Abstract
Objectives
The purposes of our study are to determine the quantitative elasticity values of normal common extensor tendon (CET) and to assess the interobserver variability of stiffness measurements using shear wave elastography (SWE).
Materials and methods
A total of 60 CETs of 30 (15 female, 15 male, mean age 30.2 years) healthy volunteers without any symptoms of lateral epicondylitis were examined by two radiologists. Age, sex, height, weight, body mass index (BMI), and dominant hand of all participants were noted. The first observer performed B-mode and SWE imaging, and the second observer performed only SWE imaging. Tendon thickness and stiffness values in kPa were measured.
Results
The mean thickness of CETs was 3.57 ± 0.36 mm. The mean stiffness values of CETs for two observers were 45.28 ± 9.82 kPa and 45.80 ± 9.72 kPa respectively. Tendon thickness had a weak correlation with weight (r = 0.281, p = 0.03), and moderate correlation with stiffness values (r = 0.429, p < 0.001). The mean interobserver difference of CET stiffness measurements was −0.5% of the mean CET stiffness values. Range of measurement error, defined as 95% limits of agreement, was ±23.5%. There was no significant difference between absolute values of interobserver measurements (p = 0.741).
Conclusion
Shear wave elastography is a reproducible imaging technique for the evaluation of CET elasticity and the standard stiffness values of normal CET can be used as reference data to differentiate normal from pathological tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Common extensor tendon (CET), which attaches to the lateral epicondyle of the humerus, arises from the fibers of extensor carpi radialis brevis, extensor digitorum, extensor digiti minimi, and extensor carpi ulnaris muscles [1, 2]. Its main function is to transmit the forces produced by these muscles to bone during wrist extension. Lateral epicondylopathy, known as “tennis elbow”, is associated with the overuse of CET and frequently causes lateral elbow pain [2, 3]. This clinical entity affects approximately 1.3% of the middle-aged population [4]. Although, the diagnosis is commonly made by the clinical findings, sonography or MRI may be performed to provide information about the differential diagnosis and severity of the disease [3, 5]. Despite the higher sensitivity of MRI in the precise evaluation of CET, sonography may be more practical in clinical routine owing to its low cost and relative short-time requirement in addition to the superficial location of the tendon [6, 7].
Elastography techniques assess the biomechanical and structural properties of tissues [3, 8]. SWE is a relatively new technique that evaluates the tissue elasticity quantitatively [9]. Shear waves move faster in harder materials [9, 10] and this technique can provide useful information for the diagnosis of pathological conditions that alter the tissue stiffness.
In recent years, assessment of tendons by ultrasound elastography has risen considerably. Achilles tendon is the most frequently investigated musculoskeletal tissue by elastography and most of our increasing knowledge about the elasticity features of tendons originates from the strongest tendon of the body [11]. Thus, investigations of specific tendons will bridge the gap in the literature by contributing to the information about tendon elasticity.
Previous studies [3, 5, 7, 12] evaluated the diagnostic performance of different elastography techniques in cases of lateral epicondylopathy. These studies revealed that the elastography imaging is a useful complementary method in the differentiation of pathological tendons from healthy tendons. A limited number of studies have evaluated the elasticity features of CET and in almost all of these studies the compression elastography technique was used. Furthermore, several investigations that assessed the reproducibility of SWE of the tendons exist [13, 14]. However, to the best of our knowledge, no study has yet assessed the reproducibility of SWE of the CET. Therefore, establishing stiffness measurements of normal CET by SWE and assessing the interobserver variability of this method will be beneficial as reference data. Our study is aimed at determining a reference data set for stiffness values of normal CET and to assess the interobserver variability of stiffness measurements by using SWE.
Materials and methods
This prospective study was approved by the institutional review board and written informed consent was obtained from all participants. Thirty healthy volunteers (15 women, 15 men) without any symptoms (pain or tenderness at the lateral aspect of elbow and painful resisted wrist extension) of lateral epicondylitis were enrolled to our study. History of previous elbow operation, elbow deformity, and systemic inflammatory disorders were used as exclusion criteria. Age, sex, height, weight, body mass index (BMI), and dominant hand of all participants were also noted.
