBE following UPE. Extensive soft tissue edema on MRI after UPE increases the likelihood of finding a residual microscopic tumor, justifying ample margins at TBE and consideration of adjuvant therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of malignant tumors comprising less than 1% of all malignancies [1]. Unfortunately, due to the rarity of these tumors, STSs may be excised without following oncologic principles. Guiliano and Eilber first coined the term ‘unplanned excision’ in 1985 to describe resections of STS without adequate suspicion for malignancy and without intent to achieve tumor-free margins [2]. The lack of knowledge of these oncologic principles results in incomplete tumor resections, inappropriate incisions, incorrectly placed drains, and cross-compartment contamination.
Patients who undergo unplanned excision (UPE) of STS should be referred to dedicated sarcoma centers for further evaluation and definitive management. Residual gross or microscopic disease rates at tumor bed excision (TBE) of 24% to 91% have been reported [2,3,4,5,6,7,8,9,10] with prognostic implications. In most cases, TBE is recommended to achieve tumor-free margins and remove potentially contaminated tissues, but larger resected areas, muscle flaps, and skin grafts may be necessary [11, 12].
MRI is the preferred imaging modality for evaluating local disease at the site of UPE, but examinations are often confounded by the presence of postoperative soft tissue edema, scar, and fluid collections. Davies et al. studied the ability of MRI to detect residual sarcoma in 111 patients who had UPE [13], determining the sensitivity, positive predictive value, and negative predictive value of MRI to be 0.64, 0.93, and 0.68, respectively. However, Davies et al. also found that when those cases of macroscopic residual disease (defined as >10 mm) were excluded, MRI performed significantly worse, identifying tumor in only 1 out of 13 cases with histologically identifiable disease on TBE.
This study aims to evaluate the association between specific MRI findings following UPE of STS in the extremities and the presence of local microscopic residual tumor (volume less than 5 cm3).
Methods
Subjects
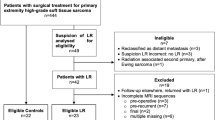
Institutional Review Board (IRB) approval was obtained for this study and in accordance with the requirements of a retrospective review; the requirement for informed consent was waived. This was a retrospective case-control study where “cases” were defined as patients with UPE and positive TBE, and “controls” were patients with UPE but negative TBE. All patients were presented at a weekly multidisciplinary conference held at our institution over a 32-month period (January 2013 through August 2015) and were retrospectively reviewed to identify those with unplanned excision of STS referred to and subsequently treated at our institution. Retrospective review of electronic medical records identified those patients with UPE in the extremities, and for those identified patients, the clinical records and radiologic imaging were examined. Exclusion criteria included TBE not performed at our institution, absence of at least one MRI study subsequent to UPE but prior to TBE, and gross residual presumed tumor volume at the time of re-excision measuring > 5 cm3. This last exclusion criterion allowed us to focus on the more radiologically challenging cases where the tumor burden was small and difficult to discern in the milieu of postoperative soft tissue changes and R1 type resections. We excluded benign lesions because intralesional or marginal excisions are usually indicated for these tumors, and we consider unplanned excision to pertain only to sarcomas where non-oncologic resection has been performed. We recorded whether subjects underwent neoadjuvant therapy, including chemotherapy, radiation therapy, or both, prior to TBE.
Histopathologic assessment
Histopathologic analysis performed at our institution of tissue collected at the time of UPE was reviewed to confirm or exclude the presence of soft tissue sarcoma. A bone and soft tissue pathologist was involved in reviewing the gross and microscopic findings in all tumor bed excision specimens. All tumor bed excision specimens were handled in a similar way using standard grossing techniques, with all surgical specimens submitted to assess for treatment effect (if applicable) and residual disease. Specifically, the specimens were entirely submitted for histologic evaluation up to a total of eight cassettes. For larger specimens, representative sections were submitted at 1-cm intervals, with sections selectively sampled from regions of fibrosis and prior surgical site changes.
