Abstract
The purpose of this retrospective study was twofold: firstly, to assess the ability of MR imaging in confirming/excluding the presence of residual tumour following inadequate primary excision of soft tissue sarcomas; and secondly, to assess the accuracy of the original radiologists report as compared with a retrospective review of the scan hard copy in confirming/excluding. A total of 111 cases were identified that fulfilled the inclusion criteria of inadequate primary surgery followed by a MR scan and subsequent wide re-excision of the surgical field. The gold standard for the assessment of the MR imaging studies was histological examination of the re-excision specimens. Histological examination revealed residual tumour in 63 (56.7%) cases. In 48 cases the residual tumour was classified macroscopic (maximum diameter >10 mm) and 15 cases microscopic (maximum diameter ≤10 mm). The original radiologists reports failed to indicate the presence or absence of tumour in 7 (6.3%) cases. In the remaining 104 cases the diagnostic performance of MR imaging gave a sensitivity of 0.64, specificity of 0.93, positive predictive value of 0.93 and negative predictive value of 0.67. In 12 of the 21 false-negative scans the residual tumour was microscopic. Subjective assessment of the radiologist’s reports indicated that the proportion of equivocal reports was much higher in both the false-negative and false-positive groups as compared with the true groups. An unblinded retrospective review of the scan hard copies only differed from the original radiologists report in 8 (7.2%) cases. Contrast-enhanced sequences were not routinely obtained in this series. The results suggest that the poor negative predictive value can be attributed more to limitations of the MR scan and not to failures in observation or interpretation by the radiologists. Despite the low negative predictive value, MR imaging remains useful in planning the re-excision surgery by identifying the site and extent of the original operation and size of major residual tumour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main treatment for a soft tissue sarcoma is surgical removal of the primary tumour with a wide margin of surrounding normal tissue, otherwise known as a wide excision [1, 2]. There are several essential pre-requisites for this to happen, notably, accurate preoperative diagnosis and assessment of the extent of the tumour using imaging [3]. First and foremost, however, clinical awareness of the possibility of a sarcoma is necessary. Soft tissue sarcomas are rare, comprising approximately 5% of all soft tissue masses presenting to non-specialist units. When confronted with a soft tissue mass, most surgeons erroneously expect to find a lipoma, lymph node, ganglion cyst or haematoma [4]. Between 25 and 43% of patients with a soft tissue sarcoma referred to a specialist centre have already undergone an inadequate surgical procedure, be it a mispositioned biopsy or marginal excision [3, 5, 6, 7]. In these situations the recommended management is wide re-excision [3, 4, 7, 8]. The purpose of this retrospective study was to establish the value of MR imaging in confirming or excluding the presence of residual tumour following inadequate primary excision of a soft tissue sarcoma.
Materials and methods
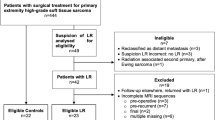
Details of all patients referred to our orthopaedic oncology service in an 8-year period (1992–2000) with the final diagnosis of a soft tissue sarcoma were obtained from the unit’s database. A review of the records identified those cases in which the pre-referral surgical management had been inadequate due to inappropriate biopsy, intralesional or marginal excision. The medical records and imaging studies of these cases for the period immediately before and after referral to our unit were reviewed. The final study group consisted of those cases in which there was a post-referral MR study of the surgical field followed by wide re-excision with histological confirmation of the presence or absence of residual tumour. All the MR scans, prior to wide re-excision, were performed on a 1-T superconducting magnet (Siemens Impact, Erlangen, Germany). A minimum standard protocol was applied comprising three sequences. T1-weighted fast-spin-echo and turbo short T1 inversion recovery (STIR) sequences oriented in the longitudinal plane of the anticipated involved compartment and a transverse T2-weighted fast-spin-echo sequence. The T2-weighted sequence was substituted for a T2-weighted fast-spin-echo fat-suppressed sequence midway through the study period after a system upgrade. A contrast-enhanced sequence with a gadolinium chelate was not routinely performed.
