Abstract
Agricultural soils of the Riotinto mining area (Iberian Pyrite Belt) have been studied to assess the degree of pollution by trace elements as a consequence of the extraction and treatment of sulphides. Fifteen soil samples were collected and analysed by ICP-OES and INAA for 51 elements. Chemical analyses showed an As–Cu–Pb–Zn association related with the mineralisation of the Iberian Pyrite Belt. Concentrations were 19–994 mg kg−1 for As, 41–4,890 mg kg−1 for Pb, 95–897 mg kg−1 for Zn and of 27–1,160 mg kg−1 for Cu. Most of the samples displayed concentrations of these elements higher than the 90th percentile of the corresponding geological dominium, which suggests an anthropogenic input besides the bedrock influence. Samples collected from sediments were more contaminated than leptosols because they were polluted by leachates or by mining spills coming from the waste rock piles. The weathering of the bedrock is responsible for high concentrations in Co, Cr and Ni, but an anthropogenic input, such as wind-blown dust, seems to be indicative of the high content of As, Cu, Pb and Zn in leptosols. The metal partitioning patterns show that most trace elements are associated with Fe amorphous oxy-hydroxides, or take part of the residual fraction. According to the results obtained, the following mobility sequence is proposed for major and minor elements: Mn, Pb, Cd, > Zn, Cu > Ni > As > Fe > Cr. The high mobility of Pb, Cu and Zn involve an environmental risk in this area, even in soils where the concentrations are not so high.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extraction of mineral resources brings with it a series of environmental alterations that can at times have very negative consequences (Merefield 1995; Salomons 1995; Gäbler and Schneider 2000; González 2003; Lee 2003). In Spain, the exploitation of metallic mineral deposits and its subsequent abandonment in recent years, particularly in the Iberian Pyrite Belt (in the southwest of the Iberian Peninsula), has had some broad repercussions. The intensive mining activity in the Riotinto area since prehistoric times (Blanco and Rothenberg 1981) has generated large amounts of scoria, pyrite ashes and other leachable materials which continue to cause acidic waters and contaminating elements to enter the Tinto and Odiel River basins (Fernández Caliani and Galán 1996; González et al. 2004; Romero et al. 2006). The abandoned mine spoils not only affects the quality of the water, but also may cause the pollution of soils by wind-blown dust (Chopin et al. 2003) or by water as a consequence of leachates or spills (Gäbler and Schneider 2000; Galán et al. 2002).
In addition to the problems of air, water and soil pollution, the abandoning of the mines in the Pyrite Belt (South Portuguese Zone) has had an important socioeconomic impact; resulting in a great loss of employment. This has led to the growth of agriculture as the new base for social development, with large planting of citrus and other fruit-bearing trees around the old waste rock piles. Correspondingly, the traditional subsistence agriculture is usually developed on floodplains near the mining sites. The level of soil pollution has not been taken into account in these cases, nor has its impact on the health of the inhabitants of these areas. At present, the only works related to this subject are those by Chopin et al. (2003) and Barba Brioso et al. (2006). Therefore, in agricultural areas close to the abandoned mines, it is of great interest to know the current state of the soils and the sources and types of contaminants derived from mining activity in order to programme its decontamination, where appropriate, according to legislation.
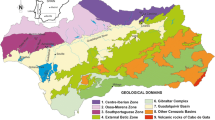
In this work, potentially contaminated soils surrounding several mines of the Iberian Pyrite Belt have been studied (Fig. 1). The trace element content has been assessed and the maximum values allowed (contamination threshold) have been compared, taking into account the results obtained by authors of other countries (Finnecy and Pearce 1986; Barth and L’Hermite 1987; Kabata Pendias and Pendias 1992; Azcue 1993; Cambier 1994; Alloway 1995; Kabata Pendias 1995; Adriano 1997; Baize 1997; Cano Parrilla et al. 1997; Llamas et al. 2000; Martín 2000; Chen et al. 2001; Navas and Machín 2002; Tarvainen and Kallio 2002) and also the regional geochemical baseline of Andalusia (http://www.juntadeandalucia.es/medioambiente/site/web/). The background levels for the geological setting (South Portuguese Zone) have also been taken into account owing to the great influence that lithology, mineralogy and granulometry have on these levels (Gregorauskine and Kadunas 1997; Salminen and Tarvainen 1997; Salminen and Gregorauskiene 2000).
