Abstract
This study reports the contents and sources of Cu, Hg, Cr, Ni, Co, Zn, Pb, Cd, As, and B pollution in soil samples from Srem in the province of Vojvodina (Republic of Serbia). They are collected in the vicinity of local industrial facilities. The main objective of this study is evaluating the impact of the industrial facilities on the eventual contamination of soils used mostly for agricultural manufacturing. This paper describes the implementation of the combination of methods to estimate the ecological status and determine potential ecological risk. This study applies sequential extraction, pollution indices, comparison with the guidelines, and statistical analysis. Other soil parameters, such as organic matter content, pH, and clay content were measured to evaluate their influence on the trace element content. The investigated soil samples exhibited the raised contents of Ni, Hg, and Cu. Elevated contents of toxic elements observed in localities accommodated within an impact zone affected by industrial complexes, indicating a correlation between the contamination of surrounding soil and potential impact on plants. The most mobile elements are Hg, Cd, and B, while Cr is the least mobile and potential least bioavailable. The results indicate Cr and Ni content increase marking the presence of bedrock, notably in the area of underlying ultramafic rocks and the surface zones influenced by diluvial-proluvial and alluvial processes. The second source of Cr and Ni in the soils of Srem is industrial activities such as leather, cement industry, as well as the metal processing factory.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils have always been important to humans and their health, providing a base for food production, prevention of landform by weathering and degradation, drinking water quality, and shelter (Ungureanu et al. 2017). The widespread contamination of soil with toxic elements represents currently one of the most severe environmental problems that can seriously affect environmental quality and human health (Khalid et al. 2017). As such, industrial zones contribute to an increase of trace elements in the natural environment, depending on the type of industrial manufacturing, by uncontrollable emissions of gas and liquid materials including disposal of solid waste. For instance, cement-derived dust can be the source of Cr, Pb, Zn, Cd, and Cu (Banat et al. 2005; Krishna and Govil 2007; Bodaghpour et al. 2012; Ghorbani et al. 2013); leather industry and tanneries commonly introduces these elements into the natural environment by solid and/or liquid waste (Sekaran et al. 1998; Kashem and Singh 1999; Tariq et al. 2005; Dheeba and Sampathkumar 2012; Gitet et al. 2013). Ni, Mn, Fe, Cu, and Co (besides Zn and Cr) can be released from metal processing plants (deposits effluent and drainage) or welding plants (Dheeba and Sampathkumar 2012; Panagopoulos et al. 2015). Moreover, industrial facilities can be a source of the set of the elements: As, Cd, Pb, Cu, Ni, Co, Mn, Fe, Zn, Cr, B, and Hg, due to fossil fuel combustion process during industrial production (Nable et al. 1997; Baez et al. 2007; Krishna and Govil 2007; Slezakova et al. 2007; Maura de Miranda et al. 2012; Cai et al. 2015).
In agricultural soils, which are the main topic of this study, the use of natural and artificial fertilizers, pesticides, and irrigation can induce the occurrence and accumulation of toxic elements: Cd, As, Pb, Hg, Cr, Zn, Cu, Fe, and Mn (Shomar et al. 2005; Huang and Jin 2008; Cai et al. 2012; Ghorbani et al. 2013; Yong et al. 2015; Mico et al. 2016).
In this manuscript, the contents of Cu, Hg, Cr, Ni, Co, Zn, Pb, Cd, As, and B in the area around industrial facilities in Srem (Vojvodina, Serbia) are studied. The Cu, Co, Zn, B, and Ni were selected because their low quantities are essential for the normal functioning of plants and humans. However, in larger quantities, these elements are toxic to living beings. The second group of elements, such as Hg, Pb, Cd, and As, are toxic and hazardous elements. One of the reason is that the Republic of Serbia recently has systematically begun an assessment of potential contamination with conditionally toxic elements (Sakan 2010), pointing this topic considerably essential for the territory of Srem as a part of Vojvodina—the area of predominant agricultural activity. Republic Serbia as a part of Europe (and an EU candidate) should complete and improve this data, focusing the attention to own agricultural areas. This study investigates soils from several localities in Vojvodina following a few previous studies. In the territory of Vojvodina, several authors (Brankov et al. 2006; Popović et al. 2008; Jakšić et al. 2012; Maksimović et al. 2012; Ninkov et al. 2012) have determined the content of trace elements, such as Pb, Ni, As, Cd, Cu, and Cr, in agricultural and nonagricultural soils and some vineyard soils (Ninkov et al. 2010). Additionally, only a few papers presented concentrations of mobile forms of the elements Ni, As, Cd, Pb, and Cu in the soil of Vojvodina (Dozet et al. 2011; Maksimović et al. 2012). The level of the potential availability of these and other more elements particularly in agricultural areas is of notable importance. In the last decade, Serbia took part in some international projects such as GEMAS project (Geological Mapping of Agricultural and Grazing Soils) (De Vivo et al. 2012) with the principal goal to established the BG and mean values in agricultural and grazing soils of Europe. However, there was paid less attention to this part of Serbia. Regarding the fact that geological matrix of Vojvodina soils differs from the other parts of Serbia, there is a reasonable presumption that there might be a difference in concentrations of the elements (Cr, Ni, Cu, and Co) established by Albanese et al. (2015).
In our research on the risk and origin assessment of the toxic elements, we started from few presumptions: (a) According to Han et al. (2001), bioavailability, toxicity, and mobility of trace elements in soils is determined by their distribution between a solution and solid phase and further redistribution among three groups (i.e., soil fractions): less labile, readily labile, and potentially labile. In the first group, water-soluble and easily exchangeable-bounding forms of metals include a bonding form in which the elements are considered bioavailable. The second group includes metals bonded to Fe-Mn oxides and organic matter, which are potentially bioavailable. In the third group, metals bonded in minerals (residual fractions) are considered almost unavailable for both plants and microorganisms (Rodriguez et al. 2009), and b) metals in less mobile fractions (e.g., extracted in the acidic residual phase) are mostly of geogenic origin (i.e., originate from a geochemical source, Relić et al. 2005), while bioavailable forms of elements originate from anthropogenic sources. On the other hand, depending on metal and soils properties, in some conditions (wetting-drying regime), trace elements of anthropogenic origin can redistribute toward stable fractions, especially Ni, Zn, and Cu in loessial soils (Han et al. 2001).
In our previous research, the level of contamination and ecological risk assessment are estimated by applying the following coefficients and indexes: ecological risk index, environmental ecological risk factor, and enrichment factor (Poznanović Spahić et al. 2018). The latter typically use the total and pseudo-total concentrations of the elements. Also, in the risk estimation, results of element distributions obtained by the sequential extraction can be used. Thus, few researchers (Lu et al. 2007; Mahanta and Bhatacharyya 2011) used the mobility factor (MF) as the ratio of the metal concentration insoluble, exchangeable, and carbonate-bounded fractions compared to the total content, given as a percentage. Similarly, the risk assessment code (RAC) was used for the ecological risk assessment (Rodriguez et al. 2009; Sakan et al. 2016). Recent studies mostly represented in the investigation of China sediments and soils (Cai et al. 2011; Yang et al. 2012;) used the secondary phase enrichment factor (Kspef). The Kspef factor evaluates the actual trace elements of contamination compared to the reference samples (uncontaminated soils). This coefficient has not presented in the studies of European and Serbian soils and sediments except study performed by Sakan et al. (2016).
Along with the geochemical characterization of the trace element and B content within the study area, additional objectives of the study include (1) defining the type of interaction between elements and soils fractions, both at the surface and subsurface layers, and the differences between them; (2) characterization of the mobility level and soil contamination risk in the agricultural soils; (3) characterization of the influence of anthropogenic activities and their effect on the trace element concentrations in the two separate layers (surface and subsurface); and (4) comparison of the obtained results with legally defined values.
One of the goal of this paper, regard the objectives and presumptions is to propose the method based mostly on the results of sequential extraction, presented within this work as a useful tool in a definition of the ecological status of soil in other investigations in Serbia and the other part of the world. That further implies a definition of the applicability of such soil in agricultural production. Furthermore, this combination of the methods should corroborate the conclusions of the previous study (Poznanović Spahić et al. 2018). Finally, investigating the accumulation of metals and the origin of pollution in soils introducing from geogenic or anthropogenic sources is the main aim of the study.
Materials and methods
Study area
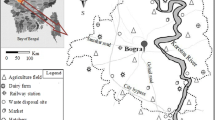
The Srem administrative area is located in the northwest part of Serbia and spreads across the south western part of Vojvodina. Northern and eastern borders are defined by the Danube River, while the Sava River defines the southern border. The western border is delineated by the Serbian-Croatian state line. The surface sediments are of Quaternary (Pleistocene and Holocene) age, while the older bedrock includes a succession of rocks of Triassic, Jurassic, and Cretaceous ages. The main geomorphological units of the Srem administrative area include Fruška Gora Mountain, loessial terraces, terraces and alluvial plains of Sava, and Bosut rivers (Stanić 2004).