The original reports, made by one of two experienced musculoskeletal radiologists (A.M.D., N.E.), on the post-referral scans were reviewed and the interpretation subjectively classified into one of five categories; these were definitely normal, equivocally normal, uncertain, equivocally abnormal or definitely abnormal with respect to the absence (normal scan) or presence (abnormal scan) of residual tumour. These results were then compared with the gold standard of the histological findings on the re-excised tumour area. The histological findings were classified as normal or, if tumour present, microscopic (i.e. aggregates of residual tumour ≤10 mm in maximum diameter) or macroscopic (i.e. one or more foci of tumour >10 mm in diameter).
Unblinded to the initial MR report or the subsequent histological findings, the hard copy of all the MR scans was reviewed to determine the site of the primary tumour, the number and type of sequences employed, whether the sequences were obtained with a surface coil (i.e. small field of view) or the body coil (i.e. large field of view), and if a gadolinium chelate had been administered. Consensus was reached between three radiologists (A.M.D., S.P., A.M.), as to whether the original report was an accurate interpretation of the findings irrespective of the subsequent re-excision histology. The re-interpretation of the scans was subjectively classified as per the original radiologists reports. The MR features considered indicative of a tumour were a soft tissue mass, hyperintense on T2-weighted and STIR images which was not uniformly homogeneous. The review also classified the MR features that could be attributed to the trauma of the surgery rather than to the possible presence of residual tumour. The three categories comprised subcutaneous scar alone, subcutaneous scar with surrounding soft tissue oedema, scar and oedema with a collection suggestive of a haematoma or seroma. Subacute haematoma was suggested by hyperintensity on T1-weighted images and a seroma by a rounded or oval, homogeneous, hyperintense mass on T2-weighted images.
Other factors recorded were the initial presurgical diagnosis, the presence or absence of pre-operative imaging and the time elapsed between the MR scan and the subsequent wide re-excision.
Results
In the study period a total of 887 patients with a final diagnosis of a soft tissue sarcoma were treated in our orthopaedic oncology service; of these, 140 (11%) had received inadequate surgical management prior to referral. Medical and imaging records could be traced in 121 patients. Ten cases were then excluded from further analysis because, for various clinical reasons, they did not undergo wide re-excision. The age range of the remaining 111 cases was 5–87 years with a mean age of 48 years. There were 63 male and 48 female patients. Preoperative imaging, undertaken before the initial inadequate surgery, was performed in only 39 (35.1%) cases. Twenty-five (22.5% of the overall series) of these underwent some form of cross-sectional imaging, with or without radiography. Four ultrasound only, 6 computed tomography (CT) only, 11 MR imaging only, 2 ultrasound and CT and 2 ultrasound and MR imaging. The commonest misdiagnosis on imaging, identified in 8 cases, was a spectrum of benign cystic lesions (e.g. ganglion, synovial cyst and bursitis).
Seventy-two (64.8%) patients did not undergo any imaging prior to initial surgery. The presumptive preoperative clinical diagnosis as stated in the letters of referral to our unit comprised lipoma (13 cases), ganglion cyst or bursa (12 cases), benign otherwise unspecified (6 cases), mass diagnosis unspecified (8 cases) and no diagnosis given (17 cases). In only 6 cases was the possibility of malignancy considered prior to surgery.
The comparison of the initial MR scan report with the subsequent histological findings are detailed in Table 1 (see Figs. 1, 2, 3, 4, 5, 6, 7). Excluding the 7 cases in which the MR scan report was classified as uncertain, the diagnostic performance of MR imaging in confirming or excluding residual tumour in the remaining 104 cases is detailed in Table 2. The calculations on which the results given in Tables 1 and 2 are based have combined the definite and equivocal reports into positive (i.e. tumour present) and negative (i.e. tumour absent) groups. The proportion of definite to equivocal MR reports were 3.75 to 1 for the true-positive group, 2 to 1 for the true-negative group, and 1 to 2 for the false-negative and false-positive groups.