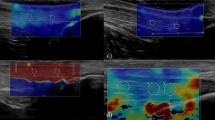
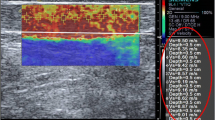
Two observers participated in this study and both of them used the same ultrasound system (LOGIQ E9; GE Medical Systems, Wisconsin, USA) with a 9-MHz linear probe. The first observer (HNS, 11 years experienced in musculoskeletal radiology) performed B-mode ultrasound and measured thickness of bilateral CETs (n = 60) of all volunteers at the level of the humeroradial joint and then used the SWE imaging to measure the stiffness values of all subjects in kPa consecutively (Figs. 1, 2). The second observer (EC, 10 years experienced in musculoskeletal radiology) performed only elastographic evaluation of all CETs just after the first examination had ended. The observers were blind to their results. All the volunteers were examined in the sitting position with 90° flexion of elbow and the thumbs up. The tip of the linear probe was covered with a generous amount of ultrasound gel and to avoid anisotropy, the probe was placed parallel to the longitudinal axis of the CET. The stiffness measurements were made by using 2-mm region of interest (ROI) at the level of the capitellar region. All ROIs included only the tendon structure. CET stiffness values were measured three times by both observers and the median of the consecutive three measurements was recorded. Consecutive measurements were made from approximately the same area of the tendon at the capitellar region on different elastography images.
All statistical analyses were performed using SPSS 22.0 software for Windows (IBM Corp, Armonk, NY, USA). The descriptive statistics of the data is composed of mean, standard deviation, median, minimum, and maximum values. The range of variables was analyzed using the Kolmogorov–Smirnov test. Pearson’s correlation test was used to evaluate correlation analysis. Bland–Altman plots were used to assess interobserver variability. The mean difference and 95% limits of agreement in CET stiffness measurements between two observers were analyzed [15]. The absolute values for interobserver variability were compared using the paired t test. P value of < 0.05 was considered significant.
Results
The demographic characteristics of the participants are presented in Table 1. Mean age of the participants was 30.2 years (age range 21 to 48). All CETs demonstrated normal fibrillar echotexture, straight contours, and none of them included any sonographic abnormality that could be associated with lateral epicondylopathy. The mean thickness of CETs was 3.57 ± 0.36 mm. Age, sex, height, and dominant hand had no significant correlation with tendon thickness. Tendon thickness had a weak correlation with weight (r = 0.281, p = 0.03). The mean stiffness values of CETs for two observers were 45.28 ± 9.82 kPa and 45.80 ± 9.72 kPa respectively. Table 2 summarizes the measurements. Age, sex, weight, height, and dominant hand had no significant effects on SWE measurements. There was a moderate correlation between tendon thickness and stiffness values (r = 0.429, p < 0.001). Figure 3 presents Bland–Altman plots of pairs of stiffness measurements that show the 95% limits of agreement and median of differences for interobserver variability. The mean interobserver difference of CET stiffness measurements was −0.5% of the mean CET stiffness values. Range of measurement error, defined as 95% limits of agreement, was ±23.5%. There was no significant difference between absolute values of interobserver measurements (p = 0.741).