The geological map of Srem (Fig. 1) indicates that the soils are predominant erosion derivative of consolidated sedimentary rocks. The main feature of the surface geology of northern Srem is a loess plateau accommodated across the major part of western Srem (Stanić 2004), further transitioning to alluvial sediments. Eastern Srem is characterized by the same plateau, including terrace plateaus and typical loess (Živković et al. 1972). In addition to current alluvial drifts along the Sava River, the surface geology of southern Srem is also represented by plateau loess. On the other hand, the surface of Fruška Gora Mountain is comprised mostly of metamorphic and partially magmatic ultramafic rocks (serpentines, serpentinized peridotites, quartz conglomerates, sericite, chlorite and epidote, chlorite, schists, quarcites…) (Fig. 1). The metamorphic and magmatic outcrops are also accompanied by sedimentary rocks such as limestones, which differentiates the sampling point of Beočin of other sampling locations. The northern slope of the mountain where Beočin city is situated consists of marly tuffites and tuffaceous marl (marine facies) with high contents of calcium carbonates (Arsikin and Čongradac 1979).
Investigated area (Geological map of Yugoslavia 1:300000, modified after Kalenić M et al. Geological Survey of Serbia, 2000)
Collecting samples and preparation
The research area includes locations of eight towns, including 15 industrial areas of Srem county and different industrial facilities (Table 1), as well as the area of southwestern Srem in which seven boreholes drilled (point BG), (Fig. 1). The aforementioned locations and associated industries include (1) Šimanovci (samples S1–S5) (plant for manufacturing cauldrons and cutting metal, printing house, plant for pesticides, and facade material); (2) Pećinci (samples S6–S8) (sugar refinery); (3) Stara Pazova (samples S9–S14) (salt plant, printing house, metal processing factory); (4) Inđija (samples S15–S20) (tobacco factory, paint and lacquer, animal feed manufacturing, battery plants, tin sheet manufacturing); (5) Ruma (samples S21–S26) (leather industry, tire industry); (6) Sremska Mitrovica (samples S27–S32) (plant for wood processing, cellulose, paper, sugar refinery); (7) Šid (samples S33–S41) (animal feed factory, paints and lacquers, insulation materials, oil refinery, printing house); and (8) Beočin (samples S42–S45) (cement industry). Šimanovci, Pećinci, Sremska Mitrovica, and Šid are in the area of alluvial terraces, with unconsolidated clays, sand, and clays, while Ruma, Inđija, and Stara Pazova situated within the area of clastites and deep water grain and limestones (Fig. 1). The sampled soils around the aforementioned industrial and associated locations are accommodate within agricultural areas, which also may represent a potential pollution source, because of the use of agrochemicals, sewage sludges, and irrigation. An additional set of pollution sources include traffic, activities within the nearby urban areas and contamination from the combustion of fossil fuels both within the industries, including private households including city stokes activity during autumn and winter periods.
The examined soil samples (S1–S45) were collected during October 2010. Sampling methodology was in accordance with that defined in the Geological Mapping of Agricultural and Grazing soils (GEMAS) project (EuroGeoSurveys Geochemistry Working Group 2008). This methodology is implemented from the first time in this part of Serbia. Samples from 45 sampling points were collected by plastic and stainless steel shovels from two separate depths. The depth varied depending on the type of soil: agricultural soils were collected from depths of 10–30 cm and 30–50 cm, after removing layer up to 10 cm including plants and roots. Grazing/nonagricultural soils were collected from depths of 0–10 cm and 10–50 cm. The depth of the holes and layers was measured precisely. For each sample, five holes were dug: four at the borders of an imaginary 20 × 50 m rectangle and one in the middle. In this manner, we collected a composite sample of 2 kg for each layer. The other composite sample (BG sample (Fig. 1) is collected from the seven boreholes (0.2/0.3–6 m depth), dug in the region of southwestern Srem, an area with oak forests, located away from industrial facilities and other sources of contamination.
Samples were manually crushed and quartered. The quartered representative composite samples were subdivided in order to determine the mineralogical characteristics of the soil samples to complement the chemical analyses. The other part was packed into polyethylene bags and frozen (− 20 °C) until the beginning of the analysis.
Defining contamination level and MAC
One of the criteria for characterization of contamination levels in this study is the comparison of the obtained results as a sum of extracted trace elements in five fractions to MAC values defined by followed standards:
-
a)
The current standard of MAC values, according to the Serbian law, is presented in the List of Systematic Monitoring of Soil Quality in the Official Gazette of the Republic Serbia (2010). The MAC and remediation values the elements in the soil defined in the aforementioned list (RV) are presented in Table 2. The limit values of metals are modified according to the measured contents of organic matter and clays, mean value of 6.84 and 4.78%, respectively, based on the standard proposition. The modified maximum allowed concentrations (MACm) and modified remediation values (RVm) are listed in Table 2.
-
b)
The standard values (MAC and RV) are in agreement with the limit values and RV defined in the Dutch Target and Intervention List (2000).
-
c)
The list of allowed values of hazardous and harmful materials in the soil, according to the Official Gazette of Serbia (1994), was valid during the time of sampling is taken as the criteria for B.
-
d)
Additionally, we consider the values of metals in the soil according to ecological investigation levels (EIL) defined by the Assessment Level for Soil, Sediment, and Water (Government of Western Australia 2010). In the present study is used the initial risk assessment based on these values. If the metal content values exceed EIL values, it requires further step: assessment of the real contamination risk by using of background (BG) values and bioavailability estimation.
Sequential extraction procedure and determination of trace element content
In order to determine the form of an element’s bonding and define their interaction with geochemical fractions, the method of sequential extraction was applied (Sakan et al. 2007, 2011). Sequential extraction procedure (SEP) is an important and widely implemented method to provide information on possible mobility (hence potential bioavailability and toxicity) of the toxic elements in the environment (Bacon and Davidson 2008). In spite of its disadvantages (e.g., metal redistribution, a formation of a new mineral during the extraction, incomplete extraction, lack of reactant selectivity) SEP method can afford that information (Bacon and Davidson 2008; Zimmerman and Weindorf 2010). The method employed in this study includes five phases (F1–F5) (Table 3). The procedure is a modified version of the extraction by Tessier (Tessier et al. 1979).
Before the extraction, samples were defrosted for 24 h. In order to avoid chemical transformation due to chelating, oxidation processes, or difficulties that can be produced by changing the physical characteristics and efficiency of extractions, the soil samples were not dried. After defrosting, material was homogenized. The duplicated sample of 1 g of mass of each defrosted sample was treated by the extractants. In order to calculate moisture content and correct the measured concentration to sample dry mass, the moisture level was determined by drying a separate single, additional 1 g sample, in an oven at 105 ± 2 °C, until constant weight limit is reached.
The extraction is performed in an overhead rotation shaker. The extract was separated from the solid residue by centrifugation at 3000 r/m for 15 min. Between the phases, the residue was washed twice with double-deionized water, shaken for 15 min and followed by decantation of the supernatant. The supernatant was decanted in a 50-ml flask, diluted with water, and stored at 4 °C until the analysis.
The content of Cu, Cd, Pb, Cr, Co, Ni, Pb, B, and As in extracts was measured by inductively coupled plasma optical emission spectrometry (ICP/OES) (iCAP-6500 Duo, Thermo Scientific). Mercury was determined by the hydride technique using a PERKIN ELMER 6500 MHS-15. Minimization of the effect of interferences was performed by using blank samples that were prepared for every matrix, i.e., extraction reagent. The instrument was calibrated using multicomponent standards. All calibration curves had a correlation coefficient of R2 ≥ 0.995. The employed wavelengths were 214.4 nm (Cd), 267.7 nm (Cr), 220.3 nm (Pb), 231.6 nm (Ni), 213.8 nm (Zn), 231.1 nm (Co), 213.5 nm (Cu), 184.9 nm (Hg), 208.9 nm (B), and 193.7 nm (As).
Organic matter content presented in a form of humus (%) was determined by the Kotzmann method involving the oxidation of potassium-permanganate followed by titration with oxalic acid. pH values were determined in the samples by the using of the pH electrode (calibrated in buffer solutions: pH 4, pH 7, and pH 10), a multicomponent instrument produced by Ejkelkamp. Clay contents were determined using a granulometric-pippete method.
Quality control
A quality control quantification of the accuracy and precision of the measurements was performed using the certified reference material “loamy soil 3” CRM 034-50G (Fluka analytical). Digestion of the CRM sample was performed in accordance with methods recommended by USA EPA 3050 b. The obtained results (Table 4) were compared using Student’s t test at a freedom level of N = 2–1 and a significance level of 95%. The results of the t test (t calculated < t critical) (12.71) indicate there is no significant difference between the obtained results of the measured elements contents and certificated CRM values.
Mineralogical analysis
The fraction of soils coarser than 0.063 mm was examined in immersion fluid under a binocular loupe or polarization microscope in emitted light (Ortolix II Pol-Bk, and Leitz-Weltzlar, Germany). The fraction of soils finer than 0.063 mm were dried, sieved, homogenized, pulverized, and prepared for X-ray powder diffraction (XRPD) analysis. The XRPD studies were performed using a PHILIPS PW-1710 automated diffractometer. A long-focus (LFF) Cu-anode was employed using a voltage and current of 40 kV and 30 mA, respectively, and monochromatic Kα1 radiation of wavelength = 1.54051 Å with a Xe proportional counter. Diffraction data were collected in the angle range 2θ from 5° to 65° with keeping back with 0.25 s on every 0.02°. The base program, PW-1877, was used for the measurement of the angle positions of diffraction maximums and their associated intensities. Precision of the diffractometer was controlled before and after experiment with metallic Si powder. Identification of the present mineral phases was done by comparison of the interplanarspacing (d) and relative intensities (I) with the literature data from the ICDD-PDF database (http://www.icdd.com).
