Abstract
Anterior cruciate ligament (ACL) reconstructions have increased over the past 25 years. The increased incidence of ACL reconstructions has translated into a larger number of graft failures and revision ACL procedures. It is important to understand the causes of graft failure when evaluating for a revision ACL reconstruction and to appreciate changes in tunnel anatomy over time prior to planning revision surgery. In this manuscript, tunnel size for ACL reconstruction and implications for single-stage versus two-stage revision ACL reconstruction will be discussed, as well as causes of tunnel enlargement, including mechanical and biological factors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There has been an increase in the number of anterior cruciate ligament (ACL) reconstructions over the past 25 years, likely attributed to the overall availability of surgeons capable of performing the procedure and the increased participation in sporting activities which are prone to ACL injuries. The incidence of ACL reconstructions is estimated to be 30–85 per 100,000 persons per year, which increases in the at-risk age group of 16–39 years [1–3]. The overall increased incidence of ACL reconstructions has translated into a larger number of graft failures and subsequent revision ACL procedures. Investigators have reported a failure rate of ACL reconstructions between 10 and 15% at short and intermediate term follow-ups, while long-term failures (greater than 10 years) have been reported as high as 27% [4, 5].
Correct tunnel placement is critical to clinical success. Evaluation of tunnel position and lysis can be evaluated on radiographs, CT and MR imaging. On lateral or sagittal views of the knee, the tibial tunnel should roughly be oriented parallel to the slope of the intercondylar roof (Blumensaat line), with the proximal opening of the tunnel posterior to the intersection of the Blumensaat line and the tibia. On AP or coronal views, the proximal tibial tunnel should be at the intercondylar eminence [6] and should form an angle ranging from 60 to 65° with respect to the medial joint line of the tibia [7]. With regards to the femoral tunnel, it is often accepted that it should be positioned at the intersection of the posterior femoral cortex and the lateral wall of the intercondylar notch [8].
It is important to understand the causes of graft failure, particularly when evaluating for a revision ACL reconstruction. Moreover, it is important to appreciate changes in the tunnel anatomy prior to planning revision surgery. Revision surgery after graft failure is generally associated with a decreased probability of returning patients to their usual athletic performance compared to primary ACL reconstruction surgery [9]. The most common cause of graft failure is traumatic re-injury (32%) [10]. Other common causes of graft failure are attributed to surgical technique (such as non-anatomic tunnel placement) or a combination of causes including biological factors and unrecognized simultaneous ligamentous injuries at the time of the initial surgery resulting in graft microinstability.
Etiology of tunnel enlargement/time dependence of tunnel enlargement
Most hamstring tunnels are drilled with a 7.5–9-mm drill bit, whereas bone-patellar tendon-bone (BPTB) tunnels are drilled with a 10-mm bit. Therefore, expected immediate post-operative ACL tunnel diameter should be around 10 mm regardless of graft selection. Several factors have been implicated in the etiology of tunnel enlargement, including: non-anatomic graft fixation and improper graft tunnel placement, foreign body immune response to allograft, heat necrosis due to drilling, ACL graft ganglia, cell necrosis due to toxic ethylene oxide and metal, and cytokine-mediated non-specific inflammatory responses [11–16]. Post-operative rehabilitation has also been implicated in tunnel widening, with some studies suggesting enlargement due to early immobilization, while others suggesting a decrease in graft micromotion and tunnel widening with nonaggressive rehabilitation [16–18]. Early aggressive rehabilitation programs may also contribute to tunnel enlargement as it subjects the graft-bone interface to early stress before biological incorporation and ligamentization is complete [19, 20]. During joint movement, enlargement gradually occurs with transverse motion of the graft at the level of the tibial tunnel—this is known as the windshield wiper effect, coined by L’insalata [19].
Tunnel widening is generally cavitary, frequently maximal in the mid-zone of the tibial tunnel and occurs in the plane of movement of a joint [21]. The etiology is proposed to be multifactorial including biological and mechanical factors, with the predominant theory being micromotion at the graft-native tunnel interface [22]. One of the main factors associated with tunnel enlargement is malposition of the tibial tunnel, which likely leads to graft micromotion [22]. Another possible cause of tunnel enlargement is graft to tunnel size mismatch, potentially leading to roof impingement, extension deficit, graft tear and/or Cyclops lesion, which if caught early, may be amenable to notchplasty; however, further studies are needed to confirm this hypothesis. The technical limitations to reproduce the shape of the ACL and its normal attachment sites have resulted in tunnel enlargement with all types of grafts and fixation techniques [20]. The further the fixation site is from the tunnel entrance in the joint, the more motion may occur at the graft-bone interface within the tunnel [19].
