Abstract
Purpose
This study was performed to assess the imaging findings in cases of parosteal osteosarcoma dedifferentiated into telangiectatic osteosarcoma. Parosteal osteosarcoma is a low-grade well-differentiated malignant tumor. Dedifferentiation into a more aggressive lesion is frequent and usually visible on imaging as a central lytic area in a sclerotic mass. Only one case of differentiation into a telangiectatic osteosarcoma has been reported. As it has practical consequences, with a need for aggressive chemotherapy, we looked for this rather typical imaging pattern.
Materials and methods
Review of 199 cases of surface osteosarcomas (including 86 parosteal, of which 23 were dedifferentiated) revealed lesions suggesting a possible telangiectatic osteosarcoma on imaging examinations in five cases (cavities with fluid). Histology confirmed three cases (the two other only had hematoma inside a dedifferentiated tumor). There were three males, aged 24, 28, and 32. They had radiographs and CT, and two an MR examination.
Results
Lesions involved the distal femur, proximal tibia, and proximal humerus. The parosteal osteosarcoma was a sclerotic, regular mass, attached to the cortex. A purely lytic mass, partially composed of fluid cavities was easily detected on CT and MR. It involved the medullary cavity twice, and remained outside the bone once. Histology confirmed the two components in each case. Two patients died of pulmonary metastases and one is alive.
Conclusion
Knowledge of this highly suggestive pattern should help guide the initial biopsy to diagnose the two components of the tumor, and guide aggressive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parosteal osteosarcoma is a well-differentiated, low-grade variant of osteosarcoma with good survival (about 90% of patients after 5 years). In our files, it accounts for 4.8% of all osteosarcomas. Dedifferentiation is a biologic phenomenon in which a high-grade sarcoma arises on a low-grade malignancy caused by a mutation that produces a high-grade malignant clone. About 15% of parosteal osteosarcomas show foci of dedifferentiation, usually evidenced by the onset of central radiolucencies. Dedifferentiation is usually towards a high-grade spindle cell sarcoma, or a high-grade surface osteosarcoma. Only one case of differentiation into a telangiectatic osteosarcoma has been reported [1]. We report three cases of dedifferentiated parosteal osteosarcoma with teleangiectatic osteosarcoma appearance and describe the clinicopathological and radiographic features of the tumors. Early recognition of these tumors would allow a better yield of the biopsy as well as for planning a more aggressive treatment.
Materials and methods
A systematic review of 199 patients diagnosed as surface osteosarcoma in our institution from 1916 to 2003 was performed by a radiologist and a pathologist. We identified 86 patients with parosteal osteosarcoma and among them, 23 dedifferentiated. Radiological review revealed five possible cases of telangiectatic dedifferentiation because of fluid cavities within the tumor mass on CT or MRI. Only one case had been diagnosed on the initial histological report, but the histopathology review of the five cases confirmed three dedifferentiation into a telangiectatic osteosarcoma, while the two other patients only had a hematoma inside the dedifferentiated part.
Results
There were three men aged 24–32 years. Two had a 2-month history of pain. The third had pain for 27 months, which became worse 5 months before the diagnosis. He was treated for phlebitis for 1 year. All had a hard, non-mobile mass. On radiographs and CT, a two-component lesion was displayed. The sclerotic part was typical of a parosteal osteosarcoma, and the lytic part corresponded to a dedifferentiation histologically. In five cases, cavities were diagnosed inside the lytic component on CT. They were multiple in three, and huge and unique in two. Fluid–fluid levels were easily recognized on one MR performed, with more difficulties on another. They were difficult to characterize on the old quality CT examinations. Histologically, the three patients with multiple small fluid cavities actually had a telangiectatis osteosarcoma, whereas the two with a big cavity, an intra-tumoral hematoma. The two patients who had neoadjuvant chemotherapy and conservative treatment died rapidly with pulmonary metastases, the third who had an amputation and post-operative chemotherapy is alive and free of disease 13 years later (Table 1; Figs. 1, 2, 3).