Statistical methods
Alongside with the descriptive statistics (mean value, standard deviation, minimum, maximum, and median), statistical data processing in this paper included the testing of the normality of data distribution using the Kolmogorov-Smirnov test and Cluster analysis. A significant deviation from a normal distribution for each of the studied variables was observed. The Wilcoxon signed-rank test, a nonparametric statistical hypothesis test, is used to compare two matched samples to assess whether their mean population ranks differ. Considering the fact that the concentration distribution deviates from a normal Gauss distribution, the median values are compared to the standard values instead of the mean values. Descriptive statistics, Kolmogorov-Smirnov test for the normality of distribution, and the Wilcoxon signed-rank test were performed using NCSS statistical software (http://www.ncss.com).
Cluster analysis is performed with no normalized values. In this manuscript was applied hierarchical cluster analysis. Details of the applied method of cluster analysis—cluster method: between-group linkage and distance measure: Pearson correlation. The cluster analysis was carried out using SPSS 21 for Windows.
Secondary phase enrichment factor (Kspef)
The secondary phase enrichment factor, Kspef, is suitable for determining the enrichment level (i.e., contamination) of a given element. The Kspef presents a ratio of the distribution of elements between the labile (secondary) and residual (primary) phases for both, investigated and reference, natural value, sample. This factor is calculated using following equation from Cai et al. (2011):
The F1–F4 phases in the current study are presented as labile forms and the F5 phase is residual. With regard to Kspef value, the level of enrichment by trace elements can be classified into five categories: 1 ≤ Kspef (no enrichment); 1 ≤ Kspef ≤3 (minor enrichment); 3 ≤ Kspef ≤5 (moderate enrichment); 5 ≤ Kspef ≤10 (moderately severe enrichment); and Kspef > 10 (severe enrichment).
The reference values calculated as (labile/residual)reference are used in Kspef equation as mean values in each sample from seven boreholes of 0.20 m/0.30 m depth (reference sample, BG). The reference values studied within this research, as well as reference values used in previous studies (Cai et al. 2011; Yang et al. 2012) are listed in Table 5. Boron and mercury were detected only in the residual phase, of the reference sample. Therefore, the reference values of these elements we cannot calculate.
Results and discussion
Mineralogical contents of soil
Accurate characterization of mineralogical contents in soils has high importance because it provides more precise information about geological settings, bedrock composition, and possible natural sources of trace elements in the investigated area.
Granulometric analysis of the samples previously studied (Poznanović Spahić et al. 2018) has shown that soil texture are composed of ~ 5% sand (fraction 0.25–0.063 mm); ~88% silt (fraction 0.063–0.005 mm); and ~ 7% clay (fraction < 0.005 mm).
Qualitative and quantitative mineral composition in fraction coarser than 0.063 mm is presented in Supplementary Table 1. The results emphasize that major minerals are mostly quartz (from 39 to 93%) and carbonates (calcite and dolomite; with up to 55–56%, samples S18/1 and S23/1) prevailing over feldspars (up to 10%, sample S29/1); micas (mostly muscovite with up to 10%, sample S41/1; and rarely biotite); and chlorites (sample S42/1). Additionally, we characterize the following minor minerals content: metallic minerals; limonite, (sample S32/1); zircon (sample S16/1); tourmalines (sample S14/1); amphiboles (sample S45/1); pyroxenes (samples S9/1, S15/1, S15/1, and S41/1); sphene (sample S44/1); rutile (samples S24/1 and S45/1); staurolite (sample S44/1); garnets (up to sample S45/1); and epidotes (only in sample S44/1).
Silt (fraction 0.063–0.005 mm) and clay (< 0.005 mm) are presumably main carriers of polluting components (Banat et al. 2005). In this paper, the XRPD technique was used to define the qualitative and semi-quantitative mineralogical composition of the fractions less than 0.063 mm (45 samples of the subsurface layer and 10 samples of the surface layer). These samples mostly consist of the silty-clayey component (Supplementary Table 2).
The obtained results indicate that a predominant mineral in this fraction is quartz, which is a primary mineral and common component in the investigated soils. The only exception is sample Ѕ41/2 where calcite has a most dominant role. In addition to quartz, most samples also contain feldspars and clay minerals. Clay minerals are mostly composed of illite-sericite, chlorite, and montmorillonite, which appears only occasionally (samples S5/2, S21/2, S43/2, and S45/2). Furthermore, in few samples, carbonates (dolomite and/or calcite) are more dominant over feldspars and clays, following quartz (samples S10/2, S12/2, S14/2, S16/2, S37/2, and S42/2). Additionally, dolomite and calcite have different ratios depending on the sample.
In addition, we characterize a set of minor mineral components: amphiboles (samples S12/2, S13/2, S14/2, S19/2, S21/2, S22/2, S31/2, S33/2, S34/2, S35/2, S36/2, S43/2, and S45/2); hematite (samples S32/2, S35/2, and S41/2); and gypsum (only in sample S37/2). It should emphasize that the XRPD analysis could not detect some other minerals because of two main reasons: either they are not present therein or the quantity is below detection of the limit (2–3%).
The findings of the analysis of the ten samples from the surface layer and their analogs from the subsurface layer (Supplementary Table 2) provide that contents of quartz and clays have a predominant increasing trend, whereas contents of carbonates and chlorites mostly decrease with depth. On the other hand, in some samples (i.e., samples S10, S14, S23, S29, S41, S43, and S45) feldspars contents decrease with the depth, whereas in others (i.e., samples S15, S26, and S39) increase. Amphiboles, hematite, and montmorillonite are determined by XRPD method only in quantity above 2–3%.
Besides quartz, most of the other detected minerals are primarily of magmatic/metamorphic origin (feldspars, micas, chlorites, and other minor or accessory phases such as pyroxenes, amphiboles, garnets, tourmalines, sphene, rutile, staurolite, epidotes, zircon). Clays as secondary minerals, most reasonably originate from some of the aforementioned magmatic/metamorphic minerals. Carbonates detected across the investigated soils originating from carbonate rocks of biogenic and dolomitized limestones. The results and the associations of minerals obtained are mostly adequate to the geological units within the study area (Fig. 1). Also, that further suggests a presence of geological matrix, most probably detached from some of the magmatic, metamorphic, and sedimentary rocks, accommodated within the Fruška Gora Mt. (Dimitrijević 1997). Moreover, it is a well-known fact that triplet of minerals tourmalines-zirconium-rutile (for example, in the sample S-22) indicates the maturity of the rocks, namely their presence indicates that rocks underwent to the following cycle: transporting-weathering-sedimentation-diagenesis, reworked through several cycles.
XRPD analyses indicate a mineralogical content which can be related to the natural source of the trace elements. It is a well-known fact that clay minerals (along with carbonates, organic matter, oxy-hydroxides of iron, and manganese) having a higher adsorption surface (especially montmorillonite) constitute an adsorptive soil complex. Moreover, trace elements such as Ni, Co, Cr, Zn, Cu, B, and Pb can be secondary content in ilites, and Cr, Ni in chlorites. Cr can be a trace element in magnetite, some amphiboles. Carbonates are minerals where elements such as Co, Cd, and Pb can exchange ions Ca (Vinogradov 1958; Hawkes and Web 1962; Huang 1967; Tančić 1994; Babič 2003; Kabata-Pendias 2011).
Physical and chemical properties of the soils
The mean pH values and the contents of organic matter (OM) do not significantly differ between the two studied layers. A mean pH value of 7.52 (Table 2) in both layers classifies soils as slightly alkaline (United States Department of Agriculture Natural Resources Conservation Service). In the surface layer, the pH ranges between 6.12 and 8.81 indicate the presence of slightly acid to severe alkaline soils. In the subsurface layer, pH values of 7.11–8.71 indicate neutral to severe alkaline reactions of the soil. Regarding the fact that pH values and organic matter content may vary due to agricultural activities and crops production, we analyzed these parameters separately in agricultural/nonagricultural soils. In the Supplementary Table 3, the pH values are exhibited in nonagricultural and agricultural soils. Applied t test to the results shows that there is no significant statistical difference (95% level of significance) between the two layers or between the sampled agricultural and nonagricultural soils.
The pH values of neutral and alkaline soil affect the reduction of soil enrichment by metals from natural sources (i.e., from the parent rock) (Krishna and Govil 2007). That implies enrichment in the shallow soil layers could originate mostly from anthropogenic sources. The maximum values (Supplementary Figure 1) are estimated in the territory of Beočin (Ѕ 42/1, Ѕ 45/2). Beočin is also characterized by organic contents that have the lowest values, with mean values of 3.40 and 2.33%, respectively. This organic content coupled with mineralogical composition makes pH values expectable. Minimum pH values were detected in the areas of Šid (Ѕ41/1) and Šimanovci (Ѕ2/2).