True positive. Axial T2-weighted fast-spin-echo image (TR/TE=3520 ms/105 ms) of the thigh showing a large mass of residual tumour (17 cm in length in the longitudinal plane) in the adductor compartment. No imaging had been performed prior to the original surgery when the clinical diagnosis had been a “haematoma”
True negatives. a Axial T2-weighted fast-spin-echo image (TR/TE=3520 ms/105 ms) showing a subcutaneous scar over the lateral aspect of the lower thigh. b Axial fat-suppressed T2-weighted fast-spin-echo image (TR/TE=2309 ms/98 ms) through the calf showing extensive subcutaneous oedema. c Axial T1-weighted fast-spin-echo image (TR/TE=637 ms/38 ms) showing a typical subacute haematoma within the subcutaneous tissues over the lateral aspect of the knee
Uncertain. a Axial T2-weighted fast spin-echo (TR/TE=5000 ms/90 ms), b T1-weighted fast spin-echo (TR/TE=600 ms/15 ms), and c contrast-enhanced T1-weighted fast-spin-echo (TR/TE=600 ms/15 ms) images. There is a heterogeneous soft tissue mass overlying the posterior muscles. The high signal intensity margins on the b precontrast image suggests subacute haematoma. There is no significant signal change on the c post-contrast medium image. The re-excision specimen revealed organizing haematoma with microscopic tumour
False negative. a Axial T2-weighted fast-spin-echo (TR/TE=4000 ms/105 ms) and b T1-weighted fast-spin-echo (TR/TE=750 ms/15 ms) showing typical features of a subacute haematoma over the medial aspect of the calf. A contrast-enhanced sequence was virtually identical to b. The re-excision specimen revealed a haematoma with miscroscopic residual tumour
The sensitivity and specificity can be combined to produce a likelihood ratio. This will give the change in odds in favour or against the presence of residual tumour for a given MR scan result; thus, a probability of the disease may be ascertained (i.e. residual tumour) before the actual disease status is known. Furthermore, it allows the use of the uncertain data included in Table 2. The likelihood ratio for the uncertain categories can be calculated utilising the test yields [9]; thus, the prior odds can be adjusted to produce probablities of the residual tumour being present/absent depending on the MR result (Table 3).
Ninety-nine (89%) patients underwent wide re-excision within 1 week of the re-staging MR scan. In the remaining 12 cases there was a delay between the MR scan and re-excision of between 2 and 24 weeks. Five of these cases were in the false-negative group with a delay ranging from 2 to 14 weeks (mean 5.5 weeks). The size of the largest focus of residual tumour on histological examination of the re-excision specimens is detailed in Table 4.
The retrospective review of the MR scan hard copy showed that the proportion of cases in each group were similar for the number of sequences and the type of coils selected. A static contrast-enhanced sequence with a gadolinium chelate was not used in any case from the true-positive, true-negative, false-negative with microscopic residual disease and false-positive groups. A contrast-enhanced sequence was obtained in 4 of the false-negative cases with microscopic disease and two of the uncertain group (Figs. 5, 7). The retrospective review agreed with the initial report in all cases in the true-positive, true-negative and false-negative with microscopic residual disease. Eight cases were reclassified. In only one scan did the retrospective review conclude that the original report had been in definite error. This was early in the study period where the typical MR features of a subacute haematoma had been misinterpreted as a mass of residual tumour and classified as definitely abnormal (Fig. 6). A much higher proportion of cases in the uncertain or false categories (84%) showed florid post-surgical changes (oedema±haematoma/seroma) as compared with the true-negative group (31%; Figs. 5, 6, 7). This indicates that the less distorted the surgical field is on the follow-up MR scan, the easier it is to exclude residual macroscopic tumour.
The proportion of true results (positives and negatives combined as compared with the false or uncertain results) varied from 4 to 1 for tumours arising in the upper limb, to 3 to 1 in the thigh, to 2 to 1 in the calf and ankle and foot, to 1.5 to 1 in the buttock. The site of the primary tumour did have a significant bearing on the incidence of residual disease. For example, 84% (16 cases) with a soft tissue sarcoma arising around the ankle and foot had evidence of residual tumour in the re-excision specimen. This compares with an incidence of only 47.6% (20 cases) in the thigh, the commonest site of a primary in this series.
