Abstract
The colonization of degrading endophytic bacteria is an effective means to reduce the residues of polycyclic aromatic hydrocarbons (PAHs) in crops. Dicarboxylic acids, as the main active components in crops, can affect the physiological activities of endophytic bacteria and alter the biodegradation process of PAHs in crops. In this study, malonic acid and succinic acid were selected as the representatives to investigate the contribution of dicarboxylic acids to pyrene biodegradation by endophytic Enterobacter sp. PRd5 in vitro. The results showed that dicarboxylic acids improved the biodegradation of pyrene and altered the expression of the functional gene of strain PRd5. Malonic acid and succinic acid reduced the half-life of pyrene by 20.0% and 27.8%, respectively. The degrading enzyme activities were significantly stimulated by dicarboxylic acids. There were 386 genes up-regulated and 430 genes down-regulated in strain PRd5 with malonic acid, while 293 genes up-regulated and 340 genes down-regulated with succinic acid. Those up-regulated genes were distributed in the functional classification of signal transduction, membrane transport, energy metabolism, carbohydrate metabolism, and amino acid metabolism. Malonic acid mainly enhanced the central carbon metabolism, cell proliferation, and cell activity. Succinic acid mainly improved the expression of degrading gene. Overall, the findings of this study provide new insights into the regulation and control of PAH stress by crops.
Key points
• Dicarboxylic acids improved the biodegradation of pyrene by Enterobacter sp. PRd5.
• The degrading enzyme activities were stimulated by dicarboxylic acids.
• There are different facilitation mechanisms between malonic acid and succinic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are mutagenic, teratogenic, carcinogenic, and cytotoxic chemicals. Their “water-hating” and “fat-loving” properties easily cause their accumulation in soil and airborne suspended particulate matter (Peng et al. 2011; Duran and Cravo-Laureau 2016; Kuppusamy et al. 2017). PAHs in the surrounding environment can migrate to crops through root uptake and foliar uptake (Jia et al. 2018; Li et al. 2019b, 2020; Zhang et al. 2020), and then accumulated in crops (Shen et al. 2020; Chen et al. 2020). The average concentration of PAHs in crops around the Yangtze River Delta industrial zone was reported to be 352.12 ~ 1218.38 μg/kg (Wang et al. 2017). Those contaminated crops increased the exposure of carcinogenic PAHs to human bodies and posed a threat to public health (Fujita et al. 2020).
The colonization of PAH-degrading endophytic bacteria is found to regulate the absorption, accumulation, and transformation of PAHs in crops and reduce PAH concentrations (Abhilash et al. 2012; Sun et al. 2015; Li et al. 2021). The degrading endophyte Pseudomonas putida PD1 colonized in willows and grass was reported to improve the growth and health of the host plants and increase 25 ~ 40% removal of phenanthrene from soil (Khan et al. 2014). Inoculation with Massilia sp. Pn2 could reduce the phenanthrene concentrations in wheat, enlarging the biomass of wheat roots (Liu et al. 2017a). In turn, host crops provided endophytes with nutrients (organic acids, alcohols, and proteins) to increase their life activities, thus stimulating the degradation of PAHs (Martin et al. 2014).
Dicarboxylic acids such as malonate acid and succinic acid are active components in crops (Sivaram et al. 2020). Pieces of evidence have shown that dicarboxylic acids could potentially promote the biodegradation of PAHs (Liu et al. 2015; Lu et al. 2017b; Sivaram et al. 2019; Zhang et al. 2021). For example, Sivaram et al. (2019) reported that dicarboxylic acids enhanced the degradation of high molecular weight PAHs by strains isolated from plant rhizospheres. Zhang et al. (2021) found that dicarboxylic acids could improve the solubility and bioavailability of phenanthrene, and relieve cell-membrane and oxidative damage. Dicarboxylic acids could also significantly increase the removal of PAH in contaminated soils by increasing the availability of phenanthrene and pyrene in soils (Lu et al. 2017b). These dicarboxylic acids can be used as a direct carbon source for bacteria, increasing cell growth and activity while improving the bioavailability of organic pollutants, ultimately accelerating the biodegradation of organic pollutants (Martin et al. 2014). Moreover, dicarboxylic acids have been reported to induce other cellular responses in microorganisms, such as increasing the intracellular NADH levels and enhancing the expression of functional enzymes (Wang et al. 2014; Nie et al. 2017). Therefore, the mechanisms of dicarboxylic acids promoting PAH degradation by endophytes remain inadequately explored. Clarification of the mechanisms of dicarboxylic acids affecting the functional endophytic bacteria would benefit the designing of strategies for improving the PAH removal efficiency in crops.
In this study, malonic acid and succinic acid, which are commonly found in crops, were selected as representatives of dicarboxylic acids (Sivaram et al. 2020). Enterobacter sp. PRd5 was used as a representative of functional endophytic bacteria. The objective of this research was to investigate the performance of pyrene biodegradation by endophytic bacteria in the presence of dicarboxylic acids. Based on the data, the degradation process of pyrene was elucidated, with a focus on transcriptomic responses of Enterobacter sp. PRd5.
Materials and methods
Strains and culture media
Enterobacter sp. PRd5 was isolated in a lab from the healthy plant Ophiopogon japonicas wild growing in PAH-contaminated sites (deposited in CGMCC ref. no.:13218). Luria–Bertani (LB) medium for cell enrichment and mineral salt (MS) medium for pyrene degradation were prepared following a previous study (Lu et al. 2019). All of the media were autoclaved at 121℃ for 20 min.
Experimental design
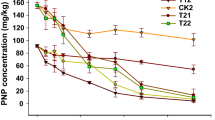
Enterobacter sp. PRd5 was cultured to a logarithmic period in the LB medium. After being centrifuged, and washing for three times with MS medium to remove the residual carbon source, the bacteria were resuspended in fresh MS medium, and adjusted to an optical density of 1.0 at 600 nm (about 2.5 × 108 colony-forming units per milliliter, CFU/mL) for inoculating. Finally, 10% (v/v) seed liquid was inoculated into 20 mL of MS (containing 50 mg/L pyrene). The concentration of dicarboxylic acids was adjusted to 0, 10, 20, 30, 40, and 50 mg/L, because the concentration of low-molecular-weight organic acids in plant tissues was about a few millimoles to tens of millimoles per liter (Sokolova 2020). Strain PRd5 grown in MS medium inoculated without dicarboxylic acids was set as the control. All groups were cultured in a shaker at speed of 180 rpm at 28 ℃ for 10 days. The samples were collected every 2 days to determine the cell growth and residual pyrene concentration. The growth of PRd5 cells was assessed by plate counts. Depending on the growth curve of the cells (Fig. 1b), the sample at the 8th day was used for subsequent analysis. All the treatments were done in triplicate.