According to the organic matter contents in the surface layer (as humus, in %), ranged from 2.08 to 7.00% (mean value of 4.71%) and from 1.05 to 7.16% (mean value of 4.85%) in the subsurface layer, we conclude that the investigated soils from Srem can be classified as moderately to very humus-type soil, excluding the sample Ѕ45/2, which is slightly humus-type soil. Bearing in mind that the organic matter content of investigated Srem soil has a low value (up to 7%), the conclusion is that most of the elements were bound to inorganic soil fraction. Correlations coefficients between pH and organic matter are presented in Supplementary Table 4. The results indicate negligible correlation (low value of coefficient R) between pH and organic matter in the sampled agricultural/nonagricultural soils. A set of factors (besides organic matter content), such as presence of carbonates (as buffer system); clays (kind and quantity); ion constituents in soil solution (Al3+, Fe3+); and vegetations (Tančić 1994), could be the reason of such weak correlation. The highest values of organic matter have remarked the soils in the territory of Pećinci (surface layer) and S. Mitrovica (subsurface layer) (Supplementary Figure 1).
Distribution of the trace elements
In this paper, we consider the two types of trace element distribution: (1) spatial distribution by areas and industrial facilities and (2) distributions by layers. Table 2 and Supplementary Figure 2 show the element contents obtained as a sum of the total extracted elements within the five phases of the sequential extractions. Respecting the fact that the silicate structure is not completely interrupted during the extraction, the obtained results present the “pseudo-total” contents.
According to Karim et al. (2014), high values of the trace element coefficient of variation (CV, Table 2), show a high variability (i.e., heterogeneity) in their content range and, according to some authors (Cai et al. 2015) a relative anthropogenic effect as well. In this study, high values of this CV coefficient for Ni in both layers, Cu in the subsurface layer and Cr in the surface layer (Table 2) can be a consequence of anthropogenic activities in addition to the differential influence of bedrocks. Additionally, high CV values (i.e., above the MACm) of Pb and Hg in the surface and in subsurface layers, respectively indicate an anthropogenic impact. Since the concentration distribution deviate from a normal Gauss distribution, the median values are compared to the standard values (Table 2). The median values for Ni, Co, and Hg are higher than the modified limit values according to the standard of the Republic of Serbia in both layers, while Cu values are higher and equivalent to the standard in the surface and subsurface layers, respectively. The set of obtained values are far below RVm, according to the same standards, as well as EIL values.
The observing range of the elements at investigated localities (Table 2, Supp. Figure 2), implies that concentrations values of Ni are both below and above values defined by all standards values (Table 2). The concentration range of Cr, Co, Zn, Hg, and Pb are below and above the modified limit values (MACm) and MAC across the entire area of study, however, below the values defined by other standards; the same refers to the Cu as well, except the locality with the extremely high content, even above intervention value RI. Concentrations of Cd in the soil are below and above MACm and MAC values in the surface layer, but below MAC in the subsurface layer, accommodated within the area surrounding industrial facilities. The contents of boron, as an element without a defined limit value by the current state law, are below MAC (50 mg kg−1) across all investigated localities. Arsenic contents in all samples are below the modified limit values except for the surface layer soils collected next to the cement factory (Ѕ41), where the maximum content of this element is observed. The concentrations of the other element (except Ni and Cu) are below the intervention values RIm, RI, and EIL.
The results of other studies (Brankov et al. 2006; Jakšić et al. 2012; Maksimović et al. 2012; Ninkov et al. 2012) indicate that the contents of these elements at the most localities are below the maximum allowed values defined by the national standard with a few exceptions: in an area of loessial terraces of Srem, the origin of the elevated values of Ni, Cr, and Pb have been interpreted as the anthropogenic in surface layers, while in the subsurface layer as geogenic (Jakšić et al. 2012). The same conclusion was met by Brankov et al. (2006), whose research showed an increased nickel content in the industrial zone of city of Pančevo (Banat).
If the received results of the contents of the element are comparing to the results in other studies of GEMAS project, it could conclude that the median value of Hg as not high as median values presented in this manuscript. However, the concentration range of Hg is within the range in agricultural soils of Europe (Ottesen et al. 2013); median value of Zn (45 mg kg−1); Pb (20 mg kg−1); Cd (0.22 mg kg−1); and As (5.69 mg kg−1) are comparable to the median values presented in the Table 2 (Reimann et al. 2012; Tarvainen et al. 2013; Ladenberger et al. 2016; Birke et al. 2017). The median values of Cu, Cr, Co, and Ni in agricultural soils of Europe presented by Albanese et al. (2015) are lower. In addition, most of the elements anomalies in those studies are explained by different geogenic sources (mineralization, ore deposits, soil type, or its bedrock) and anthropogenic sources, such as urbanization (Pb, Cd, Hg); coal combustion (As, Hg); use of the herbicides; fertilizers (As); or fungicides (Cu).
Following discussion is focused on the areas and localities around the facilities where the significant increase of the trace elements is registered (above the reference values).
According to the mean concentrations values of Cr, Ni, Co, and Cu, the soil in the areas of Beočin and Ruma are the most contaminated with regard to these elements. A higher nickel content, particularly in the alluvial-diluvial soils of Srem, can be explained by alluvial processes which may have transported nickel from ultrabasic (magmatic) rocks exposed at Fruška Gora Mountain into the hypsometrical lower area (Dozet et al. 2011). This scenario can be one of the explanations of the highest value of these elements—Ni, Cr, and Co in parts of the investigated area—alluvial (Sremska Mitrovica, Stara Pazova, Pećinci, and Šid). Namely, proluvial-diluvial processes can be the reason for the presence of fragments rocks of magmatic origin in the soils sampled in the areas—Beočin and Ruma (Poznanović Spahić et al. 2018). The association of these three elements may suggest an appearance of ultramafic rocks, presented in the other part of Serbia. The results gained in agricultural soils in Balkan and Serbia are one of the highest (Albanese et al. 2015). The increased values, presented in this paper are similar to those values. These results further support the idea of weathering of ultramafic rocks particularly rich in serpentinite which is known to produce soils and sediments with high concentrations of Cr, Ni, Mn, and Co (Venturelly et al. 1997). Finally, the geogenic origin was confirmed by the facts that these elements associated with chlorite- and serpentine-type minerals and that presence of chlorites and amphiboles identified in the samples (Supplementary Tables 1 and 2). The same conclusion withdrew Giuseppe et al. (2014).
According to the spatial distribution of the highest values of the elements, the other sources of these elements can be industrial facilities. Namely, maximum and higher values of Ni (above EIL values) in both layers are observed in the soil sampled around the cement plant: 400 m from cement plant in the urban area of Beočin (Ѕ43) and directly next to the cement plant Ѕ42/1 (106 mg kg−1); in the vicinity of the leather factory in Ruma town—the locality Ѕ22 in the surface layer (167 mg kg−1), which was collected 250 m from the leather factory and Ѕ21 (158 and 148 mg/kg), collected directly next to the factory in the urban area. The maximum of Cr content, measured in the vicinity of these two industrial objects are in the samples Ѕ21/1 and S43/2. Popović et al. (2008) observed decreasing of the values of Ni content with the distance from the cement plant of Beočin. The samples Ѕ43/1 (141 mg kg−1) and Ѕ22 in the surface layer (130 mg kg−1) are recognized by high values of Cr content (above MAC). The highest value, registered in the sample taken directly next to the waste drainage channel of the leather plant indicates increasing due to use of the Cr (VI) salts in the process (Tariq et al. 2005). Additionally, increasing of Cr values can be the consequence of amphiboles presence, as well as of wearing of the rotary kilns in cement industry (Bodaghpour et al. 2012). Maximum Co value is registered in the samples located next to the cement plant—Ѕ42/1 (surface layer).
Maximum copper values are observed again in the samples in the vicinity of cement plant - collected at a distance of 400 m (surface layer) and leather industry in the soil collected 250 m from the facility. However, the fact that latter sample is collected in an abandoned orchard implies that this extremely high copper content is not a consequence of local industrial activities. Most likely, it is the result of an application of a Cu-based fungicide of the Bordeaux mixture type that generally used for fruit growth within an orchard and local vineyards. Ninkov et al. (2010) observed increasing nickel (of geogenic-lithological origin) and copper contents in vineyards in the territory of Banat (Vojvodina). It should be pointed out that the aforementioned fungicide can contain lead and zinc (Orešković and Gašić 2005). According to the results of Cu content in the soil sample S43, and results of the Pb, Zn, Cd, As, and Hg content (Supplementary Figure 2), the cement plant can be one of the sources of contamination of soils in Beočin area (area of the highest mean values of these elements). The highest value of Cd (0.63 mg kg−1) (Supplementary Figure 2) along with the maximum value of As, and Pb in the surface layer (Table 2) are registered in the sample collected next to the cement factory Ѕ42/1. The highest concentrations of Cu, Zn, Pb, Cd, and Hg in the soils surrounding the cement plant as one of the main sources were found in other studies (Banat et al. 2005; Ghorbani et al. 2013). More precisely, these elements together with Cr can be released in fly ash, which is a product of fuel combustion as an energy source in the process of clinkering and calcinations, as well as in fugitive-derived dust from cement plants (Yongming et al. 2006).
The soils in the Ruma are contaminated by Zn and Hg. The highest value of Hg in surface layer is registered in the sample Ѕ21/1. Maximum zinc contents and higher value are registered at locality Ѕ21 (surface layer) and Ѕ22 (143 and 98.1 mg kg−1) respectively (above the limit defined by the regulation of the Republic of Serbia and below EILs), suggesting an impact of the leather industry (waste water/combustion of fossil fuels in the industrial process) or fungicide use.