MRI assessment
MRI in these patients was performed as part of routine care to identify potential areas of residual disease after UPE and also plan the extent of tumor bed excision. A fellowship-trained musculoskeletal radiologist with 4 years’ experience retrospectively reviewed the MRI studies in consensus with a senior radiology resident. The readers were blinded to the status of residual tumor from TBE. When available, MRI studies performed prior to UPE were examined to determine the initial tumor volume. Volume measurements were approximated using the formula for the volume of an ellipsoid (volume = 0.52 × length × width × height).
MRI studies performed subsequent to UPE but prior to TBE were assessed to determine the presence and size of nodular enhancement (i.e., non-linear, marginated enhancement) and maximum extent of the “edema” signal. We interpreted a T2 hyperintense signal on fat-suppressed images as soft tissue “edema” when it occurred in the surgical bed and had approximately the signal intensity of regional small veins; in determining its length, the hyperintensity in the soft tissues needed to be contiguous with <0.5 cm normal intervening fat signal.
When more than one MRI was performed between UPE and TBE, the MRI performed closest to the time of TBE was used as this was felt to be most representative of the conditions encountered at TBE. Depending on availability, these pre-TBE MRI studies were performed at either our institution or outside facilities. The minimum standard protocol for MRI studies performed at our institution consisted of T1-weighted fast-spin-echo and fluid-sensitive sequences (using either inversion recovery or chemical fat saturation techniques) oriented in the axial and longitudinal planes, as well as axial T1-weighted fast-spin-echo fat-suppressed sequences obtained before and after contrast administration. The MRI studies were also assessed to determine if multiple anatomic compartments were involved and whether tumors were superficial or deep. Superficial tumors were those tumors that did not involve the superficial investing muscular fascia or structures deep to this fascia.
The extent of soft tissue edema signal in the pre-TBE MRI studies was measured using the single greatest length of edema signal seen in any plane. To allow for more meaningful quantitative comparison between limbs of varying sizes, a normalized edema value was calculated using the ratio of the maximum length of edema signal to the sum of the anteroposterior and transverse lengths of the nearest long bone. A sum of diameters was chosen so that the methodology could be rapidly incorporated in a clinical setting, requiring use of only the linear digital caliper. This ratio was arbitrarily scaled by a factor of 10 so that values were >1 to allow for ease of presentation (Fig. 1).
A 51-year-old female status post UPE for myxofibrosarcoma in the lower leg. Axial fat-saturated proton density-weighted image illustrating how the normalized edema value is calculated. The normalized edema value is given by the ratio of the maximal length of the edema signal (yellow line, which in this example is 7.5 cm) to the sum of the AP and transverse diameters of the nearest long bone (blue lines, which in this example are 4.9 cm and 3.4 cm, respectively), multiplied by a scaling factor: \( \frac{7.5 cm}{4.9 cm+3.4 cm}x\ 10=9.0 \)
Areas of nodular or mass-like enhancement in the pre-TBE MRI studies were identified on post-contrast T1-weighted fast-spin-echo fat-suppressed sequences. Volumetric approximations of these areas of enhancement were derived using the formula for the volume of an ellipsoid, given above.
Imaging studies for patients who received neoadjuvant therapy prior to TBE were reviewed to identify those patients who got more than one MRI between UPE and TBE. For those patients, the MRI immediately following UPE was also evaluated to observe how MR characteristics changed over time.
Statistics
Comparisons between subjects with positive microscopic residual tumor vs. those without residual tumor at TBE were carried out using the two-sample Student t-test. The correlation between the extent of soft tissue edema and time interval between UPE and MRI was assessed using Pearson’s correlation coefficient. The association between the presence of nodular enhancement and microscopic residual tumor was assessed using Fisher’s exact test. Statistical analyses were performed using Stata Statistical Software: Release 13 (StataCorp LP). For all analyses, a p value of ≤ 0.05 was considered significant.