Measurement of residual concentration of pyrene
Pyrene was extracted from MSM media following the method by Wang et al. (2016) with some modifications. The residual pyrene in the collected samples was extracted by twice volume methanol with sonication for 30 min, followed by filtration through a 0.22-μm filter. Pyrene was quantified by an Agilent 1200 high-performance liquid chromatography (HPLC, Santa Clara, CA, USA) equipped with a 4.6 mm × 250 mm reverse phase C18 column and a UV detector. Methanol and water (90:10, v/v) were used as the mobile phase at a flow rate of 1.2 mL/min. Chromatography was performed at 30 °C and the detector set at 245 nm.
The biodegradation kinetics of pyrene was modeled by the primary degradation rate model (1) (Sun et al. 2019);
Note: Ct (mg/L) is the concentration of residual pyrene at the incubation time t, C0 (mg/L) is the initial concentration of pyrene, t (d) is the incubation time, k (h−1) is the biodegradation rate constant, and e is the natural constant.
The half-life of pyrene in each group was calculated using Eq. (2):
Metabolite analysis
The pyrene degradation intermediates were extracted following a previous study (Sun et al. 2019) with some modifications. The supernatant was extracted with ethyl acetate twice at neutral (pH = 7.0) and acidic (pH = 2.3), respectively. After centrifugation (8000 × g, 4℃, 5 min), the upper layer organic phases from both extractions were collected and combined, and dried over anhydrous Na2SO4. The samples thus obtained were evaporated to dryness on a rotary evaporator, the extracted pyrene degradation intermediates were reconstituted in 500 μL n-hexane and filtered through a 0.22-μm filter, followed by derivatization with 140 μL of BSTFA-TMCS (N, O-bis(trimethylsilyl) trifluoroacetamide—trimethylchlorosilane, 99:1, v/v) at 80℃ water bath for 30 min.
The intermediates were detected by an Agilent T890A/5975C gas chromatography/mass spectrometry (GC/MS, Santa Clara, CA, USA) equipped with an HP-5-MS column and an electron impact (EI) ionization source. Helium was used as the carrier gas at a flow rate of 1.0 mL/min. The injection port temperature was set at 250 ℃. The heating program was as follows: initially at 60 ℃ for 1 min, then raised to 290 ℃ at a rate of 10 ℃/min and held for 20 min. The MS was run at a full scan mode (50–550 m/z). The EI source was set at 70 eV (Li et al. 2019a). Identities of the pyrene degradation intermediates were determined by retrieving the MS of the analytes in the NIST standard MS library.
Enzyme assays
The samples were centrifuged at 6000 × g for 10 min at 4 ℃. The collected cell pellet was washed three times with phosphate buffer saline (PBS) buffer and resuspended in PBS buffer, and then crushed by ultrasonic disintegrator at a power of 450 W (150 times for 3 s with 3 s breaks) in an ice bath. The crude enzyme solution was obtained after centrifuging to remove cell debris. 1-hydroxy-2-naphthoic acid hydroxylase (1H2NH) (Deveryshetty and Phale 2009), 1-naphthol hydroxylase (1-NAH) (Sah and Phale 2011), salicylic acid hydroxylase (SAH) (Fang and Zhou 2014), phthalate dioxygenase (PDO) (Seo et al. 2009), catechol-1,2-dioxygenase (C12O) (Guzik et al. 2013), and gentisic acid-1,2-dioxygenase (G12O) (Reddy et al. 2018) were monitored as described. The protein concentrations were determined by the method of Bradford with bovine serum albumin as the standard. The specific activity was defined by the amount of product/substrate in μmol (U) oxidized per second per milligram of protein (U/mg) (Nie et al. 2017).
RNA extraction and transcriptome sequencing
The samples were centrifuged at 10,000 × g for 15 min at 4℃. Total RNA from cell samples of each group was extracted by the RNAprep Pure Bacteria kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. The concentration and purity of RNA were determined based on the absorbance at 260 nm in an ultra-micro spectrophotometer (NanoDrop 200C, Thermo Scientific, Waltham, USA). Meanwhile, the integrity of the RNAs was assessed by electrophoresis in 1% agarose. Qualified RNAs were sent to Shanghai Majorbio Bio-pharm Technology Co., Ltd., China, for subsequent mRNA purification, cDNA synthesis, library construction, and transcriptome sequencing by Illumina HiSeq 4000 system (RNA-Seq).
Differentially expressed genes (DEGs) and enrichment analysis
Quantitative software RSEM was used to assess the expression levels of the assembled transcripts by the number of uniquely mapped reads per kilobase per million reads (FPKM) (Yue et al. 2021). The fold change log2 ratio was calculated by comparing the gene expression levels of the control sample to the dicarboxylic acid–treated samples. The significance analysis of expression differences was corrected by BH (Benjamini/Hochberg) method for multiple testing. Raw counts were screened by software DESeq2 to select DEGs between the control sample and dicarboxylic acid–treated sample based on P-adjust < 0.05 and |log2 fold change|≥ 1 (Love et al. 2014). The data were analyzed on the online platform of Majorbio Cloud Platform (www.majorbio.com).
To further explore the biological functions of DEGs and the regulation mechanism of dicarboxylic acids on strain PRd5 at the molecular level, the biologically classified DEGs were clarified by gene ontology (GO) annotation, and the biological regulation pathways were found by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotations.
Validation by quantitative real-time PCR (qRT-PCR)
To confirm the reliability of RNA-Seq identification of DEGs, eight functional genes (7 up-regulated and 1 down-regulated) with significant changes were selected according to different KEGG pathways for qRT-PCR validation. These genes include two PAH degradation genes (adhE, hpcB), two central carbon metabolism genes (paaH, deoC), two ABC transport genes (mnt_B5, ydhC), and two amino acid synthesis/metabolism genes (hisA, argC). The forward primer sequence and reverse primer sequence were designed according to the gene sequence by the software primer premier 6.0 (Supplementary Table S1). The RNA samples were reverse-transcribed and qPCR performed by One-Step qRT-PCR kit (Sangon Biotech, Shanghai, China) according to the manufacturer’s instructions. The expression level of each gene was calculated by the 2−ΔΔCt method (Livak and Schmittgen 2001). Biological triplicate of qRT-PCR was performed for each group.
