Abstract
Purpose
To determine the accuracy and short-term efficacy of fluoroscopy-guided steroid/anesthetic injections for symptomatic pars interarticularis (pars) defects.
Materials and methods
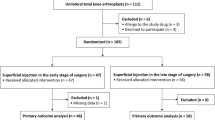
Following IRB approval, all fluoroscopically guided pars injections from a single institution (6/2010 to 3/2016) were retrospectively and independently reviewed by two MSK radiologists. The radiologists evaluated the fluoroscopic images to determine if all of the pars injections associated with each procedure were intra-pars (n = 57 procedures; 106 pars injections), peri-pars (n = 3 procedures; three pars injected), or a combination of intra-pars and peri-pars (n = 6 procedures; 12 pars injected). The patients were asked their pain score (graded on a scale of 0–10) pre-injection, 5–10 min and 1-week post-injection. Age, gender, and fluoroscopic times were recorded. Statistical analysis was performed on the all intra-pars injections only.
Results
Exact inter-reader agreement was present in 92 % (112/121) of the injections, with 57 of the procedures (106 pars injections) performed on 41 patients (mean age 36; 18 M, 23 F) all intra-pars. The mean pre-injection and 5–10 min post-injection reduction in pain for the all intra-pars injections was −3.0 units (95 % CI: [−3.9, −2.1] units; p < 0.001) with a mean 1-week post-injection (n = 21 procedures; 38 pars) reduction in pain of −0.7 units (95 % CI [−1.5, 0.0]; p = 0.06). The geometric mean fluoroscopic time per pars injected was 42 s.
Conclusions
Over 92 % of fluoroscopically guided injections for symptomatic spondylolysis are technically successful with minimum fluoroscopic time, resulting in statistically significant pain reduction immediately post-injection and a trend in pain reduction 1-week post-injection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spondylolysis, or pars interarticularis defects, are estimated to occur in 6 % of the adult population [1, 2], with the number possibly as high as 11.5 % due to the increased sensitivity of CT [3]. Most pars defects occur at the caudal-most lumbar vertebral motion segment, and they are usually bilateral. Many believe that spondylolysis is the result of a stress fracture of the pars interarticularis as pars defects may either heal with conservative measures or progress to a non-union [4–6]. Some anatomic variants, including an insufficient interfacet distance, have also been proposed as a factor in the development of pars defects [7]. In addition, athletes in sports requiring a significant amount of hyperflexion, extension, or high rotational motions may be more prone to developing spondylolysis [8–10].
Spondylolysis has been implicated as an etiology for low back pain, with a higher incidence of spondylolysis related back pain reported in younger patients [11, 12]. To our knowledge, no study has evaluated the accuracy of fluoroscopy-guided pars injections and only a few studies have directly evaluated the efficacy of therapeutic steroid injections for symptomatic spondylolysis [13, 14]. Therefore, we sought to determine if injections of painful pars defects could be accurately performed using fluoroscopic guidance and if the injections were effective in providing immediate and short-term pain relief.
Materials and methods
General
Institutional review board approval was obtained for the study. All fluoroscopy-guided pars interarticularis injection procedures performed on an outpatient basis between June 2010 and March 2016 at a single institution were retrospectively reviewed. Pre-injection confirmation of a pars defect was made by an analysis of MRI, CT, or radiographs (Fig. 1a, b). Over 95 % of the patients were referred by the orthopedic department (mostly from orthopedic spine specialists); neurosurgery and other departments accounted for the remainder. The procedures were performed by musculoskeletal (MSK) faculty and/or fellows in all cases.
