Abstract
4-hydroxyisoleucine (4-HIL) has a potential value in treating diabetes. The α-ketoglutarate (α-KG)-dependent isoleucine dioxygenase (IDO) can catalyze the hydroxylation of L-isoleucine (Ile) to form 4-HIL by consuming O2. In our previous study, the ido gene was overexpressed in an Ile-producing Corynebacterium glutamicum strain to synthesize 4-HIL from glucose. Here, a triple-functional dynamic control system was designed to regulate the activity of IDO, the supply of α-KG, O2, and Ile and the synthesis of by-product L-lysine (Lys) for promoting 4-HIL synthesis. Firstly, the codon-optimized ido was positively regulated by seven Ile biosensors Lrp-PbrnFEN with different intensities, and the resulting seven D-NI strains produced 38.7–111.1 mM 4-HIL. Then on the basis of D-NI, odhI and vgb were simultaneously regulated by three PbrnFEN with different intensities to synergistically control α-KG and O2 supply. The 4-HIL titer of twelve D-NINONV strains was more than 90 mM, with D-0I7O7V generating the highest titer of 141.1 ± 15.5 mM. Thirdly, ilvA was negatively regulated by an Ile attenuator PilvBNC on the basis of D-NI strains and some D-NINONV strains to balance the synthesis and conversion of Ile. The resulting D-NIPA strains produced 73.6–123.2 mM 4-HIL, while D-7I7O1VPA accumulated 127.1 ± 20.2 mM 4-HIL. Finally, dapA was negatively regulated by a Lys-OFF riboswitch and Lys content decreased by approximately 70% in most D-RS-NIPA strains. A strain D-RS-5IPA with the highest 4-HIL titer (177.3 ± 8.9 mM) and the lowest Lys concentration (6.1 ± 0.6 mM) was successfully obtained. Therefore, dynamic regulation of main and branch pathway by three functional biosensors can effectively promote 4-HIL biosynthesis in C. glutamicum.
Key points
• Three biosensors were coordinated for dynamic 4-HIL biosynthesis in C. glutamicum
• Bidirectional regulation of Ile synthesis and conversion promoted 4-HIL synthesis
• Negative regulation of Lys synthesis further increased 4-HIL production
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic engineering is a powerful tool for the production of valuable chemicals (Jones et al. 2015; Nielsen and Keasling 2016). Metabolic engineering mainly includes static metabolic engineering and dynamic metabolic engineering. Static metabolic engineering has been tried and proved to be effective for some targets (Holtz and Keasling 2010; Liu et al. 2015). Its main strategies include engineering promoter (Jin et al. 2019), ribosome binding site (Nowroozi et al. 2014; Salis et al. 2009), and gene copy number (Jones et al. 2000). However, when the intracellular metabolic flow is rewired, it will impair microbial growth and production (Hartline et al. 2021). Dynamic metabolic engineering can regulate gene expression according to the changes of intracellular state and environmental conditions, thereby alleviate the growth retardation and metabolic flow imbalance caused by static engineering, and significantly increase the yield of target products (Anesiadis et al. 2008; Liu et al. 2015). Dynamic control strategies mainly include optogenetics switch (Renicke and Taxis 2016), temperature sensing switch (Zhou et al. 2018), biomolecular-sensor devices (Yang et al. 2018; Zhang et al. 2012), RNA-based dynamic controller (Zhou et al. 2019), quorum sensing devices, and so on (Liu et al. 2021), which have been applied in many fields. Among them, biomolecular-sensor devices can sense the concentration of certain metabolites and thereby regulate gene expression mainly through transcription factor (TF)-promoter pairs. RNA-based dynamic controller such as riboswitch can sense the concentration of specific metabolites and thereby mediate the structural changes of RNA and regulate the expression of their downstream genes. For example, Farmer et al. constructed a dynamic control switch to regulate lycopene synthesis by using acetyl phosphate responsive TF-promoter (Farmer and Liao 2000). Zhou and Zeng used L-lysine (Lys)-responsive riboswitch to regulate Lys synthesis (Zhou and Zeng 2015).