Statistical analysis
All data analyses were performed in the statistical software SPSS 19.0 (IBM, Armonk, NY, USA). The significant differences between the control group and dicarboxylic acid–treated group were analyzed by t-test and one-way ANOVA, and p < 0.05 was considered statistical significance.
Results
Pyrene degradation and cell growth under dicarboxylic acid treatment
Prior to the analysis of the physiological activity of strain PRd5 during the pyrene degradation experiment, the optimal concentration of dicarboxylic acids was determined. The highest pyrene degradation (48.8%) was achieved in the presence of 20 mg/L succinic acid. The optimal concentration of malonic acid was also 20 mg/L, when 45.4% pyrene degradation was observed (Supplementary Fig. S1). Therefore, the dose of 20 mg/L was selected for the subsequent index determination.
The residual concentration of pyrene gradually decreased with the increasing growth of strain PRd5 (Fig. 1). Both malonic acid and succinic acid could significantly improve the degradation of pyrene by strain PRd5 (p < 0.05). Adding malonic acid or succinic acid accelerated the degradation of pyrene in the medium (Table 1). Compared with the control, adding 20 mg/L malonic acid and succinic acid significantly increased the pyrene degradation rate constants by 25.1% and 38.5%, respectively. Malonic acid and succinic acid reduced the half-life of pyrene by 20.0% and 27.8%, respectively. These results showed that a moderate amount of dicarboxylic acids increased the number of cells and removed more pollutants, and dicarboxylic acids might be used as co-metabolic carbon sources by the strain PRd5.
Identification of pyrene degradation intermediates under dicarboxylic acid treatment
Five trimethylsilyl (TMS)-derived metabolites, designated as peak P1 to peak P5, were detected and identified by comparison with the GC retention time (Rt) and mass spectra with those of the authentic standards (Table 2; Supplementary Fig. S2). Based on this, five intermediate metabolites of pyrene degradation by strain PRd5 were identified, i.e., 1,2-dihydroxynaphthalene, 1-hydroxy-2-naphthoic acid, salicylic acid, catechol, and phthalate. Typical intermediates in the phthalic pathway (phthalic) and the salicylic acid pathway (salicylic acid, catechol, and 1,2-dihydroxynaphthalene) were detected during pyrene biodegradation. Other than that, there was no difference in metabolites between the control and dicarboxylic acid–treated group, which means that dicarboxylic acids did not cause a new pathway of pyrene degradation by strain PRd5.
Enzyme activity in strain PRd5 cell extracts under dicarboxylic acid treatment
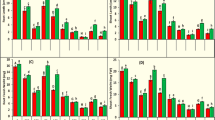
Dicarboxylic acids significantly stimulated the activities of 1H2NH, 1-NAH, SAH, C12O, and G12O enzymes, while PDO enzyme activity was reduced by malonate acid (p < 0.05) (Fig. 2). In addition, the activity of 1H2NH in strain PRd5 was higher than that of other degrading enzymes. The alteration of enzyme activities indicated that dicarboxylic acids could promote the expression of most of the relevant functional genes encoding degrading enzymes.
Transcriptome responses of Enterobacter sp. PRd5 under dicarboxylic acid treatment during pyrene biodegradation
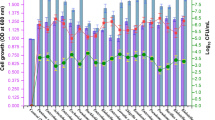
Dicarboxylic acids affected the gene expression of strain PRd5 during pyrene biodegradation. Compared with the control, 386 genes were up-regulated and 430 genes were down-regulated in strain PRd5 treated with malonic acid; 293 genes were up-regulated and 340 genes were down-regulated under succinic acid treatment (Fig. 3a and b). DEGs were functionally classified according to the KEGG database. The metabolism, cellular process, and environmental information of strain PRd5 were affected under dicarboxylic acid treatment. A large number of up-regulated and down-regulated genes were distributed in the functional classification of signal transduction, membrane transport, energy metabolism, carbohydrate metabolism, and amino acid metabolism. In addition, down-regulated genes were also enriched in the functional classification of cellular community prokaryotes and cell motility (Fig. 3c and d).
Global transcriptional response of strain PRd5 under dicarboxylic acid–treated. Volcano plot of malonic acid–treated (a) and succinic acid–treated (b). Functional classification of DEGs in strain PRd5 under dicarboxylic acid treatment. Histogram of control vs succinic acid (c) and control vs malonic aicd (d)
Eight DEGs were selected for qRT-PCR to verify the accuracy of DEGs in RNA-Seq data. Genes of adhE, hpcB, deoC, mntB_5, ydhC, hisA, and argC were up-regulated and paaH gene was down-regulated by dicarboxylic acids. The change trend of DEGs displayed by qRT-PCR was consistent with that of RNA-Seq (Fig. 4), which clarified the reliability of RNA-Seq data.
Differentially expressed genes (DEGs) in Enterobacter sp. PRd5
The gene ligA, ligB, adhE, doxA, hpcB, hcaB, and PH00798 associated with PAH degradation were up-regulated in strain PRd5 (Table 3). The up-regulation was 0.85- to 1.53-fold under malonic acid treatment and 1.10- to 1.83-fold under succinic acid treatment. These results indicate that dicarboxylic acids increased the expression of PAH-degrading genes to stimulate the intracellular biodegradation of pyrene. Comparing the two dicarboxylic acids, succinic acid induced more PAH-degrading gene expression than malonate acid, resulting in a faster pyrene removal.
DEGs related to amino acid biosynthesis and metabolism were distributed in eight pathways (Supplementary Table S2). Among them, up-regulated genes related to arginine biosynthesis, histidine metabolism, and cysteine and methionine metabolism form three complete pathways (Fig. 5). The genes related to arginine biosynthesis were up-regulated by dicarboxylic acids by 0.86- to 4.09-fold (Supplementary Table S2). These up-regulated genes form a pathway with glutamate as a precursor, and arginine is synthesized through catalyzed by various enzymes encoded by the arg gene family (Fig. 5a). The genes related to histidine metabolism were up-regulated by dicarboxylic acids by 1.04- to 1.95-fold (Supplementary Table S2). Enzymes encoded by these genes catalyze the conversion of phosphoribose pyrophosphate to L-histidine (Fig. 5b). The genes related to cysteine and methionine metabolism were up-regulated by dicarboxylic acids by 0.34- to 2.07-fold (Supplementary Table S2). Enzymes encoded by these genes could catalyze the conversion of L-aspartate to L-methionine (Fig. 5c). There were other eleven DEGs associated with other amino acid pathways, where the up-regulation was 0.36- to 2.01-fold (Supplementary Table S2).