a, b An 18-year-old female high school softball pitcher with axial low back pain worsened with activity. a Sagittal reformatted computed tomography image demonstrates bilateral L5 pars defects (right-side provided with arrow). b Fluoroscopic injection of right L5 pars defect demonstrates contrast within the pars defect (arrow). Note: Needle tip is adjacent to the inferior process of L4 (dotted arrow). Fluoroscopic time, 39 s per pars
Technique
After obtaining written informed consent, the patients were placed prone on a fluoroscopic table with a bolster placed underneath their lower abdomen to reduce the lumbar spine lordosis as described by Sarazin et al. [15]. The patients were sterilely prepped and draped, and the appropriate needle entry site on the skin was marked using the AP image (i.e., for an L5 pars defect, the skin entry site was just below the inferior articular process of L4). Using fluoroscopic guidance, a 22-gauge 3.5-inch spinal needle was placed parallel to the image intensifier and directed towards the inferior articular recess of the facet joint at the level of the spondylolysis via a posterior approach such that the needle and its hub were “bulls-eyed” on the AP view [15]. After the needle was advanced through the superficial soft tissues, the image intensifier was turned to the ipsilateral oblique position. The L4–L5 facet joint was identified between the L4 inferior and L5 superior articular processes with the pars defect visualized at the base of the L5 superior articular process. Using both the AP and oblique images, the needle was advanced further until it contacted either the base of the L5 superior articular process or the pars defect itself (Fig. 2a–c). Following needle placement, 0.5–1.5 cc of Omnipaque-300 (Iohexol, GE Healthcare Princeton, NJ, USA) was injected. Opacification of the pars defect was used as the criteria for determining an intra-pars injection; otherwise, the injection was considered to be peri-pars in location. All patients were then injected with 0.5 ml of 0.25 % bupivacaine (bupivacaine HCL, Auro Medics Pharma LLC Dayton, NJ, USA) and either 20 mg (0.5 ml) Depo-Medrol (methylprednisolone acetate, Pharmacia and Upjohn Co New York, NY, USA) or an equivalent dose of another steroid. Patients were excluded if a different concentration of steroid or if an anesthetic other than bupivacaine (i.e., lidocaine) was injected into the pars defect.
a–c A 31-year-old male patient with a bilateral L5 pars defects and low back pain. a AP fluoroscopic image with the appropriate skin needle entry site marked (X) just below the left inferior articular process of L4. b After the needle is placed parallel to the image intensifier and the needle hub “bulls-eyed” on the skin entry site on the AP view, the image intensifier is turned to the ipsilateral oblique position. The left L4–L5 facet joint is between the L4 inferior (L4 IP) and L5 superior (L5 SP) articular processes. The pars defect (circle) is at the level of the base of the L5 superior articular process. The needle (illustrated by an arrow) is advanced until it is inferior to the L4 inferior articular process and contacting either the base of the L5 superior articular process or within the pars defect itself. c Contrast is then injected and demonstrates filling of the pars defect (arrow). Fluoroscopic time, 15 s per pars
Image analysis
Two fellowship-trained MSK radiologists, with 2 and 16 years of experience in performing pars injections, independently reviewed the fluoroscopic injection images to determine if the injections were intra-pars or peri-pars in location. In the event of a discrepancy in determining the location of the injection, a consensus interpretation was rendered with the discrepant cases reanalyzed in a blinded fashion. The consensus interpretation was utilized for the statistical analyses. If more than one pars defect was injected in the same procedural setting, all injections needed to be within the pars defects for the procedure to be considered intra-pars (Table 1).
Pain analysis
All patients reported their pre-injection and immediate post-injection pain scores to a radiology nurse using an 11-point numeric pain rating scale (NRS); 0 (no pain) to 10 (worst pain imaginable). The patients were contacted by a radiology administrator via telephone 1 week following the injection and were asked to again report their level of pain using the same 0–10 pain scale.
Statistical analyses
Data summarization
Categorical data were summarized as frequencies and percentages, and continuous scaled data were generally summarized by the mean and standard deviation of the distribution.
Inter-reader agreement
The concordance between the two readers’ assessments of whether the interarticularis injection needle was all intra-pars, partially in, or all peri-pars was evaluated by way of the kappa statistic (Table 1). An exact binomial confidence interval was utilized to establish a plausible range of values for the underlying level of concordance between the two readers’ injection classifications. Only procedures where the injection(s) was all intra-pars were analyzed further.
Post-procedure pain analysis
Gaussian generalized estimating equation (GEE) regression models were utilized to estimate the immediate and the 1-week post-injection mean changes in the pain scores. Marginal (average) as well as covariate dependent estimates of the mean changes in pain were estimated when factors such as patient age, gender, pre-pain level, and procedure personal (“Attending Physician” involvement) were deemed via statistical tests to be important determinates of immediate and/or 1-week post-injection mean changes in pain. It is important to note that since only seven of the 41 patients (17 %) who underwent the pars procedure had multiple pars procedures, and since no two procedures conducted on the same patient occurred within 25 days of each other, the Huber and White sandwich variance-covariance estimator was utilized to estimate the GEE regression model variance-covariance parameters used in hypothesis testing and confidence interval construction [16, 17]. Null hypotheses related to the immediate and 1-week changes in pain were that the mean change in pain was equal to zero. A two-sided p < 0.05 decision rule was used as the null hypothesis rejection rule.
