Abstract
Angiomatoid fibrous histiocytoma is a rare soft tissue tumor of low-grade malignancy. We present the case of a 32-year-old man who complained of soreness and numbness over his left arm and hand over the previous 2 months and of having a palpable mass over his left upper back for 4 years. Magnetic resonance imaging (MRI) showed an intramuscular soft tissue mass in the left scapular region. The tumor mass was seen to have multiple cystic components with fluid-fluid levels. Histological examination showed multiple cystic spaces filled with blood lakes and hemosiderin deposits in the solid part of the tumor. After the initial surgery, the patient had local recurrences over 2.5 years. The immunohistochemical study at the second surgery showed that the recurrent tumor was strongly positive for the histiocytic marker CD68, and the myoid trait desmin. Histological diagnosis was compatible with angiomatoid fibrous histiocytoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiomatoid fibrous histiocytoma is a rare soft tissue tumor. Enzinger in 1979 [1] first designated the tumor as angiomatoid malignant fibrous histiocytoma. The tumor was later renamed angiomatoid fibrous histiocytoma because of its slow growth and rare metastasis [2]. To our knowledge, only one case of angiomatoid fibrous histiocytoma with magnetic resonance imaging (MRI) findings has been described [3]. We present a case of angiomatoid fibrous histiocytoma occurring in the muscles about the scapula with striking MRI findings.
Case report
A 32-year-old man complained of soreness and numbness over his left arm and hand over the past 2 months and of having a palpable mass over his left upper back for 4 years. Physical examination revealed a tender mass in the left scapular region, limitation of range of motion of the shoulder joint, and a normal deep tendon reflex. Laboratory examination revealed anemia (hemoglobin 7.3 gm/dl), normal serum carcinoembryonic antigen, alpha-fetoprotein and CA-199, and normal complete coagulation profiles.
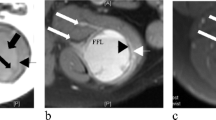
Computed tomography (CT) showed a lobulated soft tissue mass with heterogeneous density at the left scapula (Fig. 1A). MRI (1.5 T, Gyroscan ACS-NT, Philips, The Netherlands) showed a 10×10×5 cm intramuscular soft tissue mass in the left scapular region (Fig. 1B―D). The mass was seen to have multiple cystic components with fluid-fluid levels indicative of layered serum. Foci of low signal intensity were noted on both T1- and T2-weighted images, suggesting hemosiderin deposits. Gadolinium-enhanced T1-weighted images showed no enhancement of the presumed areas with hemosiderin deposition.
A Contrast-enhanced CT scan shows a soft tissue tumor with hypodense components (arrows) in the left scapular region. Note that there is no enhancement of the tumor. B Axial T1-weighted (TR/TE=554/15 ms) and C T2-weighted (TR/TE=5091/100 ms) MR images show a multiloculated soft tissue mass with fluid-fluid levels indicative of layered blood serum. The high-signal-intensity layer (curved arrows) represents serum in the nondependent position. A focal low-signal-intensity area (open arrow) is noted on both T1- and T2-weighted images, indicative of hemosiderin deposits. D Contrast-enhanced T1-weighted (TR/TE=916/15 ms) MR image with fat saturation shows no enhancement of the soft tissue mass. The high signal intensity of the cystic component (arrow) is an effect of fat suppression. E Gross specimen (cut surface) shows a lobulated mass containing multiple cystic spaces filled with blood clot. F Photomicrograph shows multiple cystic spaces of blood lakes, and hemosiderin deposits (arrow) in the solid part of the tumor (H&E, ×20). G Dense fibrous tissue (fibrous pseudocapsule, thick arrows) is noted in the periphery of the tumor. Lymphoplasmacytic infiltrate with randomly distributed lymph follicles with germinal center formation (arrow) is also noted (H&E, ×10). H The solid part of tumor is composed of irregular sheets of histiocyte-like cells with dense neutrophilic and lymphoplasmacytic infiltrate. The blood-filled cystic spaces are lined by tumor cells (H&E, ×20). I The tumor cells have eosinophilic cytoplasm, round to oval nuclei, vesicular chromatin pattern, nuclear indentation or grooving, and the tumor is considered to be a histiocytic lesion (H&E, ×40). J Immunohistochemical stain for CD68 shows a positive result (brown color in cytoplasm) (peroxidase-antiperoxidase immune complex method). K Desmin expression is also noted (brown color in cytoplasm) (peroxidase-antiperoxidase immune complex method)
The tumor was widely excised following the preoperative diagnosis of hemorrhagic soft tissue tumor. At surgery, a circumscribed, red-brown and elastic soft tissue mass was encountered. The cut surface of the tumor revealed grayish solid tissue with multiple intervening variable-sized, blood-filled cystic spaces (Fig. 1E). On microscopy, the most striking features were variable-sized blood lakes in the cystic component and prominent hemosiderin deposition in the hypocellular fibrous tissue of the solid part (Fig. 1F).