The DEGs related to central carbon metabolism were mainly concentrated in glycolysis/gluconeogenesis, pyruvate metabolism, and pentose phosphate pathway (Supplementary Table S3). The DEGs associated with glycolysis/gluconeogenesis were up-regulated 1.22- to 1.9-fold under malonic acid treatment, and up-scaled 0.78- to 1.11-fold under succinic acid treatment. The results imply that malonic acid exhibits a higher cellular growth-promoting capacity than succinic acid. The expression of six genes related to pyruvate metabolism increased under dicarboxylic acid treatment, suggesting that the dicarboxylic acids stimulated the metabolism of pyruvate. There were 3 up-regulated genes related to the pentose phosphate pathway, indicating that dicarboxylic acids promoted the multi-path metabolism of glucose and provided energy and metabolic activity required for cell growth.
Twenty-eight DEGs involved in ABC transport were up-regulated under dicarboxylic acid treatment, which mainly involve the transport of molecules such as manganese, ribose, and arginine. The genes encoding manganese transporters were up-regulated by 2.55- to 3.38-fold (Supplementary Table S4). Other up-regulated genes involved in purine metabolism (deoD, deoB, cpdB, and hiuH-2), pyrimidine metabolism (cdd, rutA, rutB-2), nitrogen metabolism (nirD, nasD), and sulfur metabolism (cysl, cysH-1) could provide energy for resisting external coercion (Wei et al. 2021). Furthermore, the down-regulated genes associated with flagellar assembly would alter cell motility, which is an energy regulation mechanism in response to changes in the growth environment (Liu et al. 2005).
Regulatory mechanism of dicarboxylic acids on the pyrene biodegradation of Enterobacter sp. PRd5
The schematic diagram shows the effects of dicarboxylic acids on pyrene degradation by Enterobacter sp. PRd5, based on the DEGs and pyrene intermediate metabolites (Fig. 6). Malonic acid induced overexpression of genes encoding ABC transporters to promote transmembrane transport of pyrene and uptake by strain PRd5. As the intracellular pyrene concentration increased, the expression of genes encoding dioxygenases related to pyrene degradation was up-regulated, which accelerated the pyrene ring-opening process. The up-regulated dehydrogenase gene promoted pyrene intermediates to be catalyzed to short-chain fatty acids into the TCA cycle. The overexpression of genes encoding amino acids and central carbon metabolism provided the corresponding substrates for the TCA cycle, enabling the complete mineralization of pyrene and the cellular process of energy production. Moreover, up-regulation of genes encoding glutamate and histidine increased the intracellular solubility of pyrene and protected cells from toxic damage. Finally, the gene encoding the ABC transporter increased the excretion of pyrene biodegradation intermediates in a timely manner to promote cell growth, reproduction, and cell activity.
Discussion
PAH-degrading endophytes colonize in the interior of crops and promote the elimination of PAH inside crop tissues (Liu et al. 2017a; Khan et al. 2014). In turn, active components in crops, such as dicarboxylic acids, provide a favorable culture environment for functional endophytic bacteria (Wang et al. 2014; Técher et al. 2011). Exploring the contribution of dicarboxylic acids to PAH biodegradation by an endophytic bacterium in vitro would reveal the feedback mechanisms of crops to endophytic colonization. In plants, dicarboxylic acids were used as a direct and easily accessible energy source during the biodegradation of pyrene (Sivaram et al. 2019; Dominguez et al. 2020), and increased the water solubility of PAHs and thus improving their bioavailability (Zhang et al. 2021; Xiao and Wu 2014; Dominguez et al. 2020). These results led to the increase of degradation efficiency of pyrene by strain PRd5 and the promotion of cell growth (Fig. 1).
An interesting phenomenon was found that the degradation of pyrene was not exactly synchronized with the cell growth between two dicarboxylic acids; the degradation efficiency of pyrene was higher in the group supplemented with succinic acid and the cell growth was higher in the group supplemented with malonic acid (Fig. 1). This suggests that different mechanisms of pyrene degradation were invoked by malonic acid and succinic acid. Similar results have been reported in recent studies that different small-molecule organic acids have different effects on PAH-degrading microorganisms. For instance, Zhang et al. (2021) found that citric acid mainly improved cell proliferation and activity, while glutaric acid and oxalic acid mainly improved enzyme expression and provided energy for cells when evaluating the role of small-molecule organic acids in the degradation of PAHs.
No new intermediate was detected under dicarboxylic acid treatment, suggesting that dicarboxylic acids would not alter the metabolic pathway of pyrene by strain PRd5. As a key enzyme in the phthalate pathway, enzyme PDO is responsible for initiating the aerobic decomposition of phthalate to form cis-4,5-dihydro-4,5-dihydroxyphthalate (Tarasev et al. 2006; Seo et al. 2009). Its activity showed significant differences between malonic acid treatment and succinic acid treatment, which means succinic acid stimulated this process and malonate acid inhibited it. The enzymes 1H2NH, 1-NAH, SAH, C12O, and G12O were related to salicylic acid metabolism, which included dual pathways of gentisic acid and catechol (Chowdhury et al. 2014). Enzyme 1H2NH is a flavoprotein monooxygenase specific for 1-hydroxy-2-naphthoic acid, which catalyzes the conversion of 1-hydroxy-2-naphthoic acid to 1,2-dihydroxynaphthalene under aerobic conditions (Deveryshetty and Phale 2010). Enzyme 1-NAH catalyzes the conversion of 1-naphthol to 1,2-dihydroxynaphthalene (Trivedi et al. 2014). Enzyme SAH catalyzes the oxidative decarboxylation of salicylic acid to catechol (Zhou et al. 2021). Enzyme C12O has high activity towards the catalytic oxidation of catechol and its methyl derivatives (Guzik et al. 2013). Enzyme G12O is one of the class III ring-cleaving dioxygenases, which catalyzes the cleavage of aromatic rings between hydroxyl and adjacent carboxyl groups in the substrate (Subbotina et al. 2021). The difference between succinic acid and malonate acid alternating enzyme activities indicated that the salicylic acid pathway was significantly promoted by dicarboxylic acids.