Corynebacterium glutamicum is a gram-positive bacterium widely used in amino acid industry (Becker et al. 2018). Products produced by C. glutamicum are generally recognized as safe. However, unlike Escherichia coli and Saccharomyces cerevisiae, there are only a few dynamic regulatory elements that have been well characterized and applied for metabolic engineering in C. glutamicum (Venayak et al. 2015). These tools mainly include the Lrp-PbrnFE biosensor that can upregulate gene expression by sensing branched chain amino acids (BCAAs) and L-methionine (Met) (Mustafi et al. 2012; Tan et al. 2020), the PilvBNC attenuator that can downregulate gene expression by responding to BCAAs (Morbach et al. 2000), the LysG-PlysE biosensor upregulating gene expression by sensing Lys, L-histidine, and L-arginine (Kortmann et al. 2019), the Lys riboswitch that can turn off gene expression by binding Lys (Zhou and Zeng 2015), and the ShiR-PshiA biosensor responding to shikimic acid (Liu et al. 2018).
The TF-based biosensor Lrp-PbrnFE has been used to dynamically regulate odhI expression and α-ketoglutarate (α-KG) supply for 4-hydroxyisoleucine (4-HIL) biosynthesis in C. glutamicum (Zhang et al. 2018). Quite recently, this biosensor has been further mutated and the modified strong biosensor Lrp-PbrnFEN was used to positively regulate the expression of odhI and vgb, resulting in a significant increase in the yield of 4-HIL (Tan et al. 2020). Besides the TF-based biosensor, attenuator and riboswitch were also used to regulate gene expression by sensing metabolite concentration. The PilvBNC promoter region of C. glutamicum contains an attenuator, which can also sense the concentration of BCAAs (Morbach et al. 2000). PilvBNC was used to attenuate and regulate icd expression, which increased the production of L-leucine (Leu) (Luo et al. 2021). PilvBNC was also used to attenuate and regulate the expression of odhA gene, which increased the production of 4-HIL (Zhang et al. 2018). The odhA gene encodes the E1 subunit of α-ketoglutarate dehydrogenase complex (ODHC). Riboswitch is a regulatory fragment located in the 5′ untranslated region (UTR) of mRNA. Riboswitch can detect and bind small molecule metabolites and then mediate the change of the RNA secondary structure, thereby affecting transcription termination and/or translation initiation (Winkler and Breaker 2005). Lys riboswitch has been successfully applied to Lys production. Zhou and Zeng used Lys-OFF riboswitch from E. coli to regulate gltA expression in C. glutamicum, which increased Lys production (Zhou and Zeng 2015). However, the Lys riboswitch has never been reported to be used in the synthesis of 4-HIL in C. glutamicum.
4-HIL can promote insulin secretion and decrease insulin resistance; thus, it is a promising drug for diabetes (Yang et al. 2020; Zafar and Gao 2016). Isoleucine dioxygenase (IDO) can catalyze the hydroxylation of C-4 of L-isoleucine (Ile) to form 4-HIL by consuming O2, and α-KG is oxidized to succinic acid at the same time (Smirnov et al. 2010). In our previous study, the ido gene was expressed in an Ile producing strain C. glutamicum SN01 for de novo biosynthesis of 4-HIL (Shi et al. 2015). Subsequently, the yield of 4-HIL was further improved by static metabolic engineering (Shi et al. 2016, 2018, 2019, 2020). However, due to the inhibition of IDO by the excess substrate Ile and the requirement of coordinated supply of three substrates, it is difficult to increase the yield of 4-HIL by using the common static metabolic strategies. Then, the Lrp-PbrnFEN biosensor was used to dynamically upregulate IDO activity and the supply of α-KG and oxygen according to Ile concentration. The 4-HIL production was increased to 135.3 mM (Tan et al. 2020). However, during fermenting in a bioreactor, Ile accumulated too fast, which exacerbated the substrate inhibition and made the yield of 4-HIL unable to increase further. Meanwhile, a large amount of Lys was produced. Therefore, in addition to the upregulation of the downstream pathway for converting Ile to 4-HIL, it is necessary to dynamically regulate the upstream pathway for synthesizing Ile to avoid substrate inhibition. Meanwhile the synthesis pathway of by-product Lys shall be dynamically downregulated.
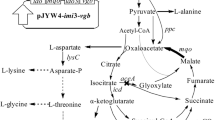
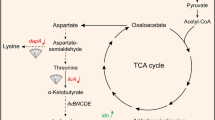
In this study, a triple-functional dynamic control system was designed to regulate the synthesis of 4-HIL. In order to accelerate the conversion of Ile to 4-HIL, the codon-optimized ido was regulated by the Lrp-PbrnFEN biosensor. This Ile biosensor was then used to dynamically upregulate the supply of other two substrates α-KG and oxygen. Meanwhile, in order to balance the supply of Ile and promote the conversion of Ile to 