One year later, local recurrence and regional metastasis to the left subclavicular region occurred. At the second operation, the recurrent tumor was noted to have more solid and cellular parts than the tumor at the initial operation (Fig. 1G―I). The tumor was composed of relatively bland-looking spindle and round tumor cells, mixed with intense infiltrates of plasma cells and lymphocytes. The tumor cells were strongly positive for the histiocytic marker CD68 and the myoid trait desmin on immunohistochemical study (Fig. 1J, K). The histological diagnosis was angiomatoid fibrous histiocytoma.
After the initial surgery, the patient experienced local recurrences over 2.5 years. He received wide excision and adjuvant radiotherapy four times in this period.
Discussion
Angiomatoid fibrous histiocytoma is a low-grade neoplasm which primarily affects children and young adults. Eighty-eight percent of patients are 30 years of age or younger [4]. The tumor usually occurs in a superficial location and most commonly in the extremities, followed by the trunk and head and neck. Symptoms of anemia, weight loss, and fever are observed in a minority of cases.
Accurate preoperative diagnosis of angiomatoid fibrous histiocytoma is difficult. CT and MRI findings of angiomatoid fibrous histiocytoma can be similar to those of malignant fibrous histiocytoma [5]. MRI is superior to CT in demonstrating fluid-fluid levels within multiple cystic components of the tumor, indicative of intralesional hemorrhage [3]. The fluid-fluid levels are nonspecific findings that may occur in malignant fibrous histiocytoma, soft tissue hemangioma, and hematoma [6]. Malignant fibrous histiocytoma, however, usually occurs in older adults. An entire lesion filled with blood is not typical for malignant fibrous histiocytoma, which may have more enhancing tissue about the hemorrhagic region.
The diagnosis of angiomatoid fibrous histiocytoma is made on the basis of histopathology and immunohistochemical studies [7, 8]. Four histological features are commonly identified. These are a fibrous pseudocapsule, round or spindle fibrohistiocytic proliferation, a pseudoangiomatous pattern, and plasmalymphocytic response. Pseudovascular blood-filled spaces have been observed in 50% of cases [9]; this can be related to multiloculated cystic components of the tumor, as shown on MRI in our case. Smith and associates [10] reported that the histiocytic marker CD68 was positive in 9 of 19 (47%) cases of angiomatoid fibrous histiocytoma. Immunopositivity for myoid or myofibroblastic markers in more than 50% of cases has also been reported [9].
Local recurrence has been reported in 11% of patients and distant metastasis in 1%; wide excision is recommended as the treatment of angiomatoid fibrous histiocytoma [4, 7]. Local recurrence is attributed to the infiltrative margin and deep location of the tumor, as in our case which had a deep location. Angiomatoid fibrous histiocytoma in the head and neck also can frequently recur, which may be a result of the difficulty of performing a wide local excision [4].
In summary, angiomatoid fibrous histiocytoma is a rare soft tissue tumor with low-grade malignancy. Although it is a nonspecific finding, angiomatoid fibrous histiocytoma should be included in the differential diagnosis in young patients having a soft tissue tumor with multiple fluid-fluid levels occurring in the extremities and trunk. Tumor location and clear surgical margins are important factors affecting local recurrence or metastasis.
References
Enzinger FM. Angiomatoid malignant fibrous histiocytoma: a distinct fibrohistiocytic tumor of children and young adults simulating a vascular neoplasm. Cancer 1979; 44:2147–2157.
Enzinger FM, Weiss SW. Fibrohistiocytic tumors of intermediate malignancy. In: Enzinger FM, Weiss SW, eds. Soft tissue tumors. St Louis: Mosby-Year Book, 1995:325–349.
Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologic-pathologic correlation. Radiographics 1994; 14:807–826.
Costa MJ, Weiss SW. Angiomatoid malignant fibrous histiocytoma: a follow-up study of 108 cases with evaluation of possible histologic predictors of outcome. Am J Surg Pathol 1990; 14:1126–1132.
De Beuckeleer L, Fibrohistocytic tumors. In: De Schepper AM, Parizel PM, De Beuckeleer L, Vanhoenacker F, eds. Imaging of soft tissue tumors. Berlin Heidelberg New York: Springer, 2001:181–193.
Tsai JC, Dalinka MK, Fallon MD, Zlatkin MB, Kressel HY. Fluid-fluid level: a nonspecific finding in tumors of bone and soft tissue. Radiology 1990; 175:779–782.
Grossman LD, White RR 4th, Arber DA. Angiomatoid fibrous histiocytoma. Ann Plast Surg 1996; 36:649–651.
Asakura S, Tezuka N, Inoue S, Kihara N, Fujino S. Angiomatoid fibrous histiocytoma in mediastinum. Ann Thorac Surg 2001; 72:283–285.
Fanburg-Smith JC, Miettinen M. Angiomatoid “malignant” fibrous histiocytoma: a clinicopathologic study of 158 cases and further exploration of the myoid phenotype. Hum Pathol 1999; 30: 1336-1343.
Smith ME, Costa MJ, Weiss SW. Evaluation of CD68 and other histiocytic antigens in angiomatoid malignant fibrous histiocytoma. Am J Surg Pathol 1991; 15: 757-763.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, CS., Chan, W.P., Chen, WT. et al. MRI of angiomatoid fibrous histiocytoma. Skeletal Radiol 33, 604–608 (2004). https://doi.org/10.1007/s00256-004-0769-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-004-0769-7