Genes related to PAH degradation were induced to express more under succinic acid treatment than under malonic acid treatment, leading to a higher biodegradation rate of pyrene. This is consistent with the previous results (Fig. 1). The protocatechuate 4,5-dioxygenase encoded by the gene ligA/ligB is a member of the class III exoglycol-type catechin dioxygenase family. It is shared with the aromatic ring-hydroxylating dioxygenase subunit alpha (PH00798) catalyzing ring opening of aromatics under dicarboxylic acid treatment (Liu et al. 2017b; Zeng et al. 2017). Naphthalene 1,2-dioxygenase encoded by the gene doxA is the main enzyme involved in the degradation of naphthalene (Dutta et al. 2018). Dioxygenases and dehydrogenases involved in the remaining genes were used throughout the metabolism of pyrene (Zada et al. 2021; Wang et al. 2018).
Amino acid metabolism is mainly used to synthesize nitrogen-containing substances unique to the body itself or to decompose into α-ketoacid through a series of biological processes, enter the tricarboxylic acid cycle, and release energy (Li et al. 2022). In addition, it is closely related to the interaction of plants with microorganisms, providing signaling molecules, nutrients, and defense compounds (Moormann et al. 2022). A by-product of arginine biosynthesis, fumarate is an important substrate of the tricarboxylic acid (TCA) cycle (Dai et al. 2019). Histidine can reduce the surface hydrophobicity of protein and improve the solubility and emulsifying of protein in salt solutions (Wang et al. 2021). Histidine metabolism is reported to lead to the synthesis of glutamate, and its metabolite can enter the TCA cycle to provide energy for cell growth and reproduction (Liu et al. 2017a; Brosnan and Brosnan 2020). Glutathione composed of cysteine, glutamate, and glycine can help the cell resist external stress, and reduce the toxic effect of PAHs on cells (Liu et al. 2017c). Cysteine was found to enhance the activities of dehydrogenase and peroxidase, alleviated the negative effects of PAH on plants, and increased the degradation of PAHs (Rostami et al. 2021). Pyruvate, a precursor of the TCA cycle, can also be derived from the breakdown of cysteine under anaerobic conditions. Furthermore, other amino acid pathways involved in DEGs also contribute to cell growth. Serine and threonine generate pyruvate through deamination. Valine, leucine, and isoleucine were metabolized to propionyl-CoA, acetoacetyl-CoA, and acetyl-CoA, resulting in NADH/FADH2 (Lu et al. 2017a). Lysine was metabolized by transamination reaction to finally generate α-ketoglutarate and acetoacetyl-CoA (Hallen et al. 2013). These metabolites eventually converged into the TCA cycle to provide energy and activity for cell growth and development, and increasing the solubility and bioavailability of pyrene in strain PRd5.
Pyruvate is a major intermediate for the mutual conversion of amino acids, fatty acids, sugars, and other substances to generate energy (Fu et al. 2021). It can be further metabolized to acetyl-coA into the TCA cycle (Liu et al. 2017b). Gene malX belonging to the glycolysis pathway encodes PTS maltose transporter subunit IICB, which is related to the biofilm formation, ensuring the supply of extracellular proteins and polysaccharides (Kong et al. 2021). Glyceraldehyde-3-phosphate dehydrogenase encoded by the gene gap-1 was reported to be the key enzyme of substrate phosphorylation with multiple intracellular functions such as apoptosis induction; receptor-associated kinase, tRNA export, and DNA repair (Ong et al. 2019). Gene-related pentose phosphate pathway can achieve direct oxidation and decarboxylation of glucose to produce nicotinamide adenine dinucleotide phosphate (NADPH) as a reducing agent for biosynthesis (Rashida and Laxman 2021). Dicarboxylic acids increased the energy supply for the growth and metabolic activity of strain PRd5, which may be one of the reasons why dicarboxylic acids significantly improve pyrene biodegradation.
ABC transporters are proteins that regulated the transport of basic substrates (such as amino acids, sugars, and essential metals) across membranes by the binding and hydrolysis of ATP (Rees et al. 2009). Up-regulated genes associated with ABC transport indicated that the dicarboxylic acids accelerated transmembrane transport of pyrene and energy substrates in response to pyrene stress in strain PRd5. Moreover, ABC transporters were found to assist the transfer of PAHs from extracellular to intracellular, and mediate the efflux of metabolites, thus preventing excessive accumulation of toxic compounds (Voica et al. 2016).
In summary, dicarboxylic acids altered the expression of the functional gene, increased cell growth and activity, stimulated the activities of pyrene degradation enzyme, and ultimately accelerated PAH degradation. Those up-regulated genes were distributed in the functional classification of signal transduction, membrane transport, energy metabolism, carbohydrate metabolism, and amino acid metabolism. Malonic acid mainly enhanced central carbon metabolism and cell proliferation and activity, while succinic acid mainly improved the expression of degrading genes, such as protocatechuate 4,5-dioxygenase and aromatic ring-hydroxylating dioxygenase subunit alpha and naphthalene 1,2-dioxygenase as well as providing energy for the PRd5 cells. These results provide new horizons for endophytic colonization techniques.
Data availability
The datasets of RNA sequencing raw data generated in this study have been submitted and made available in NCBI BioProject under the accession number PRJNA843874 (https://dataview.ncbi.nlm.nih.gov/object/PRJNA843874).