In order to know the present and future hazard posed by the soils, chemical and mineralogical speciation has been carried out, given that the availability of a metal depends on the phase in which it is found (McLean and Bledsoe 1992; Pérez et al. 2000; Galán 2003a). Bioavailability has also been assessed. This will permit the degree of actual contamination to be evaluated for contaminated soils and sediments (Lindsay 1979; Plant and Raiswell 1983; Chao 1984; Bohn et al. 1985; Kloke and Einkmann 1991; Kabata-Pendias and Pendias 1992; Van der Berg et al. 1993; O’Neill 1995; López Julián and Mandado Collado 2002).
The aim of this work is to determine the content of potentially toxic elements in the soils studied and to evaluate the consequential environmental problems. The following specific objectives have been covered: (a) chemical and mineralogical characterisation of the soils, (b) determination of potentially toxic trace element content, and (c) chemical and mineralogical speciation of the trace elements and their bioavailability. With the results obtained the potential risk incurred in the agricultural use of the studied soils has been assessed.
Materials and methods
Fifteen surface samples (0–20 cm deep) of agricultural soils were taken in the Iberian Pyrite Belt. In this area, the bedrock is composed of shales and volcanic rocks, as these are the lithological characteristics of the South Portuguese Zone (Iberian Massif) (Leistel et al. 1998), and the soils developed are usually leptosols, cambisols and luvisols. The samples were taken at random in areas close to the mines or which might have been affected by them. Some of them were taken from small-scale orchards located over quaternary sediments and with a well-developed soil cover. Those locations were Cueva de la Mora, Valdelamusa, El Lomero, Tharsis, Sotiel, and Las Delgadas (Fig. 1). Other samples come from large plantations of citrus usually located on leptosols developed over shales such us Peña de Hierro, Riotinto and Monte Sorromero. Sometimes, this intensive agriculture also covers the valleys, such as the samples taken in Embalse Zumajo. In order to assess the bedrock’s influence on the chemical characteristics of the soils, several samples of shales and volcanic rocks were collected for to be analysed. Given that the mobility of trace elements depends directly on the characteristics of the soil in which they are found (Cambier 1994; Galán 2003b), the following edaphic parameters were determined: pH, texture and mineralogy of clays.
Determination of pH was performed using a Microph 2002 (Crisson) pH-meter, previously calibrated with two buffer solutions of pH = 4 and pH = 7. In order to perform measurements, 10 g of sample was mixed with 25 ml distilled water in a mechanical shaker for 10 min, and the pH was read after 30 min of the solution at rest.
Major, minor and trace elements analyses were performed by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) and Instrumental Neutron Activation Analysis (INAA) at Activation Laboratories Ltd. (1428 Sandhill Drive, Ancaster, Ontario, Canada, http://www.actlabs.com).
Mineralogical analysis was performed by X-Ray Diffraction (XRD), using a Bruker D8 Advance powder device with Cu anticathode and standard conditions of speed 2° 2θ/min between 3° and 70° at 30 mA and 40 KV. The study of the total sample was made by crystalline powder diffraction (non-oriented powder) on a side-loading sample holder. Bulk quantitative analyses were based on the Schultz (1964) method after correcting intensities for the automatic slit. Clay-minerals were studied in oriented aggregates using standard methods involving drying at room temperature, solvation with ethylene glycol and heating to 550°C for 2 h. Phase abundances were semi-quantitatively estimated according to mineral intensity factor proposed by Martín-Pozas et al. (1969).
Chemical speciation was carried out according to the methodological scheme given by Tessier et al. (1979). Sequential extraction involves several stages of chemical extraction in which reagents of different strength are used to separate the metallic species into the different fractions (Table 1).
In the mineralogical speciation, seven samples were selected for their content in trace metal, iron oxide and phyllosilicate, and type of interstratified minerals, so as to observe their behaviour and the mineralogical evolution, once the sequential extraction had been performed. The existing mineral phases were identified in the solid residues obtained from the different extractions carried out following Tessier’s protocol.
In order to obtain the bioavailability of trace elements the samples were treated with a solution of EDTA 0.05 M in the form of sodium salt and at pH = 7 with continuous shaking for 1 h. The resulting extract was analysed with spectrophotometric techniques (in the same way as Tessier’s different fractions).
In order to obtain metals extractable in acidic water, the samples were treated with water at pH = 1 (acidified with HNO3), with continuous shaking for one hour. The extract, in the same way as the previous case, was analysed using spectrophotometric techniques.
A statistical processing was performed on the data from the surface samples with the aim of corroborating interelement links observed between elements with similar geochemical behaviour. Specifically, correlation and principal component-analyses were accomplished.