On radiographs (a lateral view), CT (b CT guided biopsy), and MR (c T2W sagittal image), the two components of the tumor are well visible: a dense part corresponding to the parosteal well-differentiated tumor, and the telangiectatic part, lytic on radiograph and CT, and with obvious fluid–fluid levels on MR. Histologically, the tumor shows proliferation of spindle cells in between well-formed bony trabeculae. The spindle cell shows marked atypia and are considered to be grade 2 according to Broder’s grading system. The major component of the tumor is a high-grade osteosarcoma with diffuse telangiectatic features. It is made of fibrous septae with sarcomatous cells separating wide lacunae full of blood. In these areas, the production of osteoid is scanty and not obvious (d HES, ×20, e HES, ×40)
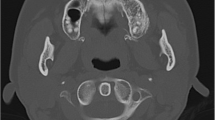
On radiographs (a AP view of the shoulder) and CT axial images (b, c), the two components are well separated: a dense distal, and a lytic proximal lesion. The latter contains multiple fluid cavities. The involvement of the medullary cavity is easily demonstrated (b). Histologically (d HES, ×40), the hard surface component of the tumor is a typical grade 1 parosteal osteosarcoma with bland spindle cells and well-formed bony trabeculae, while the hemorrhagic areas has the features of a telangiectatic osteosarcoma
On radiographs (a lateral view), the sclerotic distal and lytic proximal components of the lesion are easily separated. On CT (b), the sclerotic part is at the periphery of the lesion and the lytic part central. On MR (c sagittal T2W view), multiple fluid cavities with some fluid–fluid levels are detected. The medullary cavity is completely involved by the dedifferentiated part of the tumor. Histologically (d HES, ×40), the tumor was composed of classic parosteal grade 1 areas, areas with fibroblastic high-grade osteosarcoma areas and telangiectatic components
Discussion
Parosteal osteosarcoma (POS) is a well-differentiated, low-grade variant of osteosarcoma which arises from the surface of the bone. It was first described as a new and separate entity by Geschickter and Copeland in 1951, and was named parosteal osteoma [2]. This lesion is uncommon, and only represents 5% of all osteosarcomas. The age of presentation is between 20 to 40 years, thus more typical in adults than in teenagers, with a slight predominance in females, unlike the classic osteosarcoma [3]. Symptoms include a slow-growing, hard mass with no or slight pain, frequently associated with limitation of joint motion due to mechanical blockage. The tumor usually involves long-bone metaphysis, mostly the posterior aspect of distal femur and proximal humerus [4]. The classic radiological feature is a densely mineralized, amorphous mass attached to the cortex by a broad base [5]. The lesion is hot on bone scintigraphy. Macroscopically, it presents as a hard lobulated mass attached to the underlying cortex. Peripheral cap of cartilage may be present [6]; therefore, the lesion can be misinterpreted as osteochondroma or heterotopic ossification. Microscopically, it consists of well-formed bony trabeculae seen in a hypocellular stroma with minimal atypia of spindle cells [6]. The bony trabeculae may show osteoblastic rimming. The treatment is surgery with wide surgical margin. POS has good survival, however, if the lesion is not adequately resected, local recurrence is likely to develop. It can occur on the surface of bone or in the soft tissue [3]. Okada et al. evaluated the 5-year survival of 39 patients at 91% and the 10-year survival at 80% [7].
Telangiectatic osteosarcoma (TOS) is a high-grade variant of osteosarcoma, with a rapidly progressive course. It was first described by Paget in 1854 [8]. It accounts for about 9% of high-grade osteosarcoma with male sex preponderance and mostly occurs in younger age group between 10 and 20 years [3]. Patients may complain of rapidly increasing mass which is painful with increased local temperature. Radiograph shows a purely lytic lesion with ill-defined margin and destruction of the cortex. Sometimes, this lesion can be misdiagnosed as an aneurysmal bone cyst or giant cell tumor. CT and MRI reveal multicystic lesions with fluid–fluid levels. Isotope scan will show increase uptake beyond the osteolysis but cold area at the center due to fluid collection. Angiogram shows intense vascularity, but the findings can be adverse due to massive necrosis or hemorrhage. Treatment consists of neoadjuvant chemotherapy, surgery and adjuvant chemotherapy. Mervak et al. concluded that TOS has a similar prognosis as conventional osteosarcoma [9].
When a parosteal osteosarcoma dedifferentiates, it means that part of the tumor has transformed into a higher-grade lesion. This dedifferentiation can occur at the time of presentation (synchronous type) or at the time of recurrence (metachronous type) [10]. The early report by Unni et al in 1976 described seven cases with coexistent areas of high-grade malignancy in POS [11]. The latest review series by Bertoni et al. in 2005 described 29 cases of dedifferentiated POS (dd-POS) from 120 POS. Fourteen cases dedifferentiated into an osteoblastic, ten a fibroblastic, three a giant cell-rich and two a fibroblastic high-grade osteosarcoma [9]. Literature review revealed that telangiectatic dedifferentiation of POS was only described once, by A. Wines et al. in 2000. The case report highlighted a 28-year-old woman who was diagnosed to have telangiectatic dedifferentiation of POS on histological examination of the resected specimen [1]. Although it seems contradictory to the definition of dedifferentiation (which involves the development of a completely different tumor), low-grade parosteal osteosarcoma is different genetically and biologically from a high-grade osteosarcoma (including telangiectatic).