References
Abhilash PC, Powell JR, Singh HB, Singh BK (2012) Plant-microbe interactions: novel applications for exploitation in multipurpose remediation technologies. Trends Biotechnol 30:416–420. https://doi.org/10.1016/j.tibtech.2012.04.004
Brosnan ME, Brosnan JT (2020) Histidine metabolism and function. J Nutr 150:2570S-2575S. https://doi.org/10.1093/jn/nxaa079
Chen J, Xia X, Chu S, Wang H, Zhang Z, Xi N, Gan J (2020) Cation− π interactions with coexisting heavy metals enhanced the uptake and accumulation of polycyclic aromatic hydrocarbons in spinach. Environ Sci Technol 54(12):7261–7270. https://doi.org/10.1021/acs.est.0c00363
Chowdhury PP, Sarkar J, Basu S, Dutta TK (2014) Metabolism of 2-hydroxy-1-naphthoic acid and naphthalene via gentisic acid by distinctly different sets of enzymes in Burkholderia sp. strain BC1. Microbiology 160(5):892–902. https://doi.org/10.1099/mic.0.077495-0
Dai R, Zhou Y, Chen Y, Zhang X, Yan Y, An D (2019) Effects of arginine on the growth and microcystin-LR production of Microcystis aeruginosa in culture. Sci Total Environ 651:706–712. https://doi.org/10.1016/j.scitotenv.2018.09.213
Deveryshetty J, Phale PS (2009) Biodegradation of phenanthrene by Pseudomonas sp. strain PPD: purification and characterization of 1-hydroxy-2-naphthoic acid dioxygenase. Microbiology 155(9):3083–3091. https://doi.org/10.1099/mic.0.030460-0
Deveryshetty J, Phale PS (2010) Biodegradation of phenanthrene by Alcaligenes sp strain PPH: partial purification and characterization of 1-hydroxy-2-naphthoic acid hydroxylase. FEMS Microbiol Lett 311(1):93–101. https://doi.org/10.1111/j.1574-6968.2010.02079.x
Dominguez JJA, Inoue C, Chien MF (2020) Hydroponic approach to assess rhizodegradation by sudangrass (Sorghum x drummondii) reveals pH- and plant age-dependent variability in bacterial degradation of polycyclic aromatic hydrocarbons (PAHs). J Hazard Mater 387:121695. https://doi.org/10.1016/j.jhazmat.2019.121695
Duran R, Cravo-Laureau C (2016) Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol Rev 40:814–830. https://doi.org/10.1093/femsre/fuw031
Dutta K, Shityakov S, Khalifa I, Mal A, Moulik SP, Panda AK, Ghosh C (2018) Effects of secondary carbon supplement on biofilm-mediated biodegradation of naphthalene by mutated naphthalene 1, 2-dioxygenase encoded by Pseudomonas putida strain KD9. J Hazard Mater 357:187–197. https://doi.org/10.1016/j.jhazmat.2018.05.024
Fang T, Zhou NY (2014) Purification and characterization of salicylate 5-hydroxylase, a three-component monooxygenase from Ralstonia sp. strain U2. Appl Microbiol Biotechnol 98(2):671–679. https://doi.org/10.1007/s00253-013-4914-x
Fu A, Rooyen L, Evans L, Armstrong N, Avizonis D, Kin T, Bird GH, Reddy A, Chouchani ET, Liesa-Roig M, Walensky LD, Shapiro A, Danial NN (2021) Glucose metabolism and pyruvate carboxylase enhance glutathione synthesis and restrict oxidative stress in pancreatic islets. Cell Rep 37(8):110037. https://doi.org/10.1016/j.celrep.2021.110037
Fujita K, Haga Y, Yoshihara R, Matsumura C, Inui H (2020) Suppression of the genes responsible for transporting hydrophobic pollutants leads to the production of safer crops. Sci Total Environ 741:140439. https://doi.org/10.1016/j.scitotenv.2020.140439
Guzik U, Hupert-Kocurek K, Sitnik M, Wojcieszyńska D (2013) High activity catechol 1,2-dioxygenase from Stenotrophomonas maltophilia strain KB2 as a useful tool in cis, cis-muconic acid production. Antonie Van Leeuwenhoek 103(6):1297–1307. https://doi.org/10.1007/s10482-013-9910-8
Hallen A, Jamie JF, Cooper AJ (2013) Lysine metabolism in mammalian brain: an up-date on the importance of recent discoveries. Amino Acids 45:1249–1272. https://doi.org/10.1007/s00726-013-1590-1
Jia J, Bi C, Zhang J, Jin X, Chen Z (2018) Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: sources, exposure, and cancer risk. Environ Pollut 241:750–758. https://doi.org/10.1016/j.envpol.2018.06.002
Khan Z, Roman D, Kintz T, Alas M, Yap R, Doty S (2014) Degradation phytoprotection and phytoremediation of phenanthrene by endophyte Pseudomonas putida, PD1. Environ Sci Technol 48(20):12221–12228. https://doi.org/10.1021/es503880t
Kong X, Li C, Sun X, Niu B, Guo D, Jiang Y, Yang J, Chen Q (2021) The maltose transporter subunit IICB of the phosphor transferase system: an important factor for biofilm formation of Cronobacter. Int J Food Microbiol 370:109517. https://doi.org/10.1016/j.ijfoodmicro.2021.109517
Kuppusamy S, Thavamani P, Venkateswarlu K, Lee YB, Naidu R, Megharaj M (2017) Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: technological constraints, emerging trends and future directions. Chemosphere 168:944–968. https://doi.org/10.1016/j.chemosphere.2016.10.115
Li B, Yan ZY, Liu XN, Tang C, Zhou J, Wu XY, Wei P, Jia HH, Yong XY (2019a) Enhanced bio-electro-Fenton degradation of phenolic compounds based on a novel Fe–Mn/Graphite felt composite cathode. Chemosphere 234:260–268. https://doi.org/10.1016/j.chemosphere.2019.06.054
Li Y, Chiou CT, Li H, Schnoor JL (2019b) Improved prediction of the bioconcentration factors of organic contaminants from soils into plant/crop roots by related physicochemical parameters. Environ Int 126:46–53. https://doi.org/10.1016/j.envint.2019b.02.020
Li X, Peng D, Zhang Y, Ju D, Guan C (2020) Klebsiella sp PD3, a phenanthrene (PHE)-degrading strain with plant growth promoting properties enhances the PHE degradation and stress tolerance in rice plants. Ecotox Environ Safe 201:110804. https://doi.org/10.1016/j.ecoenv.2020.110804
Li S, Zhu X, Wang X, Zhao H, Wang D (2021) Endophytic Serratia sp. PW7 shifts bacterial endophytes in wheat (Triticum aestivum L.) to reduce pyrene contamination. Bioremediat J 26:1–13. https://doi.org/10.1080/10889868.2021.1973952