Results and discussion
Physico-chemical characterisation
The pH values of the soil samples range from 7.6 to 3.6 (Table 2). The most acidic values correspond to samples collected in the immediate vicinity of the Peña de Hierro mine (pH = 3.6, 4.2). Also showing important acidity are those samples collected at Monte Sorromero (pH = 4.9, 5.5). Those acidic pH values seem to originate from the bedrock, as those soils are leptosols and no other source of acidity has been found. Samples collected at Tharsis (pH 5–6) are well-developed soils, and the pH does not seem to be influenced by the bedrock. In this case, the source seems to be the leachates or the erosion of the adjacent waste mining piles. The rest of pH values were above 6. The values for pH are of great significance given that the concentration of hydrogen ions modifies the availability of trace elements and of nutrients, and in addition, can indicate deficiencies or alterations in the soil (Wen and Allen 1999; Galán 2003a). Most of the studied metals tend to be more available at acidic pH, with the exception of As, Mo and Cr, which are more mobile at alkaline pH. In general, the content in divalent metals such as Cu, Zn, Ni, Co, Cd and Mn decreases when pH increases (Cama et al. 2005). For this reason, the samples that may have greater availability are those which have lower pH.
Chemical analyses show values for silica ranging between 50 and 70% and for alumina between 10 and 19% (Table 2). The iron and manganese content of these soils is important owing to the fact that oxides and hydroxides of these elements, if found dispersed in the soil mass, can be very active and have a great sorption capacity for some metals (Cu, Pb) and As (Galán et al. 2003). Iron content in the studied samples ranges from 5 to 13%. The samples from Tharsis, Sotiel, Embalse Zumajo and the sample M14 in Monte Sorromero show Fe2O3 concentrations higher than the mean (8.0%). Other oxides such as CaO, K2O, MgO and Na2O are under 5%. The loss on ignition (LOI) ranges from 7 to 15%.
There were 50 minor and trace elements analysed, although only 7 were chosen for study (As, Cd, Co, Ni, Cu, Pb and Zn) owing to their abundance in soils (Bowen 1979), because they are easily mobilised (Novotny 1995), and also because they are included in the U.S. Environmental Protection Agency (EPA) priority contaminant list. Content of As ranged between 19 and 994 ppm (Table 3). The highest values appear in the samples from Tharsis (994 ppm) and Sotiel (882 ppm), which are well over the mean (227 ppm, Table 4). Lead-content oscillates between 40 and 4,900 ppm. The highest values correspond to samples from Tharsis (2,650 ppm) and Sotiel (4,890 ppm), which are also well over the mean (810 ppm). Relating to Zn, values are between 95 and 900 ppm, and of Cu between 27 and 1,160 ppm. The highest concentration of those elements also appeared in samples from Tharsis and Sotiel. Regarding Chromium, its values range between 35 and 236 ppm. Values over 200 pmm appeared in samples from Embalse Zumajo. Cadmium, a highly toxic element, is always below 2 ppm, except in Cueva de la Mora (2.3 ppm). These values are higher than those found in New Zealand (Sheppard et al. 2000) and similar to the values found in soils in Korea for Pb, Zn and Cd (Song et al. 1999) and in soils in the Iberian Pyrite Belt (Chopin et al. 2003; Barba Brioso et al 2006).
Chemical analyses of bedrock samples show that shales are poorer in MgO, CaO and Na2O (<0.1%) than volcanic rocks, but K2O is richer (3–5% in shales versus <1% in volcanic rocks) (Table 5). Iron is usually higher in volcanic rocks (up to 14.65%) as well as manganese. According to trace elements (Table 6), As and Pb (up to 58 and 69 ppm, respectively) seem to be more concentrated in shales than in volcanic rocks but the values are much lower than those noticed in the soil samples. Cadmium and S are below the detection limit in volcanic rocks, but both appear in shales. Cobalt (up to 86 ppm), Cr (up to 7.2 ppm) and Ni (up to 533) usually display higher concentrations in volcanic rocks, which are similar to or higher than those observed in soils. Copper (30–89 ppm) and Zn (20–133 pmm) show similar values in shales and volcanic rocks, but are lower than those that occur in soil samples.