For the subset of 19 patients (21 procedures) who had both immediate and 1-week pain scores available, we also tested the null hypothesis that the immediate and 1-week mean changes in pain were equal. A two-sided p < 0.05 decision rule was also used as the null hypothesis rejection rule for this test.
Statistical software
The statistical software package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), was used to conduct the aforementioned statistical analyses.
Results
General/injection
Fifty-seven procedures performed on 41 patients (18 M: 23 F; mean age 36 ± 15 years; range, 16–65 years) were considered all intra-pars accounting for a total of 106 pars defects injected (Tables 2 and 3). Three procedures (three pars) performed on three patients were considered all peri-pars and six procedures (12 pars) performed on six patients were considered to be intra-pars on one side and peri-pars on the other. In the intra-pars injections, pre-injection confirmation of a pars defect was made with MRI or CT (n = 35 patients/94 pars) or by radiographs only (n = 6 patients/12 pars) (Figs. 1a and 3a). Steroids utilized for the injection of the all intra-pars included either 20 mg (0.5 ml) Depo-Medrol (methylprednisolone acetate, Pharmacia and Upjohn Co., New York, NY, USA) (44 procedures; 88 pars), 3 mg (0.5 ml) of Celestone [betamethasone, Merck and Co., Whitehouse Station, NJ (eight procedures; nine pars) or 20 mg (0.5 ml) of Kenalog [Triamcinolone Acetonide, Bristol-Myers Squibb Co., Princeton, NJ, USA] (five procedures; nine pars).
a–b A 44-year-old female with bilateral L5 pars defects and lower back pain. a Lateral radiograph demonstrates the bilateral L5 pars defects (*) and grade II anterolisthesis (lines). b Fluoroscopic image demonstrates intra-pars injection of the right L5 pars defect (arrow). Fluoroscopic time, 53 s/pars defect
Image analysis
The exact agreement between the two readers was 92.4 % (95 % CI: [83.2, 97.5 %]) for all intra-pars with a kappa statistic of 0.65 (95 % CI: [0.40–0.90]) (Figs. 1b and 3b) (Table 1). The geometric mean fluoroscopic time required to perform an intra-pars injection was 42 s (95 % CI: [36, 49 units]; range [9, 157 s]).
Pain analysis
The pre-injection and 5–10 min post-injection 0–10 pain was recorded in all patients. One-week post-injection pain scores were only available following 37 % (21/57) of the injections even though all patients were called 1-week post-injection on at least one occasion.
Immediate change in pain
Patient age, gender, and the radiologist performing the procedure were not associated with the immediate change in pain (p= > 0.10 for all), but pre-injection pain levels were inversely associated with the immediate post-injection change in pain (p = 0.001) (Table 4). The mean pre-injection and 5–10 min post-injection pain scores for the 57 all intra-pars procedures were 6.0 and 3.0, respectively (mean Δ: −3.0 units; 95 % CI: [−3.9, −2.1 units]; p < 0.001) (Table 5).
One-week change in pain
Patient age, gender, radiologist performing the procedure, and the pre-injection pain level were not associated with the 1-week change in pain (p= > 0.30 for all) (Table 4). The 1-week post-injection mean reduction in the pain score from the pre-injection pain score was −0.7 units (95 % CI [−1.5, 0.0 units]; p = 0.06) (Table 5).
Percent pain reduction
Immediately after the injection, 35 % (20/57) of the injections resulted in 75 % or greater pain reduction with 54 % (31/57) of the injections resulting in 50 % or greater pain reduction. One-week following the injection, 5 % (1/21) of the injections resulted in 75 % or greater pain reduction with 19 % (4/21) of the injections resulting in 50 % or greater pain reduction (Table 6).
Discussion
The high concentration of free nerve endings within pars defects in patients with symptomatic spondylolysis suggests that the tissue within the pars defect could be a source of pain in these patients [18]. Treatment of patients with symptomatic pars defects often consists of nonsteroidal anti-inflammatory medications, bracing of the lower back, and the cessation of potentially aggravating activities. Since pars defects in the early or progressive phase may spontaneously heal, skeletal maturity and bone SPECT findings may occasionally alter management [19–21].