Bland–Altman plots of common extensor tendon (CET) stiffness measurements by two observers. Difference in stiffness values between observers 1 and 2 is plotted against the mean CET stiffness values of two observers. The red line indicates the mean difference of all measurements (n = 60) and the green lines denote 95% limits of agreement (mean ± 1.96 SD)
Discussion
In this study, we measured the CET thickness at the level of the humeroradial joint. The mean thickness of normal CETs was 3.57 ± 0.36 mm. Jaén-Díaz et al. [2] determined the structural and sonographic properties of CETs in the general population and measured the tendon thickness as 4.63 mm at the capitellar region for normal elbows. They found that the CET was significantly thicker in the dominant elbow and male patients, and tendon thickness correlated weakly with age and moderately with weight. However, our results revealed no significant changes with age, sex, and dominant side. In our study, we only found that tendon thickness had a significant correlation with weight. In comparison to the study by Jaén-Diaz, our study included a smaller number and narrower age range of participants. The differences in the study population may be the reason why we could not find the same relationships. Toprak et al. [16] compared the value of CET thickness measurements at the capitellar region and at the humeroradial joint level in the diagnosis of lateral epicondylopathy. They found that when using these two measurements together as an addition to qualitative findings, the sensitivity and specificity of evaluation increased to 93% and 91% respectively. They also measured the CET thickness of the control group at the humeroradial joint level to be 3.24 mm and 3.53 mm for nondominant and dominant sides respectively, which are compatible with our measurements. Lee et al. [17] reported that CET thickness measurement greater than 4.2 mm had sensitivity, specificity, and accuracy of 78.4%, 95.2%, and 87.7% respectively, in the diagnosis of lateral epicondylopathy. They measured the CET thickness at the level of the capitellar region. Thus, according to the results of our study and Toprak et al. [16], this cut-off value was estimated to be lower when measuring the tendon thickness at the humeroradial joint level. In our daily practice, we measure the tendon thickness at the humeroradial joint level. However, for stiffness measurements we preferred the capitellar region, the closer portion of the tendon to the lateral epicondyle of the humerus, which is more likely to be affected in cases of lateral epicondylitis.
Dirrichs et al. [12] evaluated the SWE of Achilles, patellar, and humeral epicondylar tendinopathies. These researchers measured the stiffness values of 25 asymptomatic and 41 symptomatic humeral epicondylar (both radialis and ulnaris) tendons. The mean stiffness measurements for asymptomatic and symptomatic tendons were 176.2 ± 18.2 kPa and 64.1 ± 29.3 kPa respectively. These results are considerably different from our measurements (45.28 ± 9.82 kPa for the first observer and 45.80 ± 9.72 kPa for the second observer in our study). According to our clinical experience, we observe that the healthy tendons are not stiff as those mentioned in that study. Arda et al. [18] measured the SWE values of various soft tissues in a healthy cohort and they found the elasticity values for Achilles tendons to be 51.5 ± 25.1 kPa, which is compatible with our stiffness measurements in this study and in clinical experience of stiffness of the tendons. However, Dirrichs et al., in the aforementioned study, performed imaging in a different position (relaxed upper extremity and slightly bent position) and used a different ultrasound system (Aixplorer; SuperSonic Imagine, Aix-en-Provence, France) which may have affected the elasticity measurements. Further studies should focus on clarifying these issues.
Tendon thickness increases in tendinopathies [1, 2, 17] and affected tendons are expected to show soft structure properties [3, 5, 12]. Our results revealed that there was a significant correlation between tendon thickness and stiffness measurements (r = 0.429, p < 0.001). Our results lead us to think that when a healthy tendon increases in size, it probably includes many more amount dense collagen fibers structurally, which may cause stiffer elasticity other than pathological tendon thickening.
The mean stiffness values of CETs for two observers were very similar (45.28 ± 9.82 kPa for the first observer and 45.80 ± 9.72 kPa for the second observer) and there were no significant differences between absolute values of interobserver measurements (p = 0.741). This significant interobserver agreement indicates that SWE is a reproducible technique for the evaluation of CET elasticity and the stiffness values of normal CET can be used as reference data to differentiate pathological conditions. Furthermore, despite the range of measurement error of ±23.5% for stiffness measurements, utilization of the same technique and learning methods by the two observers likely led to the nearly equal results. This may be an interesting investigation point and further studies should elicit the effects of institutional learning curves on the SWE measurements of tendons.
The main limitation of our study is the relatively small number of participants. On the other hand, we did not assess the intra-observer variability, which has potential differences in the evaluation of elasticity of the tendons. Shin et al. [19] demonstrated that shear wave velocities change with different ultrasound devices, transducers, and acquisition depths. Thus, it may not be possible to generalize our results for all the ultrasound devices on the market. Future researches, including inter- and intra-observer variabilities with larger study groups, different ultrasound devices, and more than two observers in the assessment of CETs by SWE, will contribute to the knowledge of the elasticity characteristics of the CET. However, our results can be considered as an initial step in the elasticity measurements of normal CET using SWE.