Stara Pazova town is mostly contaminated by Zn, B, Ni, Cr Zn, Cu, and Co, according to the mean value of these elements in the surface relative to the subsurface layer of soils. The higher contents of Ni (165 mg kg−1); Cr (146 mg kg−1); Co (20.2 mg/kg); Cu (70.4 mg kg−1); as well as Zn (145 mg kg−1) are measured in soil collected directly next to a metal processing factory (Ѕ12). The increasing of the content of this group of metals can indicate contamination due to metal processing. On the other hand, presence of amphiboles and chlorites can be the source of Ni, Co, and Cr as well, especially in the subsurface layer. In this area, it is expectable to registered higher values of Cd, Pb, Zn, Cr, Ni, and Cu in soil located in the vicinity of solid metal waste dump or steel scrap storage (Ѕ11). The content of the elements in the vicinity of the steel scrape waste that Sofilić et al. (2013) found is 3–10 times higher, because of direct but shortly contact of the soil surface with metal waste unlike the soil sample (S11). Here, Co (15.5 mg kg−1); Cu (32.6 mg kg−1); and Zn (148 mg kg−1) are above MDKm values, and background values (BG) established by Poznanović Spahić et al. (2018). Moreover, the results of the Pb (37.0 mg kg−1); Cd (0.58 mg kg−1); and Cr (51.1 mg kg−1) are higher than BG values, which is in our opinion, better indicator here for identification of the level of contamination. In such case, the combination of the element can suggest an influence of the solid metal waste, besides the fact that the results refer to the agricultural soil sample. At the same locality, the maximum boron content (below reference value) is estimated. This fact can be a consequence of agrochemicals application.
Sremska Mitrovica town is locality of higher mean values of Zn, Pb, and Hg. The locality of the urban part of Sremska Mitrovica Ѕ29 (250 m eastern from the wood and cellulose factory) is registered as the most contaminated by Pb (95.4 mg kg−1). Zn contents at the same locality of 147 mg kg−1 (surface layer) and 110 mg kg−1 (subsurface layer) are above MACm. These values of Zn and Pb could imply the impact of traffic or other urban activities at the mentioned locality. Moreover, here are registered high values (above MACm and MAC) of Ni, Cu, Cr, Co, and Hg. (Supplementary Figure 1). Within fly ash as a product of fossil fuel combustion used in industrial processes, these elements can be released in the environment. Furthermore, in this sample, amphiboles and chlorites are detected (Supplementary Figure 1 and 2).
In the sample Ѕ27/1 (agricultural soil), collected next to the cellulose industry in Sremska Mitrovica, the highest value of Hg was registered. Thus, Hg can be in soils of Sremska Mitrovica as a result of agrochemical use or use of Hg compounds in wood preservation (Kabata-Pendias 2011), released during the wood industrial treatment.
The most contaminated towns, according to the mean Hg contents are aforementioned Ruma and Inđija. The measured values were above the limit (MACm). Additionally, sample Ѕ7/2 of the agricultural soil, collected 400 m from the sugar refinery in Pećinci has the maximum value of Hg in the subsurface layer. The values range of 0.37–0.68 mg kg−1 in the surface layer (samples S15-S20, Supplementary Figure 2), and 0.28–0.47 mg kg−1 in the subsurface layer (above MACm values) in the vicinity of different industrial facilities (Table 1) in Inđija, as well as Pećinci being most likely a result of fossil fuel combustion and the use of pesticides.
The results indicate that the soil in the territories of Šimanovci is contaminated mostly by Cd. The highest value is detected in the agricultural soil sample labeled Ѕ4. The sample was collected next to the pesticide factory on one side, and the construction materials factory on the other side. The Cd content at this locality, which is higher than the modified limit value, but lower than the RVm and EILs, suggests an influence of the relevant anthropogenic sources such as agricultural activities and fossil fuel combustion.
Results of Wilcoxon’s test indicate that B, Cd, Ni, Pb, Zn, Hg, and Cu contents significantly differ within the two investigated layers having increased values in the surface layer. Such a distribution between the two layers is in accordance with the published data (Vinogradov 1958; Popović 1994; Tančić 1994; Bogdanović et al. 1997). The explanations are that the elements originating from anthropogenic activities are concentrated mostly in the surface layer; due to the high adsorption energy in the colloid particles of a soil complex, zinc is hardly washed out into deeper layers and the plants contribute to fixation across the surface layers. Boron also has higher concentrations in the shallow soil layers due to intense accumulation during the soil formation process. Moreover, biogenic elements such as Cu and Zn may be involved in biogeochemical cycling. Accordingly, their increased values in the soil surface are not necessarily non-natural (Grygar and Popelka 2016). Mercury in the surface layer accumulates more, particularly if the surface layer is rich in sulfur (e.g., organic and inorganic sulphides) (Kabata-Pendias 2011).
Geochemical affinity of the trace elements
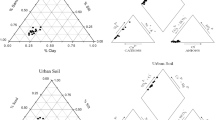
The distribution of the elements between the fractions (i.e., geochemical phases) given in % is shown in Fig. 2 and presented on diagrams.
Arsenic is a siderophile element and predominates in both layers within the residual phases, most likely iron minerals. This also accords with observations of Окоnkwo (2007) who pointed out an importance of residual phases in arsenic bounding. Copper, as a siderophile element is equally distributed within the residual (F5) and Fe-Mn-oxyhydride (F3) phases. In this study, Cu is slightly more concentrated in the surface layer in a more mobile phases (F1–F4) > F5, rather than in the subsurface layer (F1–F4) < F5. Therefore, an enrichment and the possible anthropogenic effect is more distinct in the surface layer (with the exception of the sample Ѕ22/2) (Supplementary Figure 2b).The presence of Cu is confirmed in all feldspars (Silveira et al. 2006), which suggests that the copper extracted from the residual forms may partially originate from these minerals.
The balance of lead distribution (shown in Fig. 2), suggests that lead is presented in slightly elevated level in the more mobile (F1 + F2) form from the surface layer (12.2%), compared to subsurface layer (5.47%). It could be lead content is caused mainly by an anthropogenic effect. Namely, lead ions can exchange calcium ions in carbonates (Lu et al. 2007), such that lead from anthropogenic sources (e.g., air, traffic) is often concentrated within mobile phases, particularly carbonate (Ghariani et al. 2009).
Zinc has a different fractional distribution within the two soil layers. This result agrees with those obtained from Tisa sediments in Vojvodina (Sakan et al. 2009), indicating a natural and anthropogenic origin of zinc. Additionally, Zn is a biogenic element, and Cu likewise, can become more mobile in surface layer due to biogeochemical cycling.
In both layers, chromium and nickel are distributed by a geochemical affinity in the same manner. The distribution suggests a common origin of these elements and their geochemical similarity. The distribution of Co between the phases and depth is nonselective, indicating heterogeneity in behavior of this element. This observation may support the nature of Co as a siderophile, halcophile, and lithophile element. The mobility of Ni and Co, higher than those of Cr (Fig. 3), and the soils derived on various ultrabasic bedrocks, found as well Kiercak et al. (2016).
A different distribution in the examined layers and nonselective bonding of Cd in the phases indicate a heterogeneous geochemical affinity and origin of this element. In the first layer, the dominant presence of Cd is in the carbonate phase, while in the subsurface layer, it is mostly bonded in the residual phase. It is well-known that cadmium can partition into carbonate under strong oxidation conditions (Kabata-Pendias 2011). Such bonding in an easily mobile phase makes cadmium hazardous due to its toxicity (Sakan et al. 2007). Furthermore, this distribution indicates an anthropogenic influence on its content within the surface layer.
Mercury has a very specific distribution between the F1, F3, and F4 phases in both layers. We assume that relevant substrates of mercury in the examined soils are organic matter and amorphous forms of iron oxide with differing levels of interaction that form a complex to the adsorbed phases.
Boron is mostly distributed between carbonate and residual phases. The presence of boron in more mobile forms, such as carbonates, is likely a consequence of tourmaline weathering, which results in transformation to a mobile form of boron acid, which can be lost by leaching (Nable et al. 1997). However, in a soil buffer system, calcium-carbonate, boron acid can be neutralized, and thereby boron fixed by a less soluble calcium-metaborate. The presence of boron silicates, such as tourmaline, was identified in the sampled soil but not in the reference samples. During the interaction of tourmaline with 6M HCl, its structure is partially damaged and B therefore occurs in the residual phase (F5) of the examined soil.
For most of the observed elements, the relevant bonding phase is a residual one, comprised of crystal forms of iron oxides (e.g., magnetite, hematite) and aluminosilicates, which are a significant substrate of the studied elements. This observation indicates a dominant geochemical origin for most of the element. Metals have high abundance in residual fraction indicating lithogenic origin and low availability of the metals in the soils (Ashraf et al. 2012).The same distribution of copper and arsenic between F5 and F3 phases (i.e., iron oxide of different crystallization levels) can be a result of hematite and magnetite hydration into amorphous or poor crystalline forms, including other minerals such as limonite (Nelson 2014).