Discussion
Local recurrence occurs in 10–20% of patients with a primary soft tissue sarcoma despite optimal wide excision [10]. Failure to obtain a wide margin dramatically increases the recurrence rate to between 70 and 90% [1, 4, 11, 12, 13, 14]. This not only prejudices preservation of local limb function but is also associated with the development of metastasis and decreased long-term survival [10, 13]. It is for these reasons that wide re-excision is advocated when the initial surgical procedure has been inadequate [7, 8]. When planning further surgery, with or without radiotherapy, it is important to identify the site and extent of residual disease [4]. This is particularly pertinent in those patients in whom no preoperative imaging whatsoever had been performed; this accounts for 65% of the patients in the current study. Physical examination is unlikely to identify residual tumour in a recent operative site [4]. The clinical information gleaned from the medical records and letters at the time of referral in this series proved limited and unreliable. For example, MR scanning revealed a 14-cm-diameter focus of residual tumour in 1 case that had ostensibly undergone an “excision biopsy.”
Historically, neither radiographs nor angiography proved helpful in evaluating the extent of residual disease [4, 15, 16]. Similarly, CT is of limited value in the early postoperative period where oedema, granulation tissue and scar tissue cannot be reliably distinguished from residual tumour [17, 18]. The use of MR imaging in this context has been the subject of few published studies; many either predate the routine use of MR imaging in orthopaedic oncology [4, 19] or only mention its use in passing [3] or not at all [7]. One study on re-excision following inadequate surgery deliberately excluded all cases in which a mass was palpable on clinical examination or revealed on MR imaging, in effect equivalent to excluding the true-positive group in the current study [5].
Two recent papers, limited to a total of 16 and 24 patients, respectively, have attempted assess the accuracy of MR imaging in the pre-operative work-up prior to re-excision [8, 20]. Although these studies were conducted in a different countries from the current one many of the experiences were similar. For example, in Siebenrock and coworkers’ [8] study, 11 of 16 (69%) patients underwent no imaging whatsoever before initial surgery as compared with 72 of 111 (65%). The presumptive diagnosis before primary excision was a non-malignant soft tissue mass of some description in 16 of 16 (100%) of cases as compared with 99 of 111 (89%). Residual tumour was identified in the re-excised specimens in 10 of 16 (63%) of cases as compared with 63 of 111 (57%). Other studies have reported the prevalence of residual tumour ranging from 35 to 59% [3, 5, 7]. The lower prevalences may be attributed to different treatment protocols including adjuvant chemotherapy and radiation before re-excision and by the fact that some excluded those cases with macroscopic disease [8].
The largest difference between the study published by Siebenrock et al. [8] and the current study, apart from the numbers of cases included, was in the retrospective interpretation of the original MR scan reports. In the current study, only 7 scans (6%) were classified as uncertain, i.e. the scan report did not distinguish whether the tumour was present or absent (Table 1), whereas Siebenrock et al. [8] identified 4 (25%) of the scans in an uncertain category. It is impossible to determine whether the high number of uncertain scans in that paper was due to inadequate scanning protocols. It is more likely that it reflects a degree of indecision in the wording of the original reports or caution as to how the reports were subsequently interpreted. A retrospective classification of written scan reports is bound to be subjective. It is for this reason that the subjective classification in the current study included “equivocal” categories. Although, for the purposes of calculating the sensitivity, specificity, etc., the definite and equivocal results were combined, it is of note that an equivocal report was up to four times more likely in the false-positive and false-negative groups than the true-positive and true-negative groups. An equivocal report, albeit with an emphasis towards the positive or the negative, should be viewed with circumspection. While common sense, this observation cannot necessarily be extrapolated to other centres as it is based on a subjective assessment of the wording of reports issued by two radiologists working in one specialist unit.