Ganglion cyst formation as a cause of tunnel enlargement
Ganglion cysts of the ACL are not unusual and have been described in the literature [23–27]. Cysts following ACL reconstruction occur with less frequency and have been implicated in tunnel enlargement [26]. A number of associated etiologies have been reported, including bioabsorbable screws, extra-articular fluid extravasation into the tunnel, allografts with and without ethylene oxide sterilization, and the use of non-absorbable suture [28–33]. Gonzalez-Lomas et al. reviewed seven cases of pre-tibial cysts following ACL reconstruction and found that no case had an infectious etiology, and histologic examination of the cyst-demonstrated fragments of the bioabsorbable screw and foamy histiocytes indicating foreign body reaction [34].
Radiographs of the knee usually demonstrate a large lytic lesion within the proximal tibia expanding the tunnel, with overlying soft tissue swelling anteriorly (Fig. 1). MRI of the knee shows a hyperintense lesion on fluid sensitive pulse sequences arising from or adjacent to the ACL graft, expanding the tibial tunnel with surrounding reactive marrow edema changes (Fig. 2). There is characteristic expansion of the ACL graft, with interdigitation of the lesion between the graft fibers, consistent with myxoid degeneration and ganglion cyst formation (Fig. 3). Intraoperatively, large cavitary defects may be seen (Fig. 4). Ultrasound guided soft tissue mass aspiration can be performed to exclude infection and malignancy before attempting revision ACL reconstruction. Histologic analysis reveals myxoid material with fibrous connective tissue and areas of focal histiocytes (Figs. 5, 6 and 7). Postoperative radiographs can be obtained to monitor incorporation of graft material (Fig. 8).
Pre-operative bone window NECT of patient from Fig. 6 demonstrates a cystic expansile lesion within the proximal tibial epiphysis and metaphysis at the site of the tibial tunnel measuring 3.1 × 3.4 × 3.8 cm. Also seen is an extraosseous cystic component measuring 3.1 × 2.7 × 4.5 cm
In a study of eight patients who had ACL reconstructions, Sanders et al. demonstrated that fluid collections in the osseous tunnels were common and that in seven of eight patients, these fluid collections did not develop into ganglion cysts or result in tunnel expansion at the 6–9 month follow-up period. It is unclear at this time what factors may cause a fluid collection to persist or increase in size.
Graft type dependent tunnel enlargement (e.g. BPTB vs hamstring vs allograft)
There seems to be no difference in failure rates between BPTB and hamstring (HS) autografts, with an overall failure rate of 1.5–15% [35–42]. Moreover, there is currently no evidence to suggest an increased risk of tunnel enlargement with soft tissue allografts versus soft tissue autografts [43, 44]. However, tunnel widening has been seen more commonly with the use of soft tissue grafts with suspensory fixation devices. Tunnel expansion has been found to be significantly greater following ACL reconstruction using HS autografts than those using BPTB autografts, suggesting that the bone-to-bone interface may play a role in decreasing micromotion of the graft [45–47]. Graft failure and tunnel expansion is more common with the use of bioabsorbable screws when compared with the use of metal screws [48, 49].
The ACL consists of two bundles: the anteromedial bundle and the posterolateral bundle, both contributing to the AP and rotational stability of the knee. It has been proposed that a double-bundle technique may restore the biomechanics of the knee better than the classic single-bundle technique; however, clinical data suggests that if more horizontally positioned femoral tunnels are used, single bundle technique produces comparable clinical results as double bundle [50]. This was supported by Siebold [51], that the additional bundle did not reduce the amount of tunnel enlargement in a significant way; nevertheless, it is worth noting Jarvela et al.’s [52] prospective, randomized study which showed double-bundle ACL reconstruction technique resulting in less tunnel enlargement in the tunnels on the tibial side than the single-bundle technique with similar fixation methods, graft material, and rehabilitation.