Results
Subjects
There were 544 patients presented in the multidisciplinary conference during the study period. Patients presented included those with primary STS as well as those with benign pathologies and tumors that secondarily involve soft tissues. Eighty-three of these patients underwent UPE of STS prior to referral to our institution. Of these 83 patients, 56 were excluded because of loss to follow-up, the decision not to perform TBE, or lack of MRI between unplanned excision and TBE. Of the remaining 27 patients, 5 were excluded because of the presence of gross residual disease (presumed tumor volume greater than 5 cm3) at the time of TBE, and one was excluded because of a tumor involving the lower back. The remaining 21 patients (13 male and 8 female) comprised the final study group. Mean age was 58years (range 29 to 79). The demographics and final histology of these tumors are summarized in Table 1.
Clinicopathologic features
Anatomic location of tumors consisted of 13 in the lower extremities and 8 in the upper extremities. Measurements of initial tumor size prior to UPE were obtainable for six of the tumors and ranged from 1.0 to 63.5 cm3 (mean 16.7 cm3). Original tissue margins from UPE were positive in 17 cases and inadequately assessed in the remaining 4 cases. Three of the tumors were classified as low grade (Federation Nationale des Centers de Lutte Contre le Cancer grade 1 tumors), whereas the other 18 tumors were classified as intermediate or high grade (grade 2 or grade 3 tumors).
Five out of 21 tumors involved deep soft tissue compartments, whereas the remaining 16 were superficial. Residual tumor was present at TBE in 11 of 21 cases (52%): it was present in 4 out of 5 cases within the subgroup of deep-seated tumors and in 7 out of 16 cases within the subgroup of superficial tumors. Neoadjuvant therapy (chemotherapy alone, radiation therapy alone, or both) was given in 12 cases, of which 6 had residual tumor at TBE; in the remaining 9 cases, residual tumor at TBE was found in 5 cases (Table 2).
After TBE, there were four biopsy-proven local recurrences, two in the TBE-positive group at 4 and 6 months and two in the TBE-negative group at 20 and 22 months. Mean ± SD duration of follow-up for those without local recurrence after TBE was 21 ± 18 months.
Imaging findings
Eleven out of 21 reviewed MRI studies were acquired at our institution with the remaining 10 acquired at outside facilities. After UPE, the mean of the maximum linear dimension of the soft tissue edema signal was 5.69 (range = 0 to 11.5 cm, SD = 5.46). This non-normalized linear measurement of the edema signal in the surgical bed was not significantly different between cases where the tumor bed specimen was positive for disease and cases where the tumor bed specimen was negative for residual disease (mean = 7.44, SD = 6.88 for tumor bed positive patients; mean = 4.20, SD = 2.97 for tumor bed negative patients; p = 0.185, two sample t-test). However, the presence of residual tumor was associated with greater normalized edema values (mean = 14.70, SD = 10.20 for tumor bed positive patients; mean = 6.81, SD = 4.11 for tumor bed negative patients; p = 0.034; two sample t-test). See Figs. 2, 3, and 4 for representative examples of imaging findings.
A 69-year-old male with high-grade myxofibrosarcoma of the right thigh. (a) T2-weighted fat-saturated image and (b) T1-weighted fat-saturated gadolinium-enhanced image obtained prior to TBE. Neoadjuvant radiation therapy was completed prior to this MRI. Normalized edema value was 7.5. A collapsed seroma results in the appearance of nodular enhancement (arrow), which could simulate residual disease. The tumor bed was negative for residual disease at TBE
A 68-year-old female with high-grade myxofibrosarcoma of the volar aspect of the right forearm. (a) T2 STIR and (b) T1-weighted fat-saturated gadolinium-enhanced images obtained prior to TBE. Neoadjuvant chemotherapy was completed prior to this MRI. Normalized edema value was 12.9. The tumor bed was positive for tumor at TBE. (c) T2 STIR and (d) T1-weighted fat-saturated gadolinium-enhanced images obtained 19 months after TBE demonstrate biopsy-proven tumor recurrence at the dorsal aspect of the forearm
A 63-year-old male with pleomorphic myxofibrosarcoma of the right elbow. (a) T2 STIR and (b) T1-weighted fat-saturated gadolinium-enhanced images obtained prior to TBE. The normalized edema value was 11.1. The tumor bed was positive for tumor at TBE. (c) T2 STIR and (d) T1-weighted fat-saturated gadolinium-enhanced images obtained 3 months after TBE demonstrating biopsy-proven tumor recurrence (arrow) at the ulnar proximal forearm in an area more anterior than the predominant area of edema signal present on pre-TBE images
We evaluated the normalized edema value as an indicator of residual disease at the time of TBE using receiver-operator characteristic analysis (Fig. 5). An area under the curve of 0.82 ± 0.10 (p < 0.001) was observed. Using the Youden method, an optimal cutoff point of 12.4 was obtained, yielding sensitivity and specificity of 0.64 and 1, respectively. The odds ratio characterizing the association between higher normalized edema values (12.4 or greater) and residual disease at TBE was 35.0 (95% CI = 1.6 to 752.7; p = 0.023).