The fact that MR imaging failed to reveal 25 of 63 cases (40%) with residual tumour is hardly surprising (Table 1): in over half of these the residual tumour was microscopic which would be below the expected resolution of the MR images (Figs. 5, 7). In the false-negative cases with macroscopic disease the maximum diameter of the residual tumour was smaller than that identifiable on the true-positive scans (Table 4). Also, the incidence of florid postoperative features, such as soft tissue oedema, haematoma and/or seroma formation, which might be expected to obscure residual tumour, was much higher in the uncertain with residual tumour and the false-negative groups (80%) as compared with the true-negative group (31%). A typical problematic example is the identification of a subacute haematoma at the site of previous surgery that may or may not be associated with microscopic residual tumour (Figs. 3c, 4, 5, 6, 7). Another factor to consider is the potential effect of any delay between the timing of the MR scan and subsequent re-excision. Although 89% patients underwent re-excision within 1 week of the MR scan, there was a delay in 5 of the false-negative group ranging from 2 to 14 weeks. It is possible that the residual tumour would have increased in size considerably during this period in some, if not all, cases.
We have calculated the likelihood ratios to provide the clinicians with an idea of the usefulness of MR imaging in assessing residual disease at this treatment centre. Whereas a positive scan will mean that there is residual disease in 93% cases, even scans reported as uncertain will have tumour present in 57% and a negative scan will be incorrect in 33% cases (Table 3). Magnetic resonance imaging is, therefore, not reliable in predicting the presence or absence of residual tumour.
Purists might be tempted to criticise the MR protocol in that a contrast-enhanced sequence was not routinely included. In the few cases that a static contrast-enhanced sequence was obtained it proved unhelpful (4 false-positive cases with microscopic residual tumour and 2 cases in the uncertain category; Figs. 5, 6). It can difficult to identify enhancement in the presence of subacute haemorrhage containing paramagnetic methaemoglobin. Shapeero et al. noted that conventional contrast-enhanced MR imaging may not distinguish between recurrent tumour and an inflammatory pseudotumour [21]. Kaste and coworkers in their study on MR imaging after incomplete resection of a soft tissue sarcoma used a gadolinium chelate in 16 of 24 (66%) cases [20]. The sensitivity of these presumed static contrast-enhanced images to the presence of pathologically identified tumour was 0.78 and the specificity of such studies to the absence of tumour was 0.86. The sensitivity is better than achieved in the current study, 0.78 as compared with 0.64, but not overwhelmingly so, and their specificity was lower (Table 5). Assuming that a gadolinium chelate would identify macroscopic residual disease, then a contrast-enhanced sequence would have been helpful in 11 (10%) cases in the current study but would still miss the microscopic residual tumour in 14 (13%) cases (Table 4).
It is unlikely that a dynamic contrast-enhanced sequence would be the answer because the limited image resolution, in order to obtain the necessary temporal resolution, is likely to miss microscopic tumour. In addition, relatively rapid enhancement can be expected within the soft tissues at the site of recent surgery independent of the presence or absence of residual tumour. Shapeero and coworkers had false-positive results in all 4 of their cases which had dynamic contrast-enhanced MR imaging within 2 months of resection [21].
The anatomical site of the primary tumour had a minor influence on the results of the MR scans. The proportion of true results (positives and negatives combined) varied depending on location and the incidence of residual tumour and was much higher around the ankle and foot as compared with the thigh. This reflects, firstly, the false clinical assumption that a mass arising in a superficial location, such as the ankle and foot, is benign resulting in inadequate surgery, and secondly, the difficulty in achieving a wide resection margin in relatively small anatomical compartments.
The comparison of the original scan report with the retrospective review of the hard copy showed a difference in opinion in only 8 (7.2%) cases. Such a low figure is to be expected with an unblinded review with inbuilt bias. With prior knowledge, it would take a perverse observer to reclassify one of the 80 (72%) cases from either the true-positive or true-negative groups into a false category. The value of this retrospective audit is that the results can be attributed more to the limitations of MR to demonstrate residual tumour, particularly microscopic, rather than to any major deficiencies of the original reporting radiologists both in terms of observation and interpretation. Despite the limitations of MR imaging, it remains essential prior to re-excision. There is a high positive predictive value (93%), but a negative scan does not exclude residual tumour. Nevertheless, MR imaging is useful in preoperative planning by identifying the site and extent of previous surgery. When the pre-referral clinical and surgical details are inadequate, MR imaging can alert the surgeon to the presence of major residual tumour. Clearly, the optimum treatment for all soft tissue sarcomas would be wide primary excision but, failing that, MR imaging followed by wide re-excision is the management of choice.