Cluster analysis
The cluster analysis (R-mode, Pearson method) is carried out by using the content of elements extracted in the first extraction fraction. This fraction (phase F1), chosen because it is a representative of the most mobile form of the elements, considered as the highest environmental risk/antropogenic impact carrier. Furthermore, it can indicate enrichment of the elements due to anthropogenic origin according to the main idea of their concentration in the most mobile phases. We decided to divide these two strata and observe the clustering separately because of the main presumption. Recent evidence suggests that anthropogenic sources have particularly influence at the surface of soils, and that accumulation of metals originating from the geochemical origin is present in the deeper layers. The results of the cluster analysis are shown by two similar dendrograms, in both, the surface (Fig. 4a) and the subsurface layers (Fig. 4b). In CA dendrogram (Fig. 4a), the cluster I is subdivided into the three subclusters: subcluster Ia) contains Pb and Cd; subcluster Ib) contains OM, pH, and Hg; and subcluster Ic) contains Zn, B, and Cu. The cluster II includes the two subclusters: subcluster II a) contains Cr, As, and Ni and subcluster II b) contains only Co.
CA dendrogram Fig. 4b) produces the two clusters. The first cluster (I) is divided into the three subclusters: Ia) contains Zn, Pb and Cd, and Ib) contains OM, pH and Hg. Cluster II is divided into the three subclusters; IIa) contains Cu and Ni; IIb) includes Cr, As and B; and IIc) contains Co.
Cluster I unlike cluster II (in both layers) contains the elements which much easier become mobile using the reagent 1M CH3COONH4 (Table 3). Namely, in the phase F1, the elements attacked by this reagent are water-soluble (free form), adsorbed in the adsorptive soil complex and partially, bounded to carbonates. As mentioned earlier, the elements detected in the most mobile phases may indicate the anthropogenic influence, especially in the surface layer, however not necessary. In the surface layer, the similarity between Cd and Pb suggests their correlativity—these elements originate from the same anthropogenic sources or the same manner of bounding—for example, to carbonates. The subcluster Ib groups Hg, pH, and organic matter together. The explanation can be desorption of Hg from organic matter (Leleyter et al. 2012) (bonded by Van der Waals interactions), especially depending on pH. It is probably Zn, B, and Cu have a common origin as a consequence of the application of chemicals in agriculture. Moreover, as biogenic elements, they are incorporated in biochemical cycling in more reactive forms and can be concentrated more in the surface rather than in deeper strata (where biochemical processes are less intense) (Grygar and Popelka 2016). Thus, the following conclusion imposes—these elements can be in free (water-soluble) forms and as the association suggesting similar mobility in soil profiles.
The cluster II includes less mobile elements, originating mainly from the natural sources. Separation of Co within subcluster IIb most probably indicates a predominant geochemical origin; Cr, As, and Ni are less mobile and their origin in the investigated soils is mostly natural, presuming that the main origin is weathering of rocks and minerals (silicates, iron oxide minerals) followed by adsorption at surface of amorphous structure (Mn and Fe oxy hydroxide) or clays minerals (external and interlayer surfaces of clays). Artificial minerals in soils as a product of industrial processes (for example, fossil fuel combustions) (Popović et al. 2001; Stering and Herble 2003) can be accommodated therein as well. Different forms of the elements can be present within new born structures (Sarkar et al. 2006; Shah et al. 2008; Nelson et al. 2010), wherein distribution of the elements is possible in the new environment as well.
Turning now to the subsurface layer, clustering of the elements Zn, Pb, and Cd indicates a common anthropogenic origin or translocation of Zn in the soil profile. The latter process accompanied with distribution Zn ion between soil solution and the solid surface may be the other reason for such grouping. Cu and B are less mobile in the subsurface layer, the most probably due to the same reason. Thus, Cu is placed together with Ni (making the same subcluster) and B (grouped in the same cluster) together with As and Cr, in the same substrates. Otherwise, the origin can be the same as it is explained previously (in the case of the surface layer). The same explanation of grouping is referring to Co and Hg (subcluster IIc and Ib, Fig. 4b).
Mobility and risk assessment
Mobility and risk assessment of the elements was performed using the MF and RAC both based on the ratio of soluble, ion-exchangeable and carbonate-bonded fractions (Mahanta and Bhatacharyya 2011; Rodriguez et al. 2009). In the present study, these elements contents are in the first and second phase of the extraction such that the MF and RAC factors (which are equivalent) are calculated as the ratio of the sum of element concentrations in these two phases compared to the pseudo-total element content.
Results presented in Fig. 3 indicate that the maximum MF/RAC value for boron is 52.82 (56.73%). The most mobile toxic elements are mercury (56.64 or 45.17%) and cadmium (42.19 or 34.12%). High MF values can indicate a high availability of toxic elements in soils, yet is not the only condition for uptaking them by vegetables (Ngole 2011). The results of the present study suggest that the mobility of the elements declines in the following order: As > Co > Pb > Ni > Zn > Cu > Cr, in the surface, e.g., Co > As > Pb > Ni > Cu > Zn > Cr in the subsurface layer. That further indicate a pattern if there is a change in the pH values, an increase in ion activities, e.g., increasing of the concentration of the elements in soil solution, or slightly change of Eh (redox potential), the analyzed elements in the soil solution (in given order) will become more mobile and available to plants. Consequently, these elements could become available to humans throughout the food chain. The same pattern can be used to assess the environmental risk of the toxic elements already introduced by Rodriguez et al. (2009). According to the RAC values, there is no risk for Cr contamination (RAC (Cr) < 1%). Within the low-risk group (1–10%) are Cu, Ni, and Zn, followed by moderately risk group (10–30%) of the Pb, Co, and As. The high-risk group (31–50%) involves Cd and Hg in the surface layer, and the very high-risk group (above 50%) includes B and Hg in the subsurface layer.
Secondary phase enrichment factor
The calculation of the Kspef factor includes the labile phases (first four fractions) differing from the previously measured MF/RAC, which considers the first two most mobile phases. According to the fact that mercury and boron in the reference samples are in a residual phase, the Kspef factors for Hg and B are missing.
In the Supplementary Figure 3, the Kspef values for studied elements are presented. The obtained results revealed differences in distribution among the elements and the soil samples. The Kspef values for elements decrease in the following order in surface layer: Cd (no severe) > As (no moderately severe) > Zn (minor moderately severe) > Cu (no moderate) > Pb (no moderate) > Cr (minor moderate) > Ni (no minor) > Co (no minor) enrichment. In subsurface soil layer, the Kspef values declines in the following order: Cd (no severe) > Cu (no moderately severe) > As (no moderately severe) > Zn (no moderate) > Pb (no moderate) > Cr (no minor) > Ni (no minor) > Co (no minor) enrichment.
Conclusions
In this study, we assess and characterize the contamination level, potential ecological risks, including mobility, and the origin of toxic elements in the soil of Srem (Vojvodina, Serbia). Distribution of studied elements between the geochemical phases and the sampled layers represent a balance established between the processes of redistribution of elements originating from anthropogenic activities, mostly As, Cd, Hg, Pb, and Zn, and the redistribution by mineral decomposition from parent rock (particularly Cr, Ni, and Co). The most mobile elements are Hg, Cd, and B, while Cr is the least mobile and least bioavailable. The high-risk group includes Cd and Hg in the surface layer, and the very high-risk group includes B and Hg in the subsurface layer. Different distributions of Pb and Cd within the two soil layers indicate an increase of the anthropogenic influence at the surface layer. Chromium predominantly originates from the parent rocks with the exception of the samples collected in the vicinity of a Beočin cement factory, the metal processing factory in Stara Pazova and the leather factory in Ruma.
Combination of the comparison values with MAC, BG values, completed with the results obtain after the sequential extractions (and further used calculation of MF/RAC/Kspef) with the mineralogical analyses seems to be a powerful tool for assessment of contamination level, potential risks, mobility, and origin of toxic elements in the soil of Srem. The methods coupled within this study can be applied to assess soil pollution levels by toxic elements in zones affected by relevant anthropogenic sources.
References
Albanese, S., Segedhi, M., Lima, A., Cicchella, D., Dinelli, E., Valera, P., et al. (2015). GEMAS: Cobalt, Cr, Ni Cu distribution in agricultural and grazing land soil of Europe. Journal of Geochemical Exploration, 154, 81–93.
Arsikin, P., & Čongradac, G. (1979). Nonmetallic mineral resources of Vojvodina. In 2nd conference of nonmetallic mineral resources in SFRJ, Proceedings II (pp. 441-464).
Ashraf, M. A., Maah, M. J., & Yusoff, I. (2012). Chemical speciation and potential mobility of heavy metals in the soil of former Tin mining catchement, The Scientific World Journal, 2012. Article ID 125608, 11 pages, https://doi.org/10.1100/2012/125608.
Babič, D. (2003). Mineralogy. Belgrade: Cicero.
Bacon, J. R., & Davidson, C. M. (2008). Is there a future for sequential chemical extraction? Analyst, 133, 25–46.
Baez, P. A., Garcia, M. R., Del Tores, B. M., Padilla, H. G., Belmot, R. D., Amandor, O. M., & Villalobos-Piertini, R. (2007). Origin of trace elements and inorganic ions in PM10 aerosols to the S. Mexico city. Atmospheric Research, 85, 52–63.