ROC curve for normalized edema value in classifying tumor bed positivity. Receiver-operator characteristic (ROC) curve of normalized edema value as a predictor of tumor bed positivity. AUC = 0.82 ± 0.099 (p < 0.001), optimal cutoff point (Youden method) = 12.4, sensitivity = 0.64, specificity = 1. If a cutoff point of 8.6 is selected, then sensitivity = 0.82 and specificity = 0.5
Nodular or mass-like enhancement was identified in 6 out of 21 (29%) of cases; however, residual tumor was identified in only 4 (67%) of these cases. The remaining 7 cases of residual tumor were seen in 15 cases without areas of nodular or mass-like enhancement (Table 3). Thus, nodular or mass-like enhancement as a single criterion yields only modest performance statistics in identifying residual disease at TBE: sensitivity 0.36, specificity 0.80, PPV 0.67, and NPV 0.53.
Because of the variability in presentation and disease course in our subjects, we tested whether longer delays between UPE and post-UPE MRI were associated with decreased extent of soft tissue edema signal, but found no statistically significant correlation (Pearson correlation; r = −0.20, p = 0.38). However, in the subset of patients who received neoadjuvant therapy prior to TBE (Table 4), the mean normalized edema value decreased between post-UPE and pre-TBE MRIs in 89% (post-UPE = 19.5 ± 7.0; pre-TBE mean = 9.4 ± 5.1; p = 0.003; two sample t-test).
Discussion
UPEs of STS are commonly referred to specialized sarcoma centers for management and present a therapeutic challenge, with the need to achieve negative margins at TBE superseding the desire for functional preservation. Indeed, the frequency with which such cases arise supports the need for increased awareness among practitioners.
There is general agreement that TBE is commonly recommended following UPE of soft tissue sarcomas, and the presence of residual tumor at TBE is a prognostic factor for increased local recurrence rate [12]. MRI plays a key role in identifying residual tumor following UPE, information that can help guide management, being of outmost importance in certain tumors such as myxofibrosarcomas, which had shown increased local recurrence after UPE [3], and a more infiltrative behavior, which makes assessing and achieving negative margins more difficult. However, imaging acquired following UPE is complicated by postoperative changes such as edema, scar tissue, and fluid collections. This study focuses on cases in which the potential residual tumor burden is small and difficult to discern from such postoperative changes. Although initial tumor size measurements were not available for the majority of subjects, given the eventual absence of gross residual tumor, the bulk of the tumor was presumed to have been removed at the time of UPE in these cases.
In our study, the histologic margin status at the time of UPE was positive in a majority of cases with inadequate assessment in the remaining cases, which in practice for surgical management should be assumed as positive and which in effect makes them R1 resections. For eight cases, margins were positive at UPE but subsequently had no tumor detected histologically at TBE; six of these eight patients received neoadjuvant therapy prior to TBE, and the lack of tumor at TBE is presumably due to the effects of therapy. For the other two cases who received no interval treatment, it is possible that post-surgical inflammation in the tumor bed attenuated microscopic residual tumor, given that immune modulation has recently been shown to have promising therapeutic effects in sarcoma treatment [14].