Threshold of pathologic tunnel enlargement and implications for surgical management in revision (single vs. two stage surgery)
Revision ACL reconstruction can be performed as a single-stage or a two-stage procedure. A single-stage repair is performed when the torn ACL graft is removed and the new graft is inserted in one surgical procedure into either the existing tibial and femoral tunnels or new tunnels, if the current tunnels are not in the way of newly planned tunnels. A two-stage reconstruction involves removal of the torn graft, filling the tunnels with bone graft and then waiting until the tunnels heal over a period of time. At this point, a second surgery is performed and new tunnels are drilled for the new graft.
The decision to proceed with a single-stage versus a two-stage revision is multifactorial. These factors include whether there is loss of flexion or extension as determined by the surgeon, whether the tunnels are in anatomic or non-anatomic positions, and whether the tunnels have enlarged (Fig. 9). Some authors prefer single stage revision because of the worsening cartilage degeneration that is associated with two-stage procedure due to the increased time until completed surgical management [41]; however, a two-stage procedure may be warranted in cases of significant tunnel enlargement or loss of joint mobility [53].
When initially evaluating for potential causes of failure and for preoperative planning, MRI is important in confirming ACL graft rupture and other complications (Fig. 10). The role of CT, however, is important in determining the tunnel orientation of the primary reconstruction. CT is also important in assessing the tunnel for widening, and to estimate the degree of bone graft incorporation in the case of two-stage revisions. (Fig. 11) [9, 54–56]. CT is considered more accurate and reliable than MRI for evaluation of tunnel morphology with accurate tunnel measurements in only 8% of cases with MRI, compared to 96% of cases imaged with CT [54, 55], which may be related to MRI susceptibility artifact and anatomic distortion from metallic hardware, making accurate assessment of tunnel enlargement difficult. Standard AP and lateral radiographs may serve as an inexpensive and low radiation method to assess for tunnel position, osteolysis/enlargement, and the position of fixation devices (Fig. 9); however, radiographs are severely limited in their ability to assess tunnel healing and graft injury.
Radiographic findings that suggest that a single stage procedure is achievable include—properly positioned, unexpanded, and normal shaped tunnels, or a malpositioned tunnel that is not in the way of the planned ACL reconstruction. Tunnel osteolysis can complicate an ACL revision surgery, making it necessary to perform a two-stage revision. Though it should be noted that not all osteolysis leads to graft failure. Tunnel enlargement predominantly occurs in the first 6 months following surgery, and stabilizes within 2 years [43, 57].
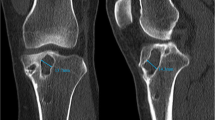
The amount of acceptable tunnel expansion for a one-stage ACL revision is debatable and relies on several factors including physician preference, planned revision graft choice, location of the expanded tunnel, and the morphology of the primary tunnels. Most hamstring tunnels are drilled with a 7.5–9-mm drill bit, whereas bone-patellar tendon-bone (BPTB) tunnels are drilled with a 10-mm bit; therefore, expected immediate post-operative ACL tunnel diameter should be around 10 mm regardless of graft selection. Radiographic findings that persuade surgeons to consider that a two-stage procedure is warranted include a tunnel greater than 15-mm, or a tunnel 10–15-mm with an irregular shape secondary to osteolysis, which limits its use in the reconstruction, and a tunnel position that is in the field of the planned ACL revision. Tibial tunnels should be measured in their sagittal and coronal diameters at their midpoint, and at their proximal and distal apertures, whereas femoral tunnels should be measured at their midpoint and at the notch aperture.
Exact measurements of bone tunnel enlargement are still undefined and the degree of tunnel enlargement that prompts a two-stage procedure is variable. It is generally accepted that tunnel enlargement greater than 15–16 mm or 100% greater than the original tunnel necessitates tunnel grafting [9, 41, 46, 58]. Anatomically correct tunnels measuring between 10 and 15-mm may need grafting depending on the shape of the tunnel or anticipated graft choice, while tunnels measuring less than 10-mm usually may be reused without grafting (permitting single-stage surgery) [9].