Most of the soil samples displayed concentrations of Cu, Pb, Zn and As exceeding the values defined for the 95th percentile of regional soils (values referred to Andalusia) (Table 3), which is indicative of an enrichment of trace elements in the soils at the studied area. In fact, the statistical processing shows a clear Pb–Zn–As–Cu association (Table 7), which is quite logical in the South Portuguese Zone where massive sulphide mineralisations exist associated to the Volcano-sedimentary Complex. Moreover, most of those values also were over the 90th percentile of the soils of South Portuguese Zone (Table 3), which suggests that the mining activity could have increased the concentration of trace elements besides the geological setting influence (Moon et al. 2000; Peng et al. 2004). On the other hand, the soils developed on sediments are more contaminated than leptosols (Table 3), which is also consistent with the theory of an anthropogenic influence. Chemical analyses of bedrock samples (Table 6), show that even their contribution may be not enough to enrich the leptosols, especially in the sample M10, which was taken close to the Riotinto Mines and could have been affected by wind-blown dust. Only the high concentration of Cr and Ni in samples from Embalse Zumajo may be influenced by the weathering of volcanic rocks (Moon et al. 2000; Zhai et al. 2003), which are rich in those elements, but an anthropogenic contribution seems to be a likely explanation for the high concentration in elements such as As, Cu or Pb.
The magnitude of anomalies can be also evaluated on the basis of the enrichment factor, defined as the relation between the total concentration and the geochemical background for the corresponding geological domain, determined as the median of the soils, in this case, of the South Portuguese Zone. The samples corresponding to the Tharsis and Sotiel mining areas are those which show the greatest degree of enrichment for As, Cu, Pb and Zn (Table 8), especially that from Sotiel which exceeds the background value of Pb by almost 200 times, and As by 50 times. The nearness of the sample location to the waste rock piles in these cases suggests that the soils might have been developed over mining spills, or they are polluted by leachates or erosion coming from the mining spoils (Fig. 2). This is consistent with the acidic pH found in samples from Tharsis. Other authors found polluted soils close to mining dumps (Lottermoser et al. 1999; Lee 2003)
Mineralogy
The mineralogy of the soil samples is made principally of quartz, phyllosilicates and feldspars (Table 9). Feldspars are Ca-plagioclase, which explains the high calcium content readings obtained in the chemical analysis for some samples despite not finding any carbonates in them. The phyllosilicate content is usually low. Only the samples from Peña de Hierro and Montesorromero have concentrations higher than 30%, but they are samples of leptosols (shales) with poor content of clay grain size, which involves a low capacity for metal adsorption. Mention should also be made of the presence of hematite in the Cuevas de la Mora, El Lomero, Tharsis, Riotinto, Embalse Zumajo and Monte Sorromero samples. The high iron oxide values (Table 2) found in the samples M5 and M7, where the presence of hematite was not detected, leads one to consider the existence of amorphous iron oxides in these samples. With regard to the fraction under 2 μm, we would highlight the fact that the samples were found to be composed of illite and of kaolinite and chlorite. Smectites, which have a high ability for metal retention, are not present generally. Only those samples corresponding to the Embalse Zumajo area show a slight content in smectite and illite-smectite interstratified minerals, but their phyllosilicate content is low and their retention ability must be limited. In some samples of shales (Peña de Hierro, Riotinto and Monte Sorromero), chlorite–vermiculite interstratified has been also identified. On the other hand, the content of gravel shows high values, over 50% in some cases, and therefore is indicative of sandy soils (Table 9). This type of soil jointed to the shortage of clay minerals lacks the ability to fix trace elements.
The correlation between Fe2O3 and the Pb–Zn–As–Cu association suggest that iron oxy-hydroxides are the main phases to store most of the trace elements (Table 7). The high content in Fe2O3 of the most contaminated samples, such as those from Sotiel, Tharsis and Embalse Zumajo, is consistent with this idea. It should also be pointed out that they are not associated with the phyllosilicates as it has been proposed by some authors (Brigatti et al. 1994; Galán 2000). The low phyllosilicate contents and, especially, the absence of clay minerals with sorption capacity support that idea. Other authors such as Rasmussen et al. (1998), Gobeil (1999) and Bilali et al. (2002) found similar results for lacustrine sediments.