An injection of local anesthetic into a pars defect is often part of an algorithmic approach used to diagnose the etiology of low back pain as well as a method to provide immediate therapeutic pain relief to these patients [19, 20, 22]. Ultimately, failed conservative measures may warrant surgical fixation with the goal of establishing solid osseous fusion across the defect in order to relieve the pain [22]. A diagnostic anesthetic injection into asymptomatic pars defect may also help determine which patients might respond favorably to surgical fusion [22].
Injection of the pars interarticularis defect may be performed by placing the needle directly into the defect using either CT or fluoroscopic guidance. This can be accomplished with fluoroscopy by using the technique that Sarazin et al. described as an “indirect” technique to perform a facet injection (i.e., target the inferior articular recess of the L4–L5 facet joint which is at the approximate level of the L5 pars defect) [15]. A pars defect can also occasionally be injected “indirectly” by directly injecting the facet joint above the defect. This is possible because the pars interarticularis is the only barrier between the inferior articular recess of the facet joint above and the superior articular recess of the facet joint below the defect, and in some but not all cases, the pars defect may subsequently fill with contrast using this “indirect” technique [13, 23, 24]. Given that the technique of directly injecting the facet joint does not result in filling of a pars defect in all cases, we prefer the technique described by Sarazin et al. that actually directly targets the pars defect (which is immediately deep to the location of the inferior facet joint recess) [15]. We have anecdotally found that this approach is well tolerated by the patient, is technically straightforward, and is less time-consuming and costly than CT guidance.
To our knowledge, the accuracy of fluoroscopically guided injection of pars defects has not been reported. We found that the technique described by Sarazin for “indirectly” injecting facet joints is highly accurate in injecting pars defects, with ∼93 % (112/121) of the injections intra-pars in location. We also found that intra-pars steroid-anesthetic injections can be performed with minimal fluoroscopic time, resulting in a statistically significant reduction in pain 5–10 min post- injection; pain relief likely attributed to the anesthetic administration.
Even though steroids are often added to the anesthetic solution with the expectation for longer pain relief [25], few studies have directly evaluated the efficacy of steroid injections in providing pain relief in patients with symptomatic pars defects. We found that intra-pars steroid-anesthetic injections were associated with a trend in pain reduction 1 week post-injection (p = 0.06); pain relief likely attributed to the steroid effect. When analyzing the number of patients with pain relief following a fluoroscopically guided pars injection using a combination steroid/anesthetic mixture (n = 8) or anesthetic alone (n = 3), Maldague et al. found that 55 % (6/11) of the patients injected had 75 % or greater pain relief [13]. This compares to 35 % (20/57) of the patients in our study with 75 % or greater pain relief 5–10 min after the injection with the difference between the percentage of patients with 75 % pain relief in our study and in Maldague et al. not statistically significant (p = 0.22). Wald et al. performed an audit of symptomatic pars defects injected with a steroid/anesthetic solution under CT guidance and reported that 43 % (18/42) of the patients had 50 % or greater pain reduction 2 weeks post-injection [14]. This compares to 19 % (4/21) of the patients in our study with 50 % or greater pain reduction 1-week post-injection with the difference between the percentage of patients with 50 % or greater pain relief in our study and in the study by Wald et al. not statistically significant (p = 0.06). Unfortunately, to our knowledge, no additional studies exist to more clearly determine the efficacy of injections for symptomatic pars defects.
This retrospective study is limited by the analysis of only 57 procedures in 41 patients. However, there is a paucity of studies in the literature evaluating the effectiveness of steroid/anesthetic injection on providing pain relief for symptomatic pars defects, the largest evaluating 59 patients [14]. In addition, our 37 % phone call response rate 1-week post-injection is lower than the response rate we usually experience following other injections. We suspect this lower response rate is partially related to the lower age group of the patients injected for pars defects as we have anecdotally found contacting university students post-injection especially challenging. Further study with additional patients would be useful to better define the 1-week post-injection trend in pain relief (p = 0.06) that we noted in our study.