If a two-stage revision is deemed necessary, the first stage involves removing the old graft and hardware; however, some authors recommend that the metal hardware should be left in place when located outside the planned reconstruction area [41]—for example, a cortical endobutton that is adherent to the lateral femoral epicondyle cortex could be left in place if asymptomatic and did not interfere the placement of the new graft. Enlarged tunnels are subsequently bone grafted using a variety of techniques and given time to heal (Fig. 12). Approximately 12–16 weeks later, the grafts are evaluated with imaging for the degree of incorporation and quality of bone (Fig. 13). If these factors are adequate, the second stage ACL reconstruction is then performed [9].
Surgical errors are seen in over 70% of ACL reconstruction failures [53]. Although it is unclear if all of these errors lead to the graft failures, Segawa and colleagues noted location and angulation to be important statistical factors contributing to tunnel enlargement likely related to micromotion of the graft [22]. Positioning of the femoral and tibial tunnels is critical for proper function of the ACL graft in order to achieve isometry of the graft [59]. Anterior placement of the femoral tunnel should be avoided to prevent excessive tightness of the graft and thus limiting full knee flexion [6]. Similarly, excessive anterior placement of the tibial tunnel may result in graft impingement in extension and early graft failure [46]. There is a trend of greater tibial tunnel enlargement in more anteriorly placed tunnels, suggesting that this leads to stretching and weakening of the graft, eventually leading to graft rupture [22, 44, 53].
Femoral tunnel malpositioning is more common than non-anatomical tibial tunnel placement and has been found to be the most commonly cited cause of surgeon related primary ACL reconstruction failure [60]; however, there is still debate about the optimal position of the femoral tunnel including anterior, isometric, and over-the-top positions of the femoral tunnel site in ACL reconstruction [19]. Over-the-top placement is not considered to be isometric, resulting in increased length and tension during knee extension. If the femoral tunnel is placed too far anteriorly, the length and tension of the graft greatly increase as the knee is flexed. The femoral tunnel should be placed as far posteriorly without disrupting the posterior cortex of the femur [6].
Graft selection in the setting of ACL revision
While ACL revisions may have good results in terms of graft stability, return to play, and decreased knee instability, they are associated with inferior clinical outcomes when compared to primary ACL reconstructions [61]. Many of the concerns in graft selection for revision ACL are similar to those for primary ACL reconstruction. The surgeon must consider double bundle versus single bundle, autograft donor site morbidity, graft to tunnel size, the small risk of disease transmission with allografts, host versus donor immunologic response to the graft and the slower rate of incorporation of allografts compared to autografts.
Allografts are more frequently used for ACL revisions (54%) when compared to the primary ACL reconstruction setting and repeated BPTP autograft is contraindicated (27%) [10, 61]. Allograft use is more common in the revision setting due to its advances in sterilization techniques and limitations in autograft options. Furthermore, allograft options such as Achilles tendon may offer a larger cross-sectional area and may be useful to fill well-positioned enlarged tunnels in a single-stage ACL revision [61]; however, similar to previous experiments looking at the failure rates of allografts in primary ACL reconstructions, recent studies have demonstrated a greater failure rate in the revision setting at 2-year follow-up with allografts [10, 61, 62]. Some investigators have suggested that autograft use in the revision setting is associated with improved sports function and patient-reported outcome measures at 2-year follow-up [10]. Quadriceps autograft, with its large cross-sectional area, may be a favorable option when BPTB and HS autograft options are not available [61]. When comparing soft tissue autografts to BPTB autografts, the Multicenter ACL Revision Study group study [10] did not find any significant differences in re-rupture or patient-reported outcomes between these autograft types.
Conclusion
Tunnel enlargement may complicate ACL revision surgery. The etiology of tunnel enlargement is multifactorial and probably is secondary to biological and mechanical factors. Revision surgery can involve single or two-stage reconstruction. A two-stage revision involves an initial bone grafting procedure to fill the tunnels, followed at least 3 months later with revision surgery. Studies published to date support that a tunnel diameter greater than 15 mm will require two-stage surgery when the original tunnels are in anatomic position, while revision with a tunnel diameter of less than 10 mm can be accomplished in a single surgery. Revision of tunnels 10–15 mm differs depending upon tunnel shape, position and the treating surgeon’s preference. The radiologist’s role is to assess potential causes of failure, surgical complications, tunnel size and anatomic or non-anatomic position. MRI remains the standard for evaluation of causes of failure. CT is important in determining tunnel orientation, widening and graft incorporation. A multicenter study including threshold of tunnel size in revision ACL reconstruction would provide further conclusive evidence in support of single versus two-stage surgery for tunnels in the 10–15 mm range.