Banat, K. M., Howary, F. M., & Al-Hamad, A. A. (2005). Heavy metals in urban soils of central Jordan: Should be worry about their environmental risks? Environmental Research, 97, 258–273.
Birke, M., Reimann, C., Rauch, U., Ladenberger, A., Demetriades, A., Jahne-Klingberg, F., Oorts, K., Gosar, M., Dinelli, E., Halamić, J., & The GEMAS project team. (2017). GEMAS: Cadmium distribution and its sources in agricultural and grazing land soil of Europe - Original data versus clr-transformed data. Journal of Geochemical Exploration, 173, 13–30.
Bodaghpour, S., Biglari, J. N., & Ahmadi, S. (2012). A review on the existence of chrome in cement and environmental remedies to control its effects. International Journal of Geology, 2(6), 62–67.
Bogdanović, D., Ubavić, M., & Hadžić, V. (1997). Heavy metals in soils. In R. Kastori (Ed.), Heavy metals in the environment (pp. 103–143). Novi Sad: Feljton.
Brankov, M., Ubavić, M., Sekulić, P., & Vasin, J. (2006). Trace elements and heavy metal contents of agricultural and nonagricultural soils in the region of Banat. Institute of Field and Vegetable Crops Proceedings, 42, 169–177.
Cai, J., Cao, Y., Tan, H., Wang, Y., & Luo, J. (2011). Fractionation and ecological risk of metals in urban river sediments in Zhongshan city, Pearl river delta. Journal of Environmental Monitoring, 13, 2450–2456.
Cai, L., Xu, Z., Ren, M., & Peng, P. (2012). Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong province, China. Ecotoxicology and Environmental Safety, 78, 2–8.
Cai, L., Xu, Z., Bao, P., He, M., Dou, L., Chen, L., Zhou, Y., & Zhu, Y. G. (2015). Multivariate and geostatistical analyses of the spatial distribution and source of arsenic and heavy metals in the agricultural soils in Shunde, Southeast China. Journal of Geochemical Exploration, 148, 189–195.
Dheeba, B., & Sampathkumar, P. (2012). Evaluation of heavy metal contamination in surface soil around industrial area.Tamil Nadu, India. International Journal of ChemTech Research, 4(3), 1229–1240.
De Vivo, B., Albanese, S., Lima, S., Reimann, C., Birke, M., Schoeters, L., Demetriades, A. & GEMAS project team. (2012).The GEMAS project: Geochemistry of European agricultural and grazing land soils, 7th EUREGEO 2012, Proceedings, session 10, 654. https://core.ac.uk/download/pdf/55071666.pdf.
Dimitrijević, M. D. (1997). Geology of Yugoslavia. Belgrade: Geological Institute GEMINI.
Dozet, D., Nešić, L.j., Belić, M., Bogdanović, D., Ninkov, J., & Zeremski, T. (2011). Origin and content of Ni in alluvial-delluvial soils of Srem, Serbia. Field and Vegetable Crops Research, 48, 369–374.
Ministerie van VolkshuisvestingRuimtelijkeOrdeningenMilieubeheer, 2000. Dutch target and intervention.
EuroGeoSurveys Geochemistry Working Group. (2008). EuroGeoSurveys geochemical mapping of agricultural and grazingland soil of Europe (GEMAS)-fieldmanual. http://www.ngu.no/upload/Publikasjoner/Rapporter/2008/2008_038.pdf. Accessed 3 Novembar 2017.
Ghariani, R. A., Gržetić, I., & Nikolić, S. (2009). Distribution and availability of potentially toxic metals in soil in central area of Belgrade (Serbia). Environmental Chemical Letters, 8, 261–269.
Ghorbani, H., Aghababaei, A., & Mirkarimi, H. R. (2013). The evaluation of industrial cement production plant on the environmental pollution using magnetic susceptibility technique. Agricultural Sciences, 4(12), 792–799.
Gitet, H., Subramanian, P. A., Minilu, D., Kiros, T., Hilawe, M., Gebremedhin, G., & Taye, K. (2013). Speciation to chromium in soils near Sheeba leather industry, Wukro Ethiopia. Talanta, 116, 626–629.
Giuseppe, D. D., Antisari, L. V., Ferronato, C., & Bianchini, G. (2014). New insights on mobility and bioavailability of heavy metals in soils of the Padanian alluvial plain (Ferrara Province, northern Italy). Chemie der Erde, 74, 615–623.
Government of Western Australia. (2010). Аssessment level for soil sediment and water. Department of Environment and: Conservation.
Grygar, M. T., & Popelka, J. (2016). Revisiting geochemical methods of distinguishing natural concentrations and pollution by risk elements in fluvial sediments. Journal of Geochemical Exploration, 170, 39–57.
Han, F. X., Banin, A., Kingery, W. L., & Triplett, G. B. (2001). Redistribution of heavy metals in arid zone soils under wetting-drying cycle soil moisture regime. Soil Science, 166(1), 18–28.
Hawkes, H. E., & Web, J. S. (1962). Geochemistry in mineral exploration. New York and Evanson: Harper and row publishers.
Huang, S. W., & Jin, J. Y. (2008). Status of heavy metals in agricultural soils as affected by different patterns of land use. Environmental Monitoring and Assessment, 139, 317–327.
Huang, W. T. (1967). Petrology. New York: Mc Graw-Hill book Co..
Jakšić, S., Sekulović, P., & Vasin, J. (2012). Content of heavy metals in gleyicchernozem of Srem loess terrace under alfalfa. Field Vegetable and Crops Research, 49(2), 189–194.
Kabata-Pendias, A. (2011). Trace elements in soils and plants. New York: CRC press, Taylor and Francis Group.
Kalenić, M., Filipović, I., Mirković, B., Dolić, D., Rakić, M., & Gajić, R. (2000). Geological map of Yugoslavia. Belgrade: Geological Survey of Serbia.
Karim, Z., Qureshi, B. A., Mumtaz, M., & Qureshi, S. (2014). Heavy metal content in urban soils as an indicator of anthropogenic and natural influences on landscape of Karachi – a multivariate spatio-temporal analysis. Ecological Indicators, 42, 20–31.
Kashem, M. D. A., & Singh, B. R. (1999). Heavy metal contamination of soil and vegetation in the vicinity of industries in Bangladesh. Water, Air, and Soil Pollution, 115, 347–361.
Khalid, S., Shahid, M., Niazi, N. K., Murtaza, B., Bibi, I., & Dumat, C. (2017). A comparison of technologies for remediation of heavy metal contaminated soils. Journal of Geochemical Exploration, 181, 247–268.
Kiercak, J., Pȩdziwiatr, A., Waroszewski, J., & Modelska, M. (2016). Mobility of Ni, Cr and Co in serpentinite soils derived on various ultrabasic bedrock under temperature climate. Geoderma, 268, 78–91.
Krishna, A. K., & Govil, P. K. (2007). Soil contamination due to heavy metals from an industrial area of Surat, Gujarat, Western India. Environmental Monitoring and Assessment, 124, 263–275.
Ladenberger, A., Reimann, C., Scanion, R., De Vivo, B., Falconi, M., & GEMAS project team (2016). GEMAS: mapping zink deficiency in agricultural and grazing land soil in Europe. 3rd International symposium on environmental & health. http://programe.exordo.com/iseh2016/delegates/presentation/423/. Accessed 29 Octobar 2018.
Leleyter, L., Rousseau, C., Biree, L., & Baraud, F. (2012). Comparison of EDTA, HCl and sequential extraction procedures, for selected metals (Cu, Mn, Pb, Zn) in soils, riverine and marine sediments. Journal of Geochemical Exploration, 116-117, 51–59.
Lu, Y., Zhu, F., Chen, J., Gan, H., & Guo, Y. (2007). Chemical fractionation of heavy metals in urban soils of Guangzhou, China. Environmental Monitoring and Assessment, 134, 429–439.
Mahanta, M. J., & Bhatacharyya, K. G. (2011). Total concentration, fractionation, mobility of heavy metals in soils of urban area of Guwahanti, India. Environmental Monitoring and Assessment, 173, 221–240.
Maksimović, L., Milošević, N., Nešić, L., Zeremski, T., Vasin, J., & Ninkov, J. (2012). Soil contamination in South Bačka Region of Serbia with dangerous and harmful substances. Field Vegetable and Crops Research, 49(2), 220–228.
Maura de Miranda, R., Andrade de Fatima, M., Fornaro, A., Astolfo, R., Afonso de Andre, P., & Saldiva, P. (2012). Urban air pollution: a representative survey of PM2.5 mass concentrations in six Brazilian cities. Air Quality Atmosphere and Health, 5, 63–77.
Mico, C., Peris, M., Sanchez, J., & Recatala, L. (2016). Heavy metal content of agricultural soils in Mediterranean semiarid area: the Segura river valley (Alicante, Spain). Spanish Journal of Agricultural Research, 4(4), 363–372.
Nable, R. O., Banuelos, G. S., & Paulli, G. J. (1997). Boron toxicity. Plant and Soil, 193, 181–198.
Nelson, S. A. (2014). Weathering and clay minerals. Tulane University. http://www.tulane.edu/~sanelson/eens211/weathering&clayminerals.htm. Accessed 10 September 2017.
Ngole, V. M. (2011). Using soil heavy metals enrichment and mobility factors to determine potential aptake by vegetables. Plant, Soil and Environment, 57(1), 75–80.