In conclusion, symptomatic pars defects can be successfully injected under fluoroscopic guidance with minimal fluoroscopic time in over 90 % of cases by targeting the inferior recess of the facet joint, which is at the level of the pars defect. Statistically significant immediate post-injection pain relief was provided by the intra-pars injections, with a trend in pain relief present 1-week post-injection.
References
Wiltse LL, Widell Jr EH, Jackson DW. Fatigue fracture: the basic lesion is isthmic spondylolisthesis. J Bone Joint Surg. 1975;57A:17–22 [Am].
Fredrickson BE. The natural history of spondylosis and spondylolisthesis. J Bone Joint Surg. 1984;66A:669–707.
Kalichman L, Kim DH, Li L, Guermazi A, Berkin V, Hunter DJ. Spondylolysis and spondylolisthesis: prevalence and association with low back pain in the adult community-based population. Spine. 2009;34(2):199–205 (Phila Pa 1976).
Fujii K. Union of defects in the pars interarticularis of the lumbar spine in children and adolescents. J Bone Joint Surg. 2004;86-B:225–31. Br.
Lusins JO, Elting JJ, Cicoria AD, Goldsmith SJ. SPECT evaluation of lumbar spondylolysis and spondylolisthesis. Spine. 1994;5:608–12.
Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6:406–11.
Ward CV, Latimer BP, Alander DH, et al. Radiographic assessment of lumbar facet distance spacing and spondylolysis. Spine. 2007;32:E85–88.
Petron DJ, Prideaux CC, Likness L. Interventional spine procedures in athletes. Curr Sports Med Rep. 2012;11(6):335–40.
Rossi F, Dragoni S. The prevalence of spondylosis and spondylolisthesis in symptomatic elite athletes: radiographic findings. Radiology. 2001;7:37–42.
Soler T, Calderon C. The prevalence of spondylolysis in the Spanish elite athlete. Am J Sports Med. 2000;1:57–62.
Mooney V, Robertson J. The facet syndrome. Clin Orthop. 1976;115:149–56.
Micheli LJ, Wood R. Back pain in young athletes. Arch Pediatr Adolesc Med. 1995;149:15–8.
Maldague B, Mathurin P, Malghem J. Facet joint arthrography in lumbar spondylolysis. Radiology. 1981;140:29–36.
Wald JT et al. A practice audit of CT-guided injections of pars interarticularis defects in patients with axial low back pain: a primer for further investigation. Pain Med. 2014;15:745–50.
Sarazin L et al. Lumbar facet joint arthrography with the posterior approach. Radiographics. 1999;19(1):93–104.
Huber PJ. The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. 1967; vol. I, pp. 221–33.
White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–38.
Schneiderman GA, McLain RF, Hambly MF, Nielsen SL. The pars defect as a pain source. A histologic study. Spine. 1995;20:1761–4.
Omidi-Kashani F, Ebrahimzadeh MH, Salari S. Lumbar spondylolysis and spondylolytic spondylolisthesis: who should behave surgery? An algorithmic approach. Asian Spine J. 2014;8(6):856–63.
Wu SS, Lee CH, Chen PQ. Operative repair of symptomatic spondylolysis following a positive response to diagnostic pars injection. J Spinal Disord. 1999;12:10–6.
Sys J, Michielsen J, Bracke P, et al. Nonoperative treatment of active spondylolysis in elite athletes with normal x-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10:498–504.
Suh PB, Esses SI, Kostuik JP. The prognostic value of pars infiltration. Spine. 1991;16:S445–448.
McCormick CC, Taylor JR, Twomey LT. Facet joint arthrography in lumbar spondylolysis: anatomic basis for spread of contrast medium. Radiology. 1989;17:193–6.
Ghelman B, Doherty JH. Demonstration of spondylolysis by arthrography of the apophyseal joint. Am J Roentgenol. 1978;130(5):986–7.
El-Khoury GY, Renfrew DL. Percutaneous procedures for the diagnosis and treatment of lower back pain: discography, facet-joint injection, and epidural injection. Am J Roentgenol. 1991;157:685–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no grants or conflicts of interest to disclose.
Rights and permissions
About this article
Cite this article
Kershen, L.M., Nacey, N.C., Patrie, J.T. et al. Accuracy and efficacy of fluoroscopy-guided pars interarticularis injections on immediate and short-term pain relief. Skeletal Radiol 45, 1329–1335 (2016). https://doi.org/10.1007/s00256-016-2427-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2427-2