References
Csintalan RP, Inacio MC, Funahashi TT. Incidence rate of anterior cruciate ligament reconstructions. Perm J. 2008;12(3):17–21.
Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–7.
Parkkari J, Pasanen K, Mattila VM, Kannus P, Rimpela A. The risk for a cruciate ligament injury of the knee in adolescents and young adults: a population-based cohort study of 46 500 people with a 9 year follow-up. Br J Sports Med. 2008;42(6):422–6.
Bach Jr BR. Revision anterior cruciate ligament surgery. Arthroscopy. 2003;19 Suppl 1:14–29.
Crawford SN, Waterman BR, Lubowitz JH. Long-term failure of anterior cruciate ligament reconstruction. Arthroscopy. 2013;29(9):1566–71.
Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009;29(7):2115–26.
Howell SM, Hull ML. Checkpoints for judging tunnel and anterior cruciate ligament graft placement. J Knee Surg. 2009;22(2):161–70.
Tomczak RJ, Hehl G, Mergo PJ, Merkle E, Rieber A, Brambs HJ. Tunnel placement in anterior cruciate ligament reconstruction: MRI analysis as an important factor in the radiological report. Skelet Radiol. 1997;26(7):409–13.
Groves C, Chandramohan M, Chew C, Subedi N. Use of CT in the management of anterior cruciate ligament revision surgery. Clin Radiol. 2013;68(10):e552–9.
Group M, Wright RW, Huston LJ, Spindler KP, Dunn WR, Haas AK, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–86.
Hantes ME, Mastrokalos DS, Yu J, Paessler HH. The effect of early motion on tibial tunnel widening after anterior cruciate ligament replacement using hamstring tendon grafts. Arthroscopy. 2004;20(6):572–80.
Hogervorst T, van der Hart CP, Pels Rijcken TH, Taconis WK. Abnormal bone scans of the tibial tunnel 2 years after patella ligament anterior cruciate ligament reconstruction: correlation with tunnel enlargement and tibial graft length. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):322–8.
Jo H, Jun DS, Lee DY, Lee SH, Seong SC, Lee MC. Tibial tunnel area changes following arthroscopic anterior cruciate ligament reconstructions with autogenous patellar tendon graft. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):311–6.
Nebelung W, Becker R, Merkel M, Ropke M. Bone tunnel enlargement after anterior cruciate ligament reconstruction with semitendinosus tendon using Endobutton fixation on the femoral side. Arthroscopy. 1998;14(8):810–5.
Romano VM, Graf BK, Keene JS, Lange RH. Anterior cruciate ligament reconstruction: the effect of tibial tunnel placement on range of motion. Am J Sports Med. 1993;21(3):415–8.
Yu JK, Paessler HH. Relationship between tunnel widening and different rehabilitation procedures after anterior cruciate ligament reconstruction with quadrupled hamstring tendons. Chin Med J. 2005;118(4):320–6.
L’Insalata JC, Klatt B, Fu FH, Harner CD. Tunnel expansion following anterior cruciate ligament reconstruction: a comparison of hamstring and patellar tendon autografts. Knee Surg Sports Traumatol Arthrosc. 1997;5(4):234–8.
Murty AN, el Zebdeh MY, Ireland J. Tibial tunnel enlargement following anterior cruciate reconstruction: does post-operative immobilisation make a difference? Knee. 2001;8(1):39–43.
Hoher J, Moller HD, Fu FH. Bone tunnel enlargement after anterior cruciate ligament reconstruction: fact or fiction? Knee Surg Sports Traumatol Arthrosc. 1998;6(4):231–40.
Wilson TC, Kantaras A, Atay A, Johnson DL. Tunnel enlargement after anterior cruciate ligament surgery. Am J Sports Med. 2004;32(2):543–9.
Robinson J, Huber C, Jaraj P, Colombet P, Allard M, Meyer P. Reduced bone tunnel enlargement post hamstring ACL reconstruction with poly-L-lactic acid/hydroxyapatite bioabsorbable screws. Knee. 2006;13(2):127–31.
Segawa H, Omori G, Tomita S, Koga Y. Bone tunnel enlargement after anterior cruciate ligament reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc. 2001;9(4):206–10.
