Abstract
Bone resorption is required for skeletal modelling during bone growth and for mineral homeostasis and bone remodelling throughout life. Osteoclasts are multinucleated cells that are uniquely specialised to carry out this physiological bone resorption. As osteolysis is a feature of most diseases of bone and joint, osteoclasts also play a role in pathological bone resorption, the extent of which is a function of the cellular and molecular mechanisms that govern their formation and function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone resorption is required for skeletal modelling during bone growth and for mineral homeostasis and bone remodelling throughout life. Osteoclasts are multinucleated cells that are uniquely specialised to carry out this physiological bone resorption. As osteolysis is a feature of most diseases of bone and joint, osteoclasts also play a role in pathological bone resorption, the extent of which is a function of the cellular and molecular mechanisms that govern their formation and function.
Osteoclast formation and function
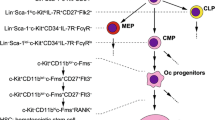
For most of the previous century osteoclasts were thought to be formed from osteoblasts. Evidence from several experimental approaches in the 1970s showed that the osteoclast is formed by fusion of circulating mononuclear precursor cells of haematopoietic origin (reviewed in [1]); these cells circulate in the (CD14+) monocyte fraction and differentiate into osteoclasts at or near the bone surface. The key molecules governing osteoclastogenesis were elucidated following the discovery that osteoprotegerin (OPG), which belongs to the TNF receptor family, induces osteopetrosis when overexpressed in transgenic mice; expression cloning found that the protein that binds OPG is receptor activator of nuclear factor κ B ligand (RANKL), a member of the TNF superfamily [2]. In bone, RANKL is expressed on the surface of bone marrow stromal cells and osteoblasts where it interacts with its receptor RANK, which is expressed by mononuclear phagocyte osteoclast precursors. In the presence of macrophage-colony stimulating factor (M-CSF) [also termed colony stimulating factor-1 (CSF-1)], this RANKL-RANK interaction results in osteoclast differentiation. OPG acts as a soluble decoy receptor for RANKL and blocks osteoclast differentiation; it also binds to RANK on mature osteoclasts to stimulate resorptive activity and to prolong cell survival [3, 4]. The RANKL:OPG ratio thus effectively determines the extent of osteoclast formation and resorption. Most calciotropic hormones and cytokines that stimulate bone resorption, such as parathyroid hormone, 1,25 (OH)2vitamin D3, glucocorticoids, TNFα, interleukin-1 (IL-1), IL-6 and prostaglandins, upregulate RANKL and downregulate OPG expression in bone cells.
The signalling pathways that are activated following RANKL-RANK binding have been a major focus of research in the past decade [4, 5]. The adapter molecule TNF receptor activating factor 6 (TRAF6) is crucial for osteoclast differentiation (Fig. 1a). TRAF6 binds to RANKL and activates several downstream signalling pathways including NFκB and the group of mitogen-activated protein kinases (MAPK) that lead to activation of c-jun terminal kinase (JNK) and P38. JNK activation leads to increased c-jun and c-fos expression. The AP-1 transcription factor consists of Fos, Jun and activating transcription factor family proteins, and RANK activates AP-1 through induction of c-fos. Another transcription factor, nuclear factor of activated T cells (NFATcl), is strongly induced by RANKL; its activation is mediated by calcineurin, a phosphatase, the activity of which is modulated by calcium signalling [6]. Dephosphorylation of NFATc1 by calcineurin results in nuclear translocation and signalling. The AP-1/NFATc1 transcription complex induces osteoclast differentiation at the genome level. NFATc1 is essential for osteoclastogenesis; it also regulates gene expression of many osteoclast-specific proteins including cathepsin K, tartrate-resistant acid phosphatase, calcitonin receptor and the osteoclast-associated receptor (OSCAR).