Reinforcing the clinical importance of determining whether residual tumor is present on post-UPE MRI, our data showed that residual tumor at re-excision increased the likelihood of earlier recurrence (on average approximately 15 months earlier) compared with negative TBE. To that point, our data show that the greater extent of soft tissue edema, normalized to extremity size (>12.4), is correlated with positive TBE and in fact 100% specific in this data set. For example, of the seven UPE of myxofibrosarcoma in our study, the six with the highest NEV (all >11) harbored residual disease at TBE. We emphasize that lower values of normalized edema do not exclude residual disease, as several cases in Table 1 illustrate with values of 2.4 or 5.2 found with positive TBE. While we caution against sole reliance on a single imaging parameter in predicting histologic or clinical outcomes, we believe that our results may guide one’s assessment of the likelihood of residual disease presence and in so doing may aid clinical decision-making.
Given the relatively low specificity and sensitivity of nodular or mass-like enhancement for residual disease at TBE, radiologists should not depend exclusively on such qualitative findings when determining the presence of residual tumor. Importantly, the absence of nodular enhancement does not exclude microscopic residual tumor in the UPE operative bed. The correlation between the extent of soft tissue edema signal and presence of tumor in TBE suggests that the edema signal serves as a marker for the area of potential contamination during UPE, a consideration in surgical planning when determining the area for subsequent TBE. Important information regarding the pre-surgical extent of peritumoral edema is largely unavailable in these cases because of the circumstances in which unplanned excisions are usually performed, with very few preoperative MRIs having been obtained prior to UPE. This is probably because the UPEs are performed predominantly on superficial soft tissue masses (16/21 cases in our data), and the superficial location leads the surgeon to underestimate the chance of malignancy. The small number of deep tumors hindered our ability to make meaningful subgroup comparisons with regard to tumor depth and normalized edema ratios, but our series does confirm a higher rate of residual disease in the TBE specimen when the original tumor was deep (80%) compared with superficial (44%). Thus, in circumstances where UPE was performed on a deep sarcoma, the deep soft tissue compartments are at additional elevated risk of harboring residual disease in the surgical bed, and scans should be accordingly interpreted with a high degree of suspicion.
We did not find that the length of time between UPE and post-UPE MRI significantly affected the normalized extent of edema; this may be because the edema-like signal related to inflammatory changes decreases over time, while the edema-like signal related to residual tumor increases over time, resulting in little net change. Alternatively, this small sample size may simply be underpowered to have detected a difference. It should be noted that in patients who received neoadjuvant therapy prior to TBE, the edema signal indeed decreased in the period between the UPE and TBE, as might be expected given the opportunity for wound healing, resolution of postoperative inflammatory changes, and eradication of residual tumor in response to therapy. This decrease in edema signal highlights the importance of obtaining an MRI close to TBE to best assess the extent of soft tissue contamination and to allow better operative planning.
Our study is limited by its small sample size and retrospective design employing consensus readings, although we believe it is comparable in size and methodology with other single-institution retrospective studies of sarcomas. While a multiple reader study design could be used to assess interobserver variability, we believe our conclusions are valid because readers were blinded and reached consensus on all cases; observer-dependent bias would have been systematic and would have affected both the TBE+ and TBE- groups. The exclusion of patients with gross residual disease following UPE contributed to the small size of our sample but allowed us to focus on R1 resections. Another limitation is that the significance of edema found in the operative bed after UPE is narrowly defined for sarcoma in this article, and the broader implications of such soft tissue changes in the operative bed after surgery for benign masses remain uncertain. In light of these considerations, our findings are best regarded as preliminary and should be substantiated by multiple-reader studies of larger, and ideally prospective, patient cohorts.