Kang CN, Lee SB, Kim SW. Symptomatic ganglion cyst within the substance of the anterior cruciate ligament. Arthroscopy. 1995;11(5):612–5.
Liu SH, Osti L, Mirzayan R. Ganglion cysts of the anterior cruciate ligament: a case report and review of the literature. Arthroscopy. 1994;10(1):110–2.
Noda M, Kurosaka M, Maeno K, Mizuno K. Case report ganglion cysts of the bilateral cruciate ligaments. Arthroscopy. 1999;15(8):867–70.
Roeser WM, Tsai E. Ganglion cysts of the anterior cruciate ligament. Arthroscopy. 1994;10(5):574–5.
Sevilla CA. Ganglion of the anterior cruciate ligament presented as a knee mass. Am J Orthop (Belle Mead NJ). 1996;25(1):46–8.
Brettler D, Soudry M. Tibial bone plug resorption with extra-articular cyst: a rare complication of anterior cruciate ligament reconstruction. Arthroscopy. 1995;11(4):478–81.
Deie M, Sumen Y, Ochi M, Murakami Y, Fujimoto E, Ikuta Y. Pretibial cyst formation after anterior cruciate ligament reconstruction using auto hamstring grafts: two case reports in a prospective study of 89 cases. Magn Reson Imaging. 2000;18(8):973–7.
Feldmann DD, Fanelli GC. Development of a synovial cyst following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17(2):200–2.
Martinek V, Friederich NF. Tibial and pretibial cyst formation after anterior cruciate ligament reconstruction with bioabsorbable interference screw fixation. Arthroscopy. 1999;15(3):317–20.
Roberts TS, Drez Jr D, McCarthy W, Paine R. Anterior cruciate ligament reconstruction using freeze-dried, ethylene oxide-sterilized, bone-patellar tendon-bone allografts: two year results in thirty-six patients. Am J Sports Med. 1991;19(1):35–41.
Victoroff BN, Paulos L, Beck C, Goodfellow DB. Subcutaneous pretibial cyst formation associated with anterior cruciate ligament allografts: a report of four cases and literature review. Arthroscopy. 1995;11(4):486–94.
Gonzalez-Lomas G, Cassilly RT, Remotti F, Levine WN. Is the etiology of pretibial cyst formation after absorbable interference screw use related to a foreign body reaction? Clin Orthop Relat Res. 2011;469(4):1082–8.
Ahn JH, Kim JG, Wang JH, Jung CH, Lim HC. Long-term results of anterior cruciate ligament reconstruction using bone-patellar tendon-bone: an analysis of the factors affecting the development of osteoarthritis. Arthroscopy. 2012;28(8):1114–23.
Asik M, Sen C, Tuncay I, Erdil M, Avci C, Taser OF. The mid- to long-term results of the anterior cruciate ligament reconstruction with hamstring tendons using Transfix technique. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):965–72.
Kaeding CC, Aros B, Pedroza A, Pifel E, Amendola A, Andrish JT, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73–81.
Leiter JR, Gourlay R, McRae S, de Korompay N, MacDonald PB. Long-term follow-up of ACL reconstruction with hamstring autograft. Knee Surg Sports Traumatol Arthrosc. 2014;22(5):1061–9.
Leys T, Salmon L, Waller A, Linklater J, Pinczewski L. Clinical results and risk factors for reinjury 15 years after anterior cruciate ligament reconstruction: a prospective study of hamstring and patellar tendon grafts. Am J Sports Med. 2012;40(3):595–605.
Maletis GB, Inacio MC, Desmond JL, Funahashi TT. Reconstruction of the anterior cruciate ligament: association of graft choice with increased risk of early revision. Bone Joint J. 2013;95-B(5):623–8.
Mayr R, Rosenberger R, Agraharam D, Smekal V, El Attal R. Revision anterior cruciate ligament reconstruction: an update. Arch Orthop Trauma Surg. 2012;132(9):1299–313.
Streich NA, Reichenbacher S, Barie A, Buchner M, Schmitt H. Long-term outcome of anterior cruciate ligament reconstruction with an autologous four-strand semitendinosus tendon autograft. Int Orthop. 2013;37(2):279–84.