A number of other cytokines and growth factors have been shown to be capable of stimulating osteoclast differentiation, including other TNF-family members such as TNFα [7]. TNFα also promotes osteoclast formation and activation by stimulating RANKL production by bone stromal cells and promoting the osteoclastogenic effect of RANKL [5, 8]. RANKL is expressed by activated T cells and activates RANK-expressing dendritic cells. The NFAT transcriptor factor family is expressed by T cells, and there are several parallels between bone and the immune system. OSCAR is an immunoglobulin-like receptor expressed by osteoclasts [9]. Costimulatory signals for RANK include the immunoreceptor tyrosine-based activation motif (ITAM)-adapter proteins FcR γ and DAP 12, which associate with OSCAR. RANKL-RANK signalling and the stimulation of OSCAR cooperatively phosphorylate ITAM, leading to NFATc1 induction and activation [6, 10] (Fig. 1a).
The other major growth factor required for osteoclast formation is M-CSF. This is produced by marrow stromal cells and osteoblasts and is required for the survival and proliferation of osteoclast precursors. M-CSF acts through a specific receptor, c-fms, which is a receptor-type tyrosine kinase that activates several signalling pathways including PI3K/Akt and ERK1/2 [5, 7]. M-CSF also stimulates the expression of RANK in mononuclear phagocyte osteoclast precursors. Other targets for M-CSF include PU.1, which is necessary for the production of marrow precursors of macrophages and osteoclasts, and microphthalmia-associated transcription factors, which promote osteoclast survival. M-CSF also activates c-src, which plays a crucial role in osteoclast attachment and motility.
In carrying out bone resorption, osteoclasts undergo cytoskeletal reorganisation and polarisation with formation of a ruffled border, an area of membrane specialisation in contact with the bone surface. The ruffled border is the resorptive organelle through which acid is secreted into a discrete resorption lacuna. The ruffled border is surrounded by a sealing zone which isolates the area undergoing resorption from surrounding bone; the sealing zone contains podosomes that consist of a core of F-actin surrounded by a rosette-like structure containing the integrin αvβ3 [5]; αvβ3 recognises an Arg-Gly-Asp amino acid motif found in several bone matrix proteins, including osteopontin and bone sialoprotein. Osteoclast motility, bone matrix adhesion and polarisation of the resorptive machinery of the osteoclast all require αvβ3. The proto-oncogene c-src associates with the β3 cytoplasmic domain of unliganded integrin, and mice that lack src contain numerous dysfunctional osteoclasts which lack ruffled borders and actin rings and are therefore not capable of lacunar resorption.
The osteoclast acidifies the extracellular compartment by secreting protons across the ruffled border membrane via a vacuolar-like H+ATPase proton pump [5, 11]. The protons are provided by the enzyme carbonic anhydrase type II, which generates H+ and HCO −3 ions from CO2. Intracellular pH is maintained by a Cl−/HC0 −3 exchanger on the anti-resorptive surface, and electroneutrality is maintained by a chloride channel (ClC-7), located in the ruffled border membrane, which transports Cl− into the resorption lacuna. The organic bone matrix is composed largely of type I collagen; this is degraded by cathepsin K, an acid protease which, like the ion-transporting molecules, polarises to the ruffled membrane when the osteoclast attaches to bone.
The osteoclast and pathologic bone resorption
Factors governing osteoclast formation and function have been shown to play a role in many bone disorders. Osteopetrosis, a heritable sclerosing bone disease resulting from diminished bone resorption, occurs rarely in man, but its many manifestations in mice have provided significant insights into the cellular and molecular pathways of osteoclast formation and function. The most common cause of human osteopetrosis is a mutation in the resorbing mechanism of the cell [12]. Mutations in the genes coding for the A3 subunit of the proton pump vaculoar H+ ATPase (TCIRG1), and the chloride-specific ion channel, chloride channel 7 (CLCN-7) account for most cases of severe osteopetrosis in man. In these forms of osteopetrosis, osteoclasts are present but unable to resorb bone. Mutations in the CAII gene coding for carbonic anhydrase II develop osteopetrosis with mild clinical abnormalities, and mutations in CLCN7 account for most cases of autosomal dominant osteopetrosis. Mutations in the cathepsin K gene result in pycnodysostosis, a disorder characterised by short stature, diffuse osteosclerosis and craniofacial abnormalities. Only rare cases of osteopetrosis due to abnormalities of the RANKL/RANK pathway have been recorded in man.