Chemical speciation
The sequential extraction confirms the low significance of phyllosilicates in the retention of metals in this study, and shows that most of the trace elements were associated significantly with the iron and manganese oxides fraction. Arsenic was extracted with the iron and manganese oxides fraction, with the rest practically remaining in the residual fraction (Fig. 3). Therefore, the amounts united to fractions of greater mobility are very discrete. Cadmium is distributed homogeneously in the different extractions but it should be mentioned that in some samples it is in greater proportion in the interchangeable fraction, although in general it is found in scant proportions in the studied samples. Chromium is distributed only into three fractions: iron and manganese oxides, organic matter and insoluble residue. Copper is present in all of the fractions, but is found mostly associated with iron and manganese oxides. In samples M11 and M12 from Embalse Zumajo Cu is also extracted with the interchangeable, carbonate and organic matter fractions. Nickel appears in all of the fractions but it is associated mainly with iron and manganese oxides and to the residual fraction. Lead appears associated to iron and manganese oxides, but also it is associated with carbonates and with the insoluble residue; a small proportion may be also interchangeable. Zinc, as in the case of Pb, was essentially found in the fraction joined to iron and manganese oxides. A small proportion can be found in the insoluble residue or in the fraction bound to the organic matter, depending on the sample type. For all the extractions, the total of the trace elements in the different fractions did not exceed ±10% of the bulk sample which is accepted as satisfactory (Tessier et al. 1980; Pickering 1986; Yan et al. 1999), except the values obtained for Pb and Zn, which exceeded those detected with ICP-OES. In these cases only the relative percentages have been considered.
Iron, as expected owing to the mineralogical composition (iron amorphous oxides and hematite), is basically found distributed into two fractions: iron and manganese oxides and insoluble residue (Fig. 3). This distribution is very similar to that obtained for As. Manganese is associated with the iron and manganese oxides fraction. It is also found in a small proportion in the fraction associated with carbonates and in the interchangeable fraction.
In general, the partitioning data obtained shows that most of the elements are found in the residual fraction or are linked to the iron and manganese oxide fraction, affirmations which are in agreement with the results obtained by Howari and Banat (2001) and by Galán et al. (2003).
Mineralogical speciation
The characterisation of the mineralogy of the different fractions does not seem to be a good tool in these types of samples, since the results are very similar for every fraction (Table 10). This is due to the fact that the mineralogical composition of the samples is mostly made up of quartz, feldspars and traces of hematite, which are resistant to different attacks. Moreover, the mineralogical characterisation method of XRD with an error of (±5%) does not allow the detection of mineral phases present in small proportions, which could be of interest owing to the ease with which they may capture trace elements (e.g. jarosite). The most relevant feature is the decrease of the difractogram background after the extraction of Mn and Fe oxides, which may be related with the dissolution of amorphous iron oxy-hydroxides. The hematite content may disappear with this step, but may sometimes also remain in the residual fraction. The mineralogy of the interchangeable fraction shows a slight decrease in the phyllosilicate content, particularly in those samples containing smectite and interstratified illite–smectite (M11, M12).
Availability and bioavailability
Extractions with acidic water have given positive readings for all cations. The highest concentrations extracted corresponded to Cu, Pb and Zn, which reached values over 100 ppm on average (Fig. 4). They were followed by As and Ni which were around 10 ppm and Cd and Cr showed values usually lower than 1 ppm. The mobility of the trace elements was assessed comparing the amounts extracted with total concentration of the samples (Fig. 5). Copper, Pb, Zn and Mn were the most mobile elements (average extraction higher than 40%, Figs. 5, 6), followed by Cd, which is also available although its concentration is very low. The most immobile elements are As, Cr and Fe.
Extractions performed with EDTA have also given a positive reading for all cations (Fig. 6). The amounts extracted are above 100 ppm for Cu, Pb and Zn, around 10 ppm for As and Ni and lower than 1 ppm for Cd (Fig. 7). The most available metals were Cd, Pb and Mn (average extraction 60–70%), followed by Cu and Zn (30–40% extraction) and As, Cr, Ni and Fe as the most immobile phases (Figs. 8, 9). According to the results obtained, the mobility sequence proposed is the following: Mn, Pb, Cd, > Zn, Cu > Ni > As > Fe > Cr.
Conclusions
Most of the samples studied were contaminated in Cu, Pb, Zn and As, especially those taken in small-scale orchards positioned over sediments close to the mining area or next to waste rock piles. Samples collected from leptosols show lower levels of pollution, but some of them surpass the 90th percentile of the corresponding geological dominium (South Portuguese Zone). This may suggest an antropogenic input of metals through a vector such us wind-blown dust. The bedrock influence may explain high values in Co, Cr and Ni, but it does not seem to be the source of As, Cu, Pb and Zn, which are associated with the ore deposits. Samples from El Lomero, Peña de Hierro and M14 in Monte Sorromero displayed concentrations of trace elements lower than the contamination threshold, but the high mobility of Cu, Pb and Zn may cause their availability and their entrance in the trophic chain. When elements such us Cr and Ni, and even As occurs in not so high concentrations, they have a low risk owing to their low mobility. Extra studies concerning the effects on plants may be required to ensure that these metals are not being assimilated.