Nelson, P. F., Shah, P., Stezov, V., Halliburton, B., & Carras, J. N. (2010). Environmental impacts of coal combustion: a risk approach to assessment of emissions. Fuel, 89, 810–816.
Ninkov, J., Zeremski-Škorić, T., Sekulić, P., Vasin, J., Milić, S., & Paprić, Đ. (2010). Heavy metals in vineyard soils of Vojvodina province. Field Vegetable and Crops Research, 47(1), 273–279.
Ninkov, J., Milić, S., Vasin, J., Kicošev, V., Sekulić, P., Zeremski, T., & Maksimović, L. (2012). Heavy metal in soil and sediments of the planned ecological network of Central Banat, Serbia. Field Vegetable and Crops Research, 49, 17–23.
Offical Gazzete, Republic Serbia 1994., No. 23.
Official Gazette of the Republic Serbia 2010., No. 88.
Окоnkwo, J. O. (2007). Arsenic status and distribution in soils at disused cattle dip in South Africa. Bulletin of Environmental Contamination and Toxicology, 79, 380–383.
Orešković, Z., & Gašić, S. (2005). Bordeaux mixture-a comparative investigation of different formulation. Pesticide and Phytomedicine, 20, 255–259.
Ottesen, R. T., Birke, M., Finne, T. R., Gosar, M., Locutura, J., Reimann, C., Tarvainen, T., & The GEMAS project team. (2013). Mercury in European agricultural and grazing land soil. Applied geochemistry, 33, 1–12.
Panagopoulos, I., Karayannis, A., Kollias, K., Xenidis, A., & Papassiopi, N. (2015). Investigations of potential soil contamination with Cr and Ni in four metal finishing facilities at Asopos industrial area. Journal of Hazardous Materials, 281, 20–25.
Popović, Z. (1994). Agrochemistry. Belgrade: University of Belgrade.
Popović, A., Đorđević, D., & Polić, P. (2001). Trace and major element pollution origination from coal ash suspension and transport processes. Environmental International, 26, 251–255.
Popović, V., Djukić, V., & Dozet, G. (2008). Distribution and accumulation of Pb in soils and wheat, 2nd joint PSU-UNS international conference in bioscience: food, agriculture, environment. Proceedings, Novi Sad. pp 292–296.
Poznanović Spahić, M., Sakan, S., Cvetković, Ž., Tančić, P., Trifković, J., Nikić, Z., & Manojlović, D. (2018). Assessment of contamination, environmental risk, and origin of heavy metals in soils surrounding industrial facilities in Vojvodina (Serbia). Environmental Monitoring and Assessment, 190, 208. https://doi.org/10.1007/s10661-018-6583-9.
Reimann, C., Flem, B., Fabian, K., Birke, M., Ladenberger, A., Negrel, P., Demetriades, A., Hoogewerff, J., & The GEMAS Project team. (2012). Lead and lead isotopes in agricultural soils of Europe-the continental perspective. Applied Geochemistry, 27, 532–542.
Relić, D., Đorđević, D., Popović, A., & Blagojević, T. (2005). Speciation of trace metals in the Danube alluvial sediment within an oil rafinery. Environmental International, 31, 661–669.
Rodriguez, L., Ruiz, E., Alonso-Azkarate, J., & Rincon, J. (2009). Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain. Journal of Environmental Management, 90, 1106–1116.
Sakan, S., Gržetic, I., & Đorđević, D. (2007). Distribution and fractionation of heavy metals in the Tisa river sediments. Environmental Science and Pollution Research, 14(4), 229–237.
Sakan, S., Đorđević, S., Manojlović, D., & Polić, P. (2009). Assessment of heavy metal pollutants accumulation in the Tisza river sediments. Journal of Environmental Management, 90, 3382–3390.
Sakan, S. (2010). A new approach of microelements utilization as tracers for identifications and differentiation of anthropogenic influence and background values. Chemical Faculty: University of Belgrade.
Sakan, S., Djordjević, S. D., & Trifunović, S. S. (2011). Geochemical and statistical methods in the evaluation of trace elements contamination: an application on canal sediments. Polish Journal of Environmental Studies, 20(1), 187–199.
Sakan, S., Popović, A., Anđelković, I., & Đorđević, D. (2016). Aquatic sediments pollution estimate using the metal fractionation secondary phase enrichment factor calculation and used statistical methods. Environmental Geochemistry and Health, 38, 855–867.
Sarkar, A., Rano, R., Udaybhanu, G., & Basu, A. K. (2006). A comprehensive characterization of fly ash from thermal power plant in Eastern India. Fuel Processing Technology, 87, 259–277.
Sekaran, G., Shanmugasundaram, K. A., & Mariappan, M. (1998). Characterization and utilization of buffing dust generated by the leather industry. Journal of Hazardous Materials B, 63(1), 53–68.
Shah, P., Stezov, V., Prince, K., & Nelson, P. T. (2008). Speciation of As, Cr, Se and Hg under coal fired power station conditions. Fuel, 87, 1859–1869.
Shomar, B. H., Müller, G., & Yahya, A. (2005). Geochemical features of topsoil in Gaza Strip: natural occurrence and anthropogenic inputs. Environmental Research, 98, 375–382.
Silveira, M. L., Alleoni, L. R. F., O’Connor, G. A., & Chang, A. C. (2006). Heavy metal sequential extraction methods-a modification for tropical soils. Chemosphere, 64, 1929–1938.
Slezakova, K., Pereira, M. C., & Reis, M. A. (2007). Influence of traffic emissions on the composition of atmospheric particles of different sizes – Part 1: Concentrations and elemental characterization. Journal of Atmospheric Chemistry, 58, 55–68.
Sofilić, T., Bertić, B., Šimunić-Meznarić, V., Brnardić, I. (2013). Soil pollution as the results of temporary steel scrap storage at the melt shop. Ecologica Balkanica, 5, 21-30. http://web.uni-plovdiv.bg/mollov/EB/2013_vol5_iss1/021-030_eb13101.pdf. Accessed 3 Decembar 2018.
Stanić, M. M. (2004). Hydrochemical distribution of water bearing layers per zones in Srem. Serbia: Geological Faculty, University of Belgrade.
Stering, R. O., & Herble, J. J. (2003). Reaction of arsenic vapor species with fly ash compounds: kinetics and speciation of the reaction with calcium silicates. Chemosphere, 51, 1111–1119.
Tančić, N. (1994). Pedology. Belgrade: University of Belgrade.
Tariq, S. R., Shah, M. H., Shaheen, N., Khalique, A., Manzoor, S., & Jaffar, M. (2005). Multivariate analysis of selected metals in tannery effluents and related soil. Journal of Hazardous Material A, 122, 17–22.
Tarvainen, T., Albanese, S., Birke, M., Ponavič, M., Reimann, C., & The GEMAS project team. (2013). Arsenic in agricultural and grazing land soils of Europe. Applied Geochemistry, 28, 2–12.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry, 51(7), 844–851.
Ungureanu, T., Iancu, G. O., Pintilei, M., & Chicoş, M. M. (2017). Spatial distribution and geochemistry of heavy metals in soils: a case study from the NE area of Vasluicounty, Romania. Journal of Geochemical Exploration, 176, 20–32.
Venturelly, G., Contini, S., & Bonazzi, A. (1997). Weathering of ultramafic rocks and element mobility at Mt. Prinzera, Noirthern Apennines, Italy. Mineralogical Magazine, 61, 765–778.
Vinogradov, A. P. (1958). The geochemistry of rare and dispersed chemical elements in soils. New York: Consultants Bureau.
Yang, Y., Chen, F., Zhang, L., Lin, J., Wu, S., & Kang, M. (2012). Comprehensive assessment of heavy metal contamination in sediment of the Pearl river estuary and adjacent shelf. Marine Pollution Bulletin, 64, 1947–1955.
Yong, L., Huifeng, W., Xiaoting, L., & Jinchang, L. (2015). Heavy metal contamination of agricultural soil in Tayiuan, China. Pedosphere, 25(6), 901–909.
Yongming, H., Peixuan, D., Junji, C., & Postmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi’an, Central China. Science of the Total Environment, 355, 176–186.
Zimmerman, A. J., & Weindorf, D. C. (2010). Heavy metal and trace metal analysis in soil by sequential extraction: a review of procedures. International Journal of Analytical Chemistry. https://doi.org/10.1155/2010/387803.
Živković, B., Nejgebauer, V., & Tanasijević, D. (1972). Soils of Vojvodina. Novi Sad: Institute for agricultural research.
Acknowledgements
We would like also to thank Sandra Škrivanj for helping in obtained analytical results, Dr. Darko Spahić and Dr. Jovan Kovačević for kindness, helpful advice, and instructions.
Funding
This study was partly funded by the Provincial Secretariat for Energy and Mineral Resources. Dr. Sanja Sakan thanks for the support of the Ministry of Science and Technological Development of the Republic of Serbia (grant no. 172001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 413 kb)
Rights and permissions
About this article
Cite this article
Spahić, M.P., Manojlović, D., Tančić, P. et al. Environmental impact of industrial and agricultural activities to the trace element content in soil of Srem (Serbia). Environ Monit Assess 191, 133 (2019). https://doi.org/10.1007/s10661-019-7268-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7268-8