Abnormalities of RANKL/RANK/OPG modulation of osteoclast formation and function have been shown to play a role in many osteolytic disorders of bone. Activating mutations in exon1 of TNFRSF11A, the gene encoding RANK, are associated with familial expansile osteolysis [12]. Mutations in TNFRS11B, the gene encoding OPG, are associated with idiopathic hyperphosphatasia (juvenile Paget’s disease). Paget’s disease in adults is a focal disorder of bone remodelling characterised by both sclerosis and lysis with increased osteoclast formation and activity. In approximately 30% of cases of familial Paget’s disease and a few sporadic cases, there is an activating mutation in the sequestosome I gene (SQSTM1), an adaptive protein important for activating NFκB, P38 and MAPK. In a recent study, genetic variations in the CSF-1 and TNFRSF11A genes were found to be associated with an increased risk of Paget’s disease. Osteoclast formation in Paget’s disease is hypersensitive to RANKL, and M-CSF is elevated in the circulation of patients with Paget’s disease [13, 14].
Post-menopausal osteoporosis is characterised by increased osteoclast resorption due to estrogen deficiency. In post-menopausal females, estrogen stimulates OPG production by osteoblasts, and marrow stromal cells express high levels of RANKL. Several genetic variations, including multiple single nuclear type polymorphisms in OPG, RANKL and RANK genes, have been associated with low bone mass and bone turnover in osteoporosis. Anti-RANKL treatment has been shown to increase mineral density in osteoporotic patients. It has been suggested that estrogen deficiency may promote osteoclast formation through T cell production of TNFα, which synergistically induces RANKL-mediated differentiation of osteoclast precursors. Steroid-induced osteoporosis is also associated with increased RANKL and decreased OPG expression by osteoblasts. There is decreased bone formation in patients who receive steroids, and it has been proposed that osteoclasts may play a role either directly or indirectly in inhibiting osteoblast function [5, 15].
Marginal bone erosions and juxta-articular osteopaenia are commonly seen in rheumatoid and other inflammatory arthropathies. The inflamed synovium and inflammatory pannus contain synovial fibroblasts and activated T cells, which express RANKL, as well as macrophages, which express RANK. It has been shown that synovial macrophages and synovial fluid macrophages can differentiate into bone-resorbing osteoclasts and that OPG and anti-RANKL antibodies can prevent arthritis-associated bone destruction [16]. Both TNFα and RANKL may play a role in promoting osteoclast differentiation and function in inflammatory arthritis. Osteoclasts and immune cells share common signalling pathways, such as NFκB. Both classical and alternative NFκB pathways are activated in inflammatory arthritis. Classical pathways are activated by most NFκB-inducing cytokines, including RANKL and TNFα, whereas the latter is activated by RANKL but not TNFα. Many of the cytokines and growth factors produced by synovial inflammatory cells, such as TNFα, IL-1, IL-6 and IL-17, enhance osteoclast resorptive activity. Abundant M-CSF is produced in this inflammatory microenvironment, and antibody-mediated blockade of c-fms, the receptor for M-CSF, prevents periarticular bone erosion. TNFα inhibitors are effective in treating rheumatoid disease but are not always curative, perhaps reflecting the role of other factors in promoting RANKL-independent pathways of osteoclast formation.