A methodology similar to that proposed in this paper is necessary to assess the degree and origin of pollution in soils. Values of local or geological dominium background rather than regional background allow us to assess the degree of pollution in soils, and comparisons with the bedrock show the anthropogenic contribution. The mineralogical characterisation and mineralogical speciation have not been very useful tools to find phases hosting metals, as they can be stored by accessories or amorphous matter, which are difficult to identify and quantify. The chemical speciation allows quantifying the percentages of trace elements associated with the different extractions, but does not supply information about the total mobility. Availability and bioavailability studies are necessary to assess real risk of the trace elements’ contamination in soils. A high mobility may involve that elements below the contamination threshold are dangerous, or a low mobility may allow the agricultural usage of a soil with high values in several elements. Although the bioavailability shows the amount of metals that plants may assimilate, other studies about the concentration in leaves or fruits may be necessary to carry out a more accurate evaluation.
References
Adriano DC (1997) Biogeochemistry of trace metals. Science Reviews, Georgia, p 432
Alloway BJ (1995) Heavy metals in soils. Blackie Academic and Profesional, Londres, p 368
Azcue JM (1993) Metales en el medio ambiente. In: Mas A, Azcue JM (eds) Metales en Sistemas Biológicos. Promociones y publicaciones Universitarias, Barcelona, pp 163–186
Baize D (1997) Teneur totales en elements traces metalliques dans les sols (France). INRA editions, Paris, Francia, p 408
Barba Brioso C, Sánchez Blanco E, Fernández Caliani JC (2006) Efectos del drenaje ácido de minas sobre la composición química y mineralógica de suelos agrícolas. Una aproximación experimental. In: Bellinfante N, Parrales IA, Jordán A, Martínez-Zavala L (eds) II Congreso Ibérico de la Ciencia del Suelo. Huelva, Spain, p 61
Barth H, L´Hermite P (1987) Scientific basis for soil protection in the European Community. Comission of the European Communities. Elsevier, Londres
Bilali L, Rasmussen PE, Hall GEM, Fortin D (2002) Role of sediment composition in trace metal distribution in lake sediments. Appl Geochem 17:1174–1181
Blanco F, Rothenberg B (1981) Exploración arquemetalúrgica en Huelva. Labor S.A., Barcelona, p 312
Bohn H, McNeal B, O’Connor G (1985) Soil chemistry, 2nd edn. Wiley, New York
Bowen HJM (1979) Environmental chemistry of the elements. Academic, London, p 333
Brigatti MF, Corradini F, Franchini G, Pacchioni MG, Poppi L (1994) Interaction of exchanged Zn2+ montmorillonite with alkaline and earth alkaline cations. Appl Clay Sci 9:121–128
Cama J, Ayora C, Querol X, Moreno N (2005) Metal adsortion on cáliz from pyrite contaminated soil. J Environ Eng 131(7):1052–1056
Cambier P (1994) Contamination of soils by heavy metals and other trace elements. A chemical perspective Analysis 22(2):21–24
Cano Parrilla MA, Moreno García AM, González Parra J (1997) Evaluación de la contaminación por metales pesados en suelos de cultivo. Ecología 11:83–89
Chao TT (1984) Use of partial dissolution techniques in geochemical exploration. J Geochem Explor 20:101–135
Chen PY, Wang MK, Yang DS (2001) Mineralogy of dickite and nacrite from northen Taiwan. Clays Clay Minerals 49:568–595
Chopin EIB, Black S, Hodson ME, Coleman ML, Alloway BJ (2003) A preliminary investigation into mining and smelting impacts on trace element concentrations in the soils and vegetation around Tharsis, SW Spain. Mineral Mag 67(2):279–288
Fernández Caliani JC, Galán E (1996) Impacto ambiental de la minería en el devenir histórico de la comarca de Riotinto (Huelva). Geogaceta 20:1168–1169
Finnecy EE, Pearce KK (1986) Land contamination and reclamation. In: Understanding our environment. Royal Society of Chemistry, Londres, Reino Unido, pp 329–337
Gäbler HE, Schneider J (2000) Assessment of heavy-metal contamination of floodplain soils due to mining and mineral processing in the Harz Mountains, Germany. Environ Geol 39:774–782