Linn RM, Fischer DA, Smith JP, Burstein DB, Quick DC. Achilles tendon allograft reconstruction of the anterior cruciate ligament-deficient knee. Am J Sports Med. 1993;21(6):825–31.
Zijl JA, Kleipool AE, Willems WJ. Comparison of tibial tunnel enlargement after anterior cruciate ligament reconstruction using patellar tendon autograft or allograft. Am J Sports Med. 2000;28(4):547–51.
Clatworthy MG, Annear P, Bulow JU, Bartlett RJ. Tunnel widening in anterior cruciate ligament reconstruction: a prospective evaluation of hamstring and patella tendon grafts. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):138–45.
Magnussen RA DJ, Spindler KP. Bone-patellar tendon-bone autograft anterior cruciate ligament reconstruction. In: WN IJS, editor. Surgery of the Knee. 5th edn. New York: Saunders Elsevier; 2012.
Webster KE, Feller JA, Elliott J, Hutchison A, Payne R. A comparison of bone tunnel measurements made using computed tomography and digital plain radiography after anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(9):946–50.
Laxdal G, Kartus J, Eriksson BI, Faxen E, Sernert N, Karlsson J. Biodegradable and metallic interference screws in anterior cruciate ligament reconstruction surgery using hamstring tendon grafts: prospective randomized study of radiographic results and clinical outcome. Am J Sports Med. 2006;34(10):1574–80.
Moisala AS, Jarvela T, Paakkala A, Paakkala T, Kannus P, Jarvinen M. Comparison of the bioabsorbable and metal screw fixation after ACL reconstruction with a hamstring autograft in MRI and clinical outcome: a prospective randomized study. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1080–6.
Streich NA, Friedrich K, Gotterbarm T, Schmitt H. Reconstruction of the ACL with a semitendinosus tendon graft: a prospective randomized single blinded comparison of double-bundle versus single-bundle technique in male athletes. Knee Surg Sports Traumatol Arthrosc. 2008;16(3):232–8.
Siebold R. Observations on bone tunnel enlargement after double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2007;23(3):291–8.
Jarvela T, Moisala AS, Paakkala T, Paakkala A. Tunnel enlargement after double-bundle anterior cruciate ligament reconstruction: a prospective, randomized study. Arthroscopy. 2008;24(12):1349–57.
Denti M, Lo Vetere D, Bait C, Schonhuber H, Melegati G, Volpi P. Revision anterior cruciate ligament reconstruction: causes of failure, surgical technique, and clinical results. Am J Sports Med. 2008;36(10):1896–902.
Hoser C, Tecklenburg K, Kuenzel KH, Fink C. Postoperative evaluation of femoral tunnel position in ACL reconstruction: plain radiography versus computed tomography. Knee Surg Sports Traumatol Arthrosc. 2005;13(4):256–62.
Marchant Jr MH, Willimon SC, Vinson E, Pietrobon R, Garrett WE, Higgins LD. Comparison of plain radiography, computed tomography, and magnetic resonance imaging in the evaluation of bone tunnel widening after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1059–64.
Meuffels DE, Potters JW, Koning AH, Brown Jr CH, Verhaar JA, Reijman M. Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta Orthop. 2011;82(6):699–703.
Peyrache MD, Djian P, Christel P, Witvoet J. Tibial tunnel enlargement after anterior cruciate ligament reconstruction by autogenous bone-patellar tendon-bone graft. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):2–8.
Tredinnick TJ, Friedman MJ. Revision anterior cruciate ligament reconstruction: technical considerations. Am J Knee Surg. 2001;14(3):193–200.
Fineberg MS, Zarins B, Sherman OH. Practical considerations in anterior cruciate ligament replacement surgery. Arthroscopy. 2000;16(7):715–24.
Morgan JA, Dahm D, Levy B, Stuart MJ, Group MS. Femoral tunnel malposition in ACL revision reconstruction. J Knee Surg. 2012;25(5):361–8.
Wilde J, Bedi A, Altchek DW. Revision anterior cruciate ligament reconstruction. Sports Health. 2014;6(6):504–18.
Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Rizer, M., Foremny, G.B., Rush, A. et al. Anterior cruciate ligament reconstruction tunnel size: causes of tunnel enlargement and implications for single versus two-stage revision reconstruction. Skeletal Radiol 46, 161–169 (2017). https://doi.org/10.1007/s00256-016-2535-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2535-z