In our series, the three telangiectatic dedifferentiations were synchronous, patients were males with a mean age of 28. Lesions involved long-bone metaphyses. A central radiolucency in the highly mineralized areas, indicating dedifferentiation, as well as multiple fluid–fluid levels suggesting telangiectatic dedifferentiation were found on CT and/or MR examinations. They were better seen on MR examinations. The correct histological diagnosis was made immediately only once on the histology, and corrected retrospectively in two more cases after review. Bertoni et al., in 1985, compared radiological and histological findings of 18 parosteal osteosarcoma patients with intralesional radiolucencies within the tumor. They found that the majority of high-grade dedifferentiated areas of tumors corresponded to deep radiolucencies [12].
The prognostic implications of intramedullary extension of parosteal osteosarcoma remains controversial. Campanacci et al., in 1984, reported that 33% of grade I POS, 65% of grade II POS, and 90% of grade III POS had medullary involvement. Their series did not have metastasis without involvement of the medullary cavity. They suggested that POS with medullary involvement is more aggressive [13]. Other authors however concluded that medullary canal invasion cannot be considered a sign of aggressiveness. Sheth et al., in 1996, found no significant prognosis difference in medullary canal invasion [14]. The Rizzoli group in 2005 found medullary canal invasion in 65% (19 patients) of 29 dedifferentiated POS. Eight of nine patients who died had medullary canal invasion, however, this invasion was also present in 11 patients without evidence of disease after a long follow-up [9].
In our center, a patient diagnosed with dedifferentiated POS, is considered as a high-grade osteosarcoma. The treatment regime will follow the osteosarcoma regimen. The adjuvant chemotherapy is given to patients with dd-POS after histopathological post-tumor resection confirmation. If the histology of dedifferentiation could be confirmed earlier during biopsy, a more aggressive treatment with neoadjuvant chemotherapy could be planned. Therefore, the ability to detect telangiectatic dedifferentiation in POS can contribute to a better yield from the biopsy and better planning from more aggressive treatment.
References
Wines A, Bonar F, Lam P, McCarthy S, Stalley P. Telangiectatic dedifferentiation of a parosteal osteosarcoma. Skeletal Radiol 2000; 29: 597–600.
Geschickter CF, Copeland MM. Parosteal osteoma of bone. A new entity. Ann Surg 1951; 133: 790–807.
Campanacci M. Bone and soft tissue tumours, 2nd ed. New York: Springer, 1999. 491–548.
Mirra JM. Bone tumours: clinical, radiologic and pathologic correlations. Philadelphia: Lea and Febiger, 1989.
Fechner RE, Mills SE. Tumours of the bones and joints. Washington DC: Armed Forces Institute of Pathology, 1993.
Christopher DM, Fletcher K, Unni K, Martens F. Pathology and genetics of tumours of soft tissue and bone. World Health Organization Classification of Tumours: WHO 2002. 279-281.
Okada K, Frassica FJ, Sim FH, Beabout JW, Bond JR, Unni KK. Parosteal osteosarcoma: a clinicopathological study. J Bone Joint Surg Am 1994; 76: 366–378.
Paget J. Lectures on surgical pathology. Philadelphia: Lindsay and Blackiston, 1854.
Mervak TR, Unni KK, Pitchard DJ, Mc Leod RA. Telangiectatic osteosarcoma. Clin Orthop 1991; 270: 135–139.
Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer 2005; 103(11): 2373–2382.
Unni KK, Dahlin DC, Beabout JW, Ivins JC. Parosteal osteogenic sarcoma. Cancer 1976; 37: 2466–2475.
Bertoni F, Present D, Hudson T, Enneking WF. The meaning of radiolucencies in parosteal osteosarcoma. J Bone Joint Surg 1985; 67(6): 901–910.
Campanacci M, Picci P, Gherlinzoni F, Guerra A, Bertoni F, Neff JR. Parosteal osteosarcoma. J bone Joint Surg Br 1984; 66: 313–321.
Sheth DS, Yasko AW, Raymond AK, et al. Conventional and dedifferentiated parosteal osteosarcoma: diagnosis, treatment and outcome. Cancer 1996; 78: 2136–2145.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azura, M., Vanel, D., Alberghini, M. et al. Parosteal osteosarcoma dedifferentiating into telangiectatic osteosarcoma: importance of lytic changes and fluid cavities at imaging. Skeletal Radiol 38, 685–690 (2009). https://doi.org/10.1007/s00256-009-0672-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0672-3