In conclusion, SWE is a reproducible imaging technique for the evaluation of the elasticity of CET and the data obtained for stiffness values of normal CET from our study can be used as reference data to differentiate normal from pathological tissues.
References
Connell D, Burke F, Coombes P, et al. Sonographic examination of lateral epicondylitis. AJR Am J Roentgenol. 2001;176:777–82.
Jaén-Díaz JI, Cerezo-López E, López-de Castro F, et al. Sonographic findings for the common extensor tendon of the elbow in the general population. J Ultrasound Med. 2010;29:1717–24.
Kocyigit F, Kuyucu E, Kocyigit A, et al. Association of real-time sonoelastography findings with clinical parameters in lateral epicondylitis. Rheumatol Int. 2016;36(1):91–100.
Shiri R, Viikari-Juntura E, Varonen H, Heliövaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol. 2006;164:1065–74.
De Zordo T, Lill SR, Fink C, et al. Real-time sonoelastography of lateral epicondylitis: comparison of findings between patients and healthy volunteers. AJR Am J Roentgenol. 2009;193(1):180–5.
Miller TT, Shapiro MA, Schultz E, Kalish PE. Comparison of sonography and MRI for diagnosing epicondylitis. J Clin Ultrasound. 2002;30:193–202.
Ahn KS, Kang CH, Hong SJ, Jeong WK. Ultrasound elastography of lateral epicondylosis: clinical feasibility of quantitative elastographic measurements. AJR Am J Roentgenol. 2014;202(5):1094–9.
Domenichini R, Pialat JB, Podda A, Aubry S. Ultrasound elastography in tendon pathology: state of the art. Skeletal Radiol. 2017;46(12):1643–55.
Taljanovic MS, Gimber LH, Becker GW, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics. 2017;37(3):855–70.
Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: musculoskeletal applications. Radiology. 2014;272(3):622–33.
Fusini F, Langella F, Busilacchi A, et al. Real-time sonoelastography: principles and clinical applications in tendon disorders. A systematic review. Muscles Ligaments Tendons J. 2018;7(3):467–77.
Dirrichs T, Quack V, Gatz M, Tingart M, Kuhl CK, Schrading S. Shear wave elastography (SWE) for the evaluation of patients with tendinopathies. Acad Radiol. 2016;23(10):1204–13.
Payne C, Watt P, Cercignani M, Webborn N. Reproducibility of shear wave elastography of the Achilles tendon. Skeletal Radiol. 2018;47(6):779–84.
Tas S, Onur MR, Yılmaz S, Soylu AR, Korkusuz F. Shear wave elastography is a reliable and repeatable method for measuring the elastic modulus of the rectus femoris muscle and patellar tendon. J Ultrasound Med. 2017;36(3):565–70.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Toprak U, Baskan B, Ustuner E, et al. Common extensor tendon thickness measurements at the radiocapitellar region in the diagnosis of lateral elbow tendinopathy. Diagn Interv Radiol. 2012;18(6):566–70.
Lee MH, Cha JG, Jin W, et al. Utility of sonographic measurement of the common extensor tendon in patients with lateral epicondylitis. AJR Am J Roentgenol. 2011;196:1363–7.
Arda K, Ciledag N, Aktas E, Aribas BK, Kose K. Quantitative assessment of normal soft-tissue elasticity using shear wave ultrasound elastography. AJR Am J Roentgenol. 2011;197(3):532–6.
Shin HJ, Kim MJ, Kim HY, Roh YH, Lee MJ. Comparison of shear wave velocities on ultrasound elastography between different machines, transducers, and acquisition depths: a phantom study. Eur Radiol. 2016;26(10):3361–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Şendur, H.N., Cindil, E., Cerit, M. et al. Interobserver variability and stiffness measurements of normal common extensor tendon in healthy volunteers using shear wave elastography. Skeletal Radiol 48, 137–141 (2019). https://doi.org/10.1007/s00256-018-3021-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-018-3021-6