That said, while a prospective study might better elucidate the evolution of MR signal changes and their significance in the setting of UPE by standardizing the MR protocols used and timing of examinations prior TBE, such a design would likely be confounded by the high variability in interval between UPE and referral to tertiary care centers for definitive therapy. A more direct comparison of MRI and histopathologic findings would also be needed to corroborate our hypothesis that the edema signal on MRI corresponds to areas of tumor contamination. While defining the extent of edema signal and distinguishing it from infiltrating tails of tumor, particularly in myxofibrosarcomas, are acknowledged challenges [15], one study by White et al. demonstrated that peritumoral areas of high signal on T2-weighted MRI images corresponded to areas of histologically identified sarcoma cells [16]. A similar methodology could be utilized to assess the edema signal in the setting of UPE, possibly identifying the existence of residual tumor within those corresponding areas of high signal; this would be of particular importance in myxofibrosarcomas since these tumors show the highest rate of local recurrence after UPE [12]. Other future directions also include evaluating the role of functional imaging (dynamic contrast-enhanced MRI, diffusion-weighted imaging, and positron emission tomography) in assessing the residual tumor burden, although such imaging techniques may be limited in the absence of gross residual tumor.
In conclusion, unplanned excision of soft tissue sarcomas results in a high rate of residual disease in the operative bed, which has prognostic factors regarding local recurrence rates. MRI is an imaging study potentially useful for planning extension of TBE based on a potential area of soft tissue contamination, as marked by soft tissue edema (normalized edema signal), and the radiologist as well as the orthopedic oncologist should be aware that microscopic residual disease could be present even in the absence of discrete nodular enhancement.
References
Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8–29.
Giuliano AE, Eilber FR. The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol. 1985;3(10):1344–8.
Chandrasekar CR, Wafa H, Grimer RJ, Carter SR, Tillman RM, Abudu A. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90(2):203–8.
Davis AM, Kandel RA, Wunder JS, et al. The impact of residual disease on local recurrence in patients treated by initial unplanned resection for soft tissue sarcoma of the extremity. J Surg Oncol. 1997;66(2):81–7.
Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13(1):110–7.
Goodlad JR, Fletcher CD, Smith MA. Surgical resection of primary soft-tissue sarcoma. Incidence of residual tumour in 95 patients needing re-excision after local resection. J Bone Joint Surg Br. 1996;78(4):658–61.
Lewis JJ, Leung D, Espat J, Woodruff JM, Brennan MF. Effect of reresection in extremity soft tissue sarcoma. Ann Surg. 2000;231(5):655–63.
Manoso MW, Frassica DA, Deune EG, Frassica FJ. Outcomes of re-excision after unplanned excisions of soft-tissue sarcomas. J Surg Oncol. 2005;91(3):153–8.
Noria S, Davis A, Kandel R, et al. Residual disease following unplanned excision of soft-tissue sarcoma of an extremity. J Bone Joint Surg Am. 1996;78(5):650–5.
Potter BK, Adams SC, Pitcher JD Jr, Temple HT. Local recurrence of disease after unplanned excisions of high-grade soft tissue sarcomas. Clin Orthop Relat Res. 2008;466(12):3093–100.
Mankin HJ, Lange TA, Spanier SS. The hazards of biopsy in patients with malignant primary bone and soft-tissue tumors. J Bone Joint Surg Am. 1982;64(8):1121–7.
Pretell-Mazzini J, Barton MD Jr, Conway SA, Temple HT. Unplanned excision of soft-tissue sarcomas: current concepts for management and prognosis. J Bone Joint Surg Am. 2015;97(7):597–603.
Davies AM, Mehr A, Parsonage S, Evans N, Grimer RJ, Pynsent PB. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol. 2004;14(3):506–13.
D'Angelo SP, Tap WD, Schwartz GK, Carvajal RD. Sarcoma immunotherapy: past approaches and future directions. Sarcoma. 2014;2014:391967.
Lefkowitz RA, Landa J, Hwang S, et al. Myxofibrosarcoma: prevalence and diagnostic value of the "tail sign" on magnetic resonance imaging. Skelet Radiol. 2013;42(6):809–18.
White LM, Wunder JS, Bell RS, et al. Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2005;61(5):1439–45.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, L., Pretell-Mazzini, J., Kerr, D.A. et al. MRI findings associated with microscopic residual tumor following unplanned excision of soft tissue sarcomas in the extremities. Skeletal Radiol 47, 181–190 (2018). https://doi.org/10.1007/s00256-017-2762-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2762-y