References
Enneking WF, Spanier SS, Malawer MM (1981) The effect of anatomic setting on the results of surgical procedures for soft-part sarcoma of the thigh. Cancer 47:1005–1022
Westbury G (1989) The management of soft-tissue sarcomas. J Bone Joint Surg (Br) 71B:2–3
Goodlad JR, Fletcher CDM, Smith MA (1996) Surgical resection of primary soft-tissue sarcoma. J Bone Joint Surg (Br) 78B:658–661
Giuliano AE, Eilber FR (1985) The rationale for planned reoperation after unplanned total excision of soft-tissue sarcomas. J Clin Oncol 3:1344–1348
Noria S, Davis A, Kandel R, Levesque J, O’Sullivan B, Wunder J, Bell R (1996) Residual disease following unplanned excision of a soft-tissue sarcoma of an extremity. J Bone Joint Surg (Am) 78A:650–655
Peabody TD, Monson D, Montag A, Schell MJ, Finn H, Simon MA (1994) A comparison of the prognosis for deep and subcutaneous sarcomas of the extremities. J Bone Joint Surg (Am) 76A:1167–1173
Zornig C, Peiper M, Schröder SS (1995) Re-excision of soft-tissue sarcoma after inadequate initial operation. Br J Surg 82:278–279
Siebenrock KA, Hertel R, Ganz R (2000) Unexpected resection of soft-tissue sarcoma. Arch Orthop Trauma Surg 120:65–69
Simel DL, Samsa GP, Matchar DB (1991) Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol 44:763–770
Lewis JJ, Leung D, Heslin M, Woodruff JM, Brennan MF (1997) Association of local recurrence with subsequent survival in extremity soft-tissue sarcoma. J Clin Oncol 15:646–652
Markhede G, Angervall L, Stener B (1982) A multivariate analysis of the prognosis after surgical treatment of malignant soft-tissue tumors. Cancer 49:1721–1733
Essner R, Selch M, Eilber FR (1991) Re-irradiation for extremity soft-tissue sarcomas. Cancer 67:2813–2817
Gustafson P (1994) Soft-tissue sarcoma: epidemiology and prognosis in 508 patients. Acta Orthop Scand (Suppl) 259:1–31
Shinozaki T, Kato K, Watanabe H, Yanagawa T, Ahmed AR, Takagishi K (2001) Discriminant analysis of prognostic factors for malignant fibrous histiocytoma in soft tissue. J Orthop Sci 6:339–342
Hudson TM, Haas G, Enneking W et al. (1975) Angiography in the management of musculoskeletal tumors. Surg Gynecol Obstet 141:11–21
Martel W, Abell MR (1973) Radiologic evaluation of soft-tissue tumors: a retrospective study. Cancer 32:352–366
Jones ET, Kuhns LR (1981) Pitfalls in the use of computed tomography for musculoskeletal tumors in children. J Bone Joint Surg (Am) 63A:1297–1304
Hudson TM, Schakel M, Springfield DS (1985) Limitations of computed tomography following excisional biopsy of soft-tissue sarcomas. Skeletal Radiol 13:49–54
Potter DA, Glenn J, Kinsella TJ, Glatstein E, Lack EE, Restrepo C, White DE, Seipp CA, Wesley R, Rosenberg SA (1985) Patterns of recurrence in patients with high-grade soft-tissue sarcomas. J Clin Oncol 3:353–366
Kaste SC, Hill A, Conley L, Shidler TJ, Rao BN, Neel MM (2002) MR imaging after incomplete resection of soft-tissue sarcoma. Clin Orthop 397:204–211
Shapeero L, Vanel D, Verstarte KL, Bloem JL (2002) Fast MR imaging with contrast for soft-tissue sarcoma viability. Clin Orthop 397:212–227
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davies, A.M., Mehr, A., Parsonage, S. et al. MR imaging in the assessment of residual tumour following inadequate primary excision of soft tissue sarcomas. Eur Radiol 14, 506–513 (2004). https://doi.org/10.1007/s00330-003-2023-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-003-2023-4