It has been conclusively shown that osteolysis associated with the growth of primary and secondary tumours in bone is not produced by tumour cells but by osteoclasts. Tumour cells produce numerous cytokines and growth factors that promote osteoclast formation and function, including prostaglandins, TNFα, IL-6, IL-8, IL-11 and parathyroid hormone-related peptide (PTHrP). Many of these factors promote RANKL expression by osteoblasts and, in the tumour microenvironment, there is an increase in the RANKL:OPG ratio. Both primary and secondary metastatic tumours of bone have a significant tumour-associated macrophage (TAM) population, and these TAMs are capable of osteoclast differentiation [7]. Physical factors such as hypoxia, acidic pH and extracellular calcium modulate the growth and activity of bone and tumour cells in primary and secondary bone tumours. Osteoclast resorption results in the release of matrix-derived growth factors which promote osteoclast formation and activity. Transforming growth factor β (TGF-β) is abundant in the bone matrix; it stimulates cancer cells to produce PTHrP and other cytokines which promote osteoblast RANKL expression; this leads to a vicious cycle of tumour osteolysis resulting in TGF-β release from the bone matrix, consequent increased osteoblast RANKL expression, and stimulation of osteoclastogenesis and osteolysis [17]. It has also been shown by some investigators that a number of tumour cells, including myeloma, breast cancer, Ewing and other sarcoma cells can express RANKL and in this way may directly promote RANKL-mediated osteoclast formation and function. Giant cell tumour of bone is characterised by the presence of numerous large osteoclast-like giant cells and mononuclear cells that include macrophage-like osteoclast precursors and RANKL-expressing stromal cells [7]. Recently denosumab, a human monoclonal antibody that binds with high affinity and specificity to RANKL, has been used in the treatment of giant cell tumour of bone [18]. This treatment may also find applications in other giant cell-rich lesions of bone and synovium (e.g. pigmented villonodular synovitis) in which there is RANKL expression and pathological bone resorption.
Conclusion
Progress in understanding the cellular and molecular mechanisms of osteoclast formation and function have led to the identification of key molecules which play a role in pathological bone resorption. Further investigation of the signalling pathways governing osteoclast generation, motility, matrix attachment and resorption is likely to lead to the development of new therapies to treat osteolytic bone and joint disorders.
References
Athanasou NA. Cellular biology of bone-resorbing cells. J Bone Joint Surg Am. 1996;78:1096–112.
Lacey DL, Timms E. Tan HL, Kelley MJ, Dunstan CR, Burgess T et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76.
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Miner Res. 2000;15:2–12.
Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42.
Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am J Pathol. 2007;170:427–35.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–64.
Knowles HJ, Athanasou NA. Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol. 2009;24:337–46.
Lam J, Takeshita S, Barker JE, Kenagawa O, Ross FP, Teitelbaum SL, et al. TNFα induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–88.
Merck E, Gaillard C, Gorman DM, Montero-Julian F, Durand I, Zurawhis M, et al. OSCAR is an FcRgamma-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood. 2004;104:1386–95.
Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–63.
Bruzzaniti A, Baron R. Molecular regulation of osteoclast activity. Rev Endocr Metab Disord. 2006;7:123–39.
Helfrich MH. Osteoclast diseases. Microsc Res Tech. 2003;61:514–32.
Roodman GD, Windle JJ. Paget disease of bone. J Clin Invest. 2005;115:200–8.
Singer FR, Leach RJ. Do all Paget disease risk genes incriminate the osteoclast? Nat Rev Rheumatol. 2010;6:502–3.
Weitzmann MN, Pacifici R. Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest. 2006;116:1186–94.
Karmakar S, Kay J, Gravallese MEM. Bone damage in rheumatoid arthritis: mechanistic insights and approaches to prevention. Rheum Dis Clin North Am. 2010;36:385–404.
Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011;48:23–9.
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, et al. Denosumab in patients with giant cell tumour of bone: an open-label phase 2 study. Lancet Oncol. 2010;11:275–80.
Acknowledgements
N.A.A. is a member of the EuroBoNet consortium, a Network of Excellence funded by the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Athanasou, N.A. The osteoclast—what’s new?. Skeletal Radiol 40, 1137–1140 (2011). https://doi.org/10.1007/s00256-011-1180-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-011-1180-9