Li C, Lai X, Luo K, Zheng Y, Liu K, Wan X (2022) Integrated metabolomic and transcriptomic analyses of two peanut (Arachis hypogaea L.) cultivars differing in amino acid metabolism of the seeds. Plant Physiol Biochem 185:132–143. https://doi.org/10.1016/j.plaphy.2022.05.037
Liu Y, Gao W, Wang Y, Wu L, Liu X, Yan T, Alm E, Arkin A, Thompson DK, Fields MW, Zhou J (2005) Transcriptome analysis of Shewanella oneidensis MR-1 in response to elevated salt conditions. J Bacteriol 187:2501–2507. https://doi.org/10.1128/JB.187.7.2501-2507.2005
Liu W, Hou J, Wang Q, Yang H, Luo Y, Christie P (2015) Collection and analysis of root exudates of Festuca arundinacea L. and their role in facilitating the phytoremediation of petroleum-contaminated soil. Plant Soil 389(1–2):109–119. https://doi.org/10.1007/s11104-014-2345-9
Liu J, Xiang Y, Zhang Z, Ling W, Gao Y (2017a) Inoculation of a phenanthrene-degrading endophytic bacterium reduces the phenanthrene level and alters the bacterial community structure in wheat. Appl Microbiol Biotechnol 101(12):5199–5212. https://doi.org/10.1007/s00253-017-8247-z
Liu S, Guo C, Lin W, Wu F, Lu G, Lu J, Dang Z (2017b) Comparative transcriptomic evidence for Tween80-enhanced biodegradation of phenanthrene by Sphingomonas sp. GY2B. Sci Total Environ 609:1161–1171. https://doi.org/10.1016/j.scitotenv.2017.07.245
Liu S, Zeng G, Niu Q, Liu Y, Zhou L, Jiang L, Tan X, Xu P, Zhang C, Cheng M (2017c) Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: a mini review. Bioresour Technol 224:25–33. https://doi.org/10.1016/j.biortech.2016.11.095
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome biol 15(12):1–21. https://doi.org/10.1186/s13059-014-0550-8
Lu J, Feng J, Cai S, Chen Z (2017a) Metabolomic responses of Haliotis diversicolor to organotin compounds. Chemosphere 168:860–869. https://doi.org/10.1016/j.chemosphere.2016.10.124
Lu H, Sun J, Zhu L (2017b) The role of artificial root exudate components in facilitating the degradation of pyrene in soil. Sci Rep 7:7130. https://doi.org/10.1038/s41598-017-07413-3
Lu X, Wang W, Zhang L, Hu H, Xu P, Wei T, Tang H (2019) Molecular mechanism of N,N-dimethylformamide degradation in Methylobacterium sp. strain DM1. Appl Environ Microbiol 85(12):e00275-19. https://doi.org/10.1128/AEM.00275-19
Martin BC, George SJ, Price CA, Ryan MH, Tibbett M (2014) The role of root exuded low molecular weight organic anions in facilitating petroleum hydrocarbon degradation: current knowledge and future directions. Sci Total Environ 472:642–653. https://doi.org/10.1016/j.scitotenv.2013.11.050
Moormann J, Heinemann B, Hildebrandt TM (2022) News about amino acid metabolism in plant–microbe interactions. Trends Biochem Sci 47(10):839–850. https://doi.org/10.1016/j.tibs.2022.07.001
Nie H, Nie M, Xiao T, Wang Y, Tian X (2017) Hexadecane degradation of Pseudomonas aeruginosa NY3 promoted by glutaric acid. Sci Total Environ 575:1423–1428. https://doi.org/10.1016/j.scitotenv.2016.09.223
Ong JS, Taylor TD, Wong CB, Khoo BY, Sasidharan S, Choi SB, Ohno H, Liong MT (2019) Extracellular transglycosylase and glyceraldehyde-3-phosphate dehydrogenase attributed to the anti-staphylococcal activity of Lactobacillus plantarum USM8613. J Biotechnol 300:20–31. https://doi.org/10.1016/j.jbiotec.2019.05.006
Peng C, Chen W, Liao X, Wang M, Ouyang Z, Jiao W, Bai Y (2011) Polycyclic aromatic hydrocarbons in urban soils of Beijing: status, sources, distribution and potential risk. Environ Pollut 159(3):802–808. https://doi.org/10.1016/j.envpol.2010.11.003
Rashida Z, Laxman S (2021) The pentose phosphate pathway and organization of metabolic networks enabling growth programs. Curr Opin Struct Biol 28:100390. https://doi.org/10.1016/j.coisb.2021.100390
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Bio 10(3):218–227. https://doi.org/10.1038/nrm2646
Reddy PV, Karegoudar TB, Monisha TR, Mukram I, Nayak AS (2018) Biodegradation of fluoranthene by Paenibacillus sp. strain PRNK-6: a pathway for complete mineralization. Arch Microbiol 200(1):171–182. https://doi.org/10.1007/s00203-017-1431-9
Rostami S, Azhdarpoor A, Rostami M, Mohammadi F, Jaskulak M, Dehghani M, Samari MR, Baghapour MA (2021) Improvement of the Rhizoremediation efficiency of PAHs contaminated soil under cysteine treatment along with modeling. Environ Nanotechnol Monit Manag 16:100519. https://doi.org/10.1016/j.enmm.2021.100519
Sah S, Phale PS (2011) 1-Naphthol 2-hydroxylase from Pseudomonas sp. strain C6: purification, characterization and chemical modification studies. Biodegradation 22(3):517–526. https://doi.org/10.1007/s10532-010-9424-2
Seo J, Keum Y, Li Q (2009) Bacterial degradation of aromatic compounds. Int J Environ Res Public Health 6(1):278–309. https://doi.org/10.3390/ijerph6010278
Shen Y, Sheng Y, Li J, Zhu J, Shi S, Zhan X (2020) The role of temperature in phenanthrene transfer and accumulation in crop leaves. Environ Pollut 258:113827. https://doi.org/10.1016/j.envpol.2019.113827
Sivaram AK, Logeshwaran P, Lockington R, Naidu R, Megharaj M (2019) Low molecular weight organic acids enhance the high molecular weight polycyclic aromatic hydrocarbons degradation by bacteria. Chemosphere 222:132–140. https://doi.org/10.1016/j.chemosphere.2019.01.110
Sivaram AK, Logeshwaran P, Lockington R, Naidu R, Megharaj M (2020) The impact of low molecular weight organic acids from plants with C3 and C4 photosystems on the rhizoremediation of polycyclic aromatic hydrocarbons contaminated soil. Environ Technol Innov 19:100957. https://doi.org/10.1016/j.eti.2020.100957
Sokolova TA (2020) Low-molecular-weight organic acids in soils: sources, composition, concentrations, and functions: a review. Eurasian Soil Sci 53(4):580–594. https://doi.org/10.1134/S1064229320050154
Subbotina NM, Chernykh AM, Taranov AI, Shebanova AD, Moiseeva OV, Ferraroni M, Kolomytseva MP (2021) Gentisate 1, 2-dioxygenase from the gram-positive bacteria Rhodococcus opacus 1CP: identical active sites vs. different substrate selectivities. Biochimie 180:90–103. https://doi.org/10.1016/j.biochi.2020.10.016