Galán E (2000) The role of clay minerals in removing and immobilising heavy metals from contaminated soils. In: Gomes C (ed) Proceedings of the 1st Latin American Clay Conference, vol 1, pp 351–361
Galán E (2003a) Aportaciones de la mineralogía a la evaluación y tratamiento de suelos y sedimentos contaminados por elementos traza. Boletín Soc Española Mineral 26:1–28
Galán E (2003b) Mineralogía Aplicada. Síntesis S.A, Madrid, p 429
Galán E, González I, Fernández-Caliani JC (2002) Residual pollution load of soils impacted by the Aznalcóllar (Spain) mining spill after clean-up operations. Sci Total Environ 286:167–179
Galán E, Gómez Ariza JL, González I, Fernández Caliani JC, Morales E, Giraldez I (2003) Heavy metals partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. Appl Geochem 18:409–421
Gobeil C (1999) Silver in sediments from the St. Lawrence River and Estuary and the Saguenay Fjord. Environ Sci Technol 33:2953–2957
González I (2003) Impacto ambiental provocado por la extracción de minerales. In: Galán E (ed) Mineralogía Aplicada. Síntesis S.A., Madrid, pp 225–249
González I, Romero A, Galán E (2004) Environmental Aspects of Waste Dumps at the Peña del Hierro Mine (Iberian Pyrite Belt SW Spain). In: Pecchio M, Andrade FRD, D’agostino LZ, Kahn H, Sant’agostino LM, Tassinari MMML (eds) Applied mineralogy: developments in science and technology ICAM 2004. Sao Paulo, pp 419–422
Gregorauskiene V, Kadunas V (1997) Experience and goals of geochemical mapping for environmental protection in Lithuania. J Geochem Explor 60:67–76
Howari FM, Banat KM (2001) Assessment of Fe, Zn, Cd, Hg, and Pb in the Jordan and Yarmouk river sediments in relation to their physicochemical properties and sequential extraction characterization. Water Air Soil Pollut 132:43–59
Kabata-Pendias A (1995) Agricultural problems related to excesive trace metals contents in soils. In: Salomons W, Forstner U, Mader P (eds) Heavy metals. Problems and solutions. Spinger, Berlin, pp 3–18
Kabata-Pendias A, Pendias H (1992) Trace elements in solis and plants, 2 edn. CRC Press Inc., Boca Raton, p 365
Kloke A, Eikmann Th (1991) Nutzung-und schutzbezogene Orientierungsdaten für (Shad) Stoffe in Böden, sonderdruck aus Heft 1, 8
Lee CH (2003) Assessment of contamination load on water, soil and sediment affected by the Kongjujeil mine drainage, Republic of Korea. Environ Geol 44:501–515
Leistel JM, Marcoux E, Thiéblemont D, Quesada C, Sánchez A, Almodóvar GR, Pascual E, Sáez R (1998) The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt. Review and preface to the Thematic Issue Mineral Deposita 33:2–30
Lindsay WL (1979) Chemical equilibria in soils. Wiley-Interscience, New York, p 449
Llamas JM, Hervás L, Martínez Escriche F, Otero F (2000) Suelos contaminados. Medioambiente 34:54–57
López Julián PL, Mandado Collado JM (2002) Extracciones químicas secuenciales de metales pesados. Aplicaciones en C.C. Geológicas. Estudios Geológicos 58:133–144
Lottermoser BG Ashley PM, Lawie DC (1999) Environmental geochemistry of the Gulf Creek copper mine area, north-eastern New South Wales, Australia. Environ Geol 39:61–74
Martín CW (2000) Heavy metal trends in floodplain sediments and valley fill, River Lahn. Germany Catena 39:53–68
Martín-Pozas JM, Martín-Vivaldi JL, Rodríguez-Gallego M (1969) Análisis cuantitativo de filosilicatos de la arcilla por difracción de rayos X. Anuario de la Real Sociedad Española de Física y Química. Serie B. L. V:109–112
McLean JE, Bledsoe BE (1992) Behaviour of metals in soils. USEPA Ground Water Issue, EPA/540/S-92/018
Merefield JR (1995) Sediment mineralogy and the enviromental impact of mining. In: Foster IDL, Gurnell AM, Webb BW (eds) Sediment and water quality in river catchments. Wiley, Chichester, pp 145–160
Moon JW, Moon HS, Woo NC, Hahn JS, Won JS, Song Y, Lin X, Zhao Y (2000) Evaluation of heavy metal contamination and implication of multiple sources from Hunchun basin, northeastern China. Environ Geol 39:1039–1052
Navas A, Machín J (2002) Spatial distribution of heavy metals and arsenic in soils of Aragón (norheast Spain): controlling factors and environmental implications. Appl Geochem 17:961–973
Novotny V (1995) Diffuse sources of pollution by toxic metals and impact on receiving waters. In: Salomons W, Förstner U, Mader P (eds) Heavy metals. Problems and solutions. Springer, Berlin, pp 33–35
O’Neill P (1995) Arsenic. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, London, p 368
Peng B, Song Z, Tu X, Xiao M, Wu F, Lv H (2004) Release of heavy metals during weathering of the Lower Cambrian Blck Shales in western Hunan, China. Environ Geol 45:1137–1147
Pérez L, Moreno A, González J (2000) Valoración de la calidad de un suelo en función del contenido y disponibilidad de los metales pesados. Edafología 7–3:113–120
Pickering WF (1986) Metal ion speciation—soils and sediment. Ore Geol Rev 1:83–146
Plant JA, Raiswell R (1983) Principles of environmental geochemistry. In: Thornton I (ed) Applied environmental geochemistry. Academic Press, London, pp 1–39
Rasmussen PE, Villard DJ, Gardner HD, Fortescue JAC, Schiff SL, Shits WW (1998) Mercury in lake sediments of the Precambian Shield near Huntsville, Ontario, Canadá. Environ Geol 33:170–181
Romero A, González I, Galán E (2006) Estimation of potential pollution of waste mining dumps at Peña del Hierro (Pyrite Belt, SW Spain) as a base for future mitigation actions. Appl Geochem 21:1093–1108
Salminen R, Gregorauskiene G (2000) Considerations regarding the definition of a geochemical baseline of elements in the surficial materials in areas differing in basic geology. Appl Geochem 15:647–653
Salminen R, Tarvainen T (1997) The problem of defining geochemical baseline. A case study of selected elements and geological material in Finland. J Geochem Explor 60:91–98
Salomons W (1995) Environmental impact of metals derived from mining activities. J Geochem Explor 53:53–56
Schultz LC (1964) Quantitative interpretation of mineralogical composition from X-ray and chemical data for the Pierre Shale. Geological Survey, Professional Paper, p 391
Sheppard DS, Claridge GGC, Campbell IB (2000) Metal contamination of soils at Scott Base, Antarctic. Appl Geochem 15:513–530
Song Y, Wilson MJ, Moon HS, Bacon JR, Bain DC (1999) Chemical and mineralogical forms of lead, zinc and cadmium in particle size fractions some wastes, sediments and soils in Korea. Appl Geochem 14:621–633
Tarvainen T, Kallio E (2002) Baselines of certain bioavailable and total heavy metal concentrations in Finland. Appl Geochem 17:975–980
Tessier A, Campbell PGC, Bisson M (1979) Sequencial extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Tessier A, Campbell PGC, Bisson M (1980) Trace metal speciation in the Yamaska and St. Francois River (Quebec). Can J Earth Sci 17:90–105
van den Berg R, Dennenman CAJ, Roels JM (1993) Risk assessment of contaminated soil: Proposal for adjusted, toxicologically based Dutch soil cleanup criteria. In: Arendt F, Annokkée GJ, Bosman R, van den Brink WJ (eds) Contaminated Soil ‘93. Fourth international KfK/TNO conference on contaminated soil. Kluwer, London, pp 349–364
Wen X, Allen EH (1999) Mobilization of heavy metals from Le An River sediment. Sci Total Environ 227:101–108
Yan X-P, Kerrich R, Hendry MJ (1999) Sequencial leachates of multiple grain size fractions from a clay-rich till, Sakatchewan, Canada: implications for controls on the rare earth element gepchemistry of porewaters in an aquitard. Chem Geol 158:53–79
Zhai M, Kampunzu HAB, Modisi MP, Totolo O (2003) Distribution of heavy metals in Gaborone urban soils (Botswana) and its relationship to soil pollution and bedrock composition. Environ Geol 45:171–180
Acknowledgments
The authors thank an anonymous reviewer who did much to improve the content of this paper.This work was supported by the Ministry of Education and Science through the Project CTM 2005-05832/TECNO and by the Junta de Andalucía through Research Group RNM 135.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López, M., González, I. & Romero, A. Trace elements contamination of agricultural soils affected by sulphide exploitation (Iberian Pyrite Belt, Sw Spain). Environ Geol 54, 805–818 (2008). https://doi.org/10.1007/s00254-007-0864-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-007-0864-x