Sun K, Liu J, Gao Y, Jin L, Gu Y, Wang W (2015) Isolation, plant colonization potential and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp. Sci Rep Uk 4(1):1–8. https://doi.org/10.1038/srep05462
Sun S, Wang H, Chen Y, Lou J, Wu L, Xu J (2019) Salicylate and phthalate pathways contributed differently on phenanthrene and pyrene degradations in Mycobacterium sp. WY10. J Hazard Mater 364:509–518. https://doi.org/10.1016/j.jhazmat.2018.10.064
Tarasev M, Pinto A, Kim D, Elliott SJ, Ballou DP (2006) The “bridging” aspartate 178 in phthalate dioxygenase facilitates interactions between the Rieske center and the Fe(II)-mononuclear center. Biochemistry 45(34):10208–10216. https://doi.org/10.1021/bi060219b
Técher D, Laval-Gilly P, Henry S, Bennasroune A, Formanek P, Martinez-Chois C, Falla J (2011) Contribution of Miscanthus x giganteus root exudates to the biostimulation of PAH degradation: an in vitro study. Sci Total Environ 409(20):4489–4495. https://doi.org/10.1016/j.scitotenv.2011.06.049
Trivedi VD, Majhi P, Phale PS (2014) Kinetic and spectroscopic characterization of 1-naphthol 2-hydroxylase from Pseudomonas sp strain C5. Appl Biochem Biotechnol 172(8):3964–3977. https://doi.org/10.1007/s12010-014-0815-4
Voica DM, Bartha L, Banciu HL, Oren A (2016) Heavy metal resistance in halophilic bacteria and archaea. FEMS Microbiol Lett 363(14):1–9. https://doi.org/10.1093/femsle/fnw146
Wang Y, Fang L, Lin L, Luan T, Tam NFY (2014) Effects of low molecular-weight organic acids and dehydrogenase activity in rhizosphere sediments of mangrove plants on phytoremediation of polycyclic aromatic hydrocarbons. Chemosphere 99:152–159. https://doi.org/10.1016/j.chemosphere.2013.10.054
Wang H, Lou J, Gu H, Luo X, Yang L, Wu L, Liu Y, Wu J, Xu J (2016) Efficient biodegradation of phenanthrene by a novel strain Massilia sp. WF1 isolated from a PAH-contaminated soil. Environ Sci Pollut Res 23:13378–13388. https://doi.org/10.1007/s11356-016-6515-6
Wang J, Zhang X, Ling W, Liu R, Liu J, Kang F, Gao Y (2017) Contamination and health risk assessment of PAHs in soils and crops in industrial areas of the Yangtze River Delta region, China. Chemosphere 168:976–987. https://doi.org/10.1016/j.chemosphere.2016.10.113
Wang W, Wang L, Shao Z (2018) Polycyclic aromatic hydrocarbon (PAH) degradation pathways of the obligate marine PAH degrader Cycloclasticus sp. strain P1. Appl Environ Microbial 84(21):e01261-18. https://doi.org/10.1128/AEM.01261-18
Wang Y, Ma T, Liu C, Guo F, Zhao J (2021) l-Histidine improves solubility and emulsifying properties of soy proteins under various ionic strengths. LWT 152:112382. https://doi.org/10.1016/j.lwt.2021.112382
Wei R, Hui C, Zhang Y, Xu L, Zhao Y, Du L, Jiang H (2021) Response of heterotrophic nitrification-aerobic denitrification bacterium Pseudomonas aeruginosa P-1 to Cd2+ and Pb2+ on ammonium removal performance, physiology, and transcriptome analysis. Int Biodeterior Biodegradation 165:105326. https://doi.org/10.1016/j.ibiod.2021.105326
Xiao M, Wu F (2014) A review of environmental characteristics and effects of low-molecular weight organic acids in the surface ecosystem. J Environ Sci (china) 26:935–954. https://doi.org/10.1016/S1001-0742(13)60570-7
Yue Z, Chen Y, Chen C, Ma K, Tian E, Wang Y, Liu H, Sun Z (2021) Endophytic Bcillus altitudinid WR10 alleviates Cu toxicity in wheat by augmenting reactive oxygen species scavenging and phenylpropanoid biosynthesis. J Hazard Mater 405:124272. https://doi.org/10.1016/j.jhazmat.2020.124272
Zada S, Zhou H, Xie J, Hu Z, Ali S, Sajjad W, Wang H (2021) Bacterial degradation of pyrene: biochemical reactions and mechanisms. Int Biodeterior Biodegrad 162:105233. https://doi.org/10.1016/j.ibiod.2021.105233
Zeng J, Zhu Q, Wu Y, Chen H, Lin X (2017) Characterization of a polycyclic aromatic ring-hydroxylation dioxygenase from Mycobacterium sp. NJS-P. Chemosphere 185:67–74. https://doi.org/10.1016/j.chemosphere.2017.07.001
Zhang L, Li X, Zuo W, Li S, Sun G, Wang W, Yu Y, Huang H (2021) Root exuded low-molecular-weight organic acids affected the phenanthrene degrader differently: a multi-omics study. J Hazard Mater 414:125367. https://doi.org/10.1016/j.jhazmat.2021.125367
Zhang Y, Huang H, Xiong G, Duan Y, Cai C, Wang X, Li J, Tao S, Liu W (2020) Structural equation modeling of PAHs in surrounding environmental media and field yellow carrot in vegetable bases from Northern China: in comparison with field cabbage. Sci Total Environ 717:137261. https://doi.org/10.1016/j.scitotenv.2020.137261
Zhou F, Last RL, Pichersky E (2021) Degradation of salicylic acid to catechol in Solanaceae by SA 1-hydroxylase. Plant Physiol 185(3):876–891. https://doi.org/10.1093/plphys/kiaa096
Acknowledgements
The authors would like to thank Professor Lu Junhe, Professor Yang Xinping, and the reviewers for improving the manuscript substantially.
Funding
This work was financially supported by the National Key R & D Plan Key Project (2019YFC1804301) and the National Natural Science Foundation of China (31870489).
Author information
Authors and Affiliations
Contributions
ZX and LC conceived and designed the research. LC and CR performed the experiments; LC, ZF, SL, and ZZ analyzed the data; ZX coordinated and supervised this study; LC drafted the manuscript. ZX revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval.
This article does not contain any studies with human participants or animals that were performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, C., Zhang, F., Sun, L. et al. Contribution of dicarboxylic acids to pyrene biodegradation and transcriptomic responses of Enterobacter sp. PRd5. Appl Microbiol Biotechnol 106, 7949–7961 (2022). https://doi.org/10.1007/s00253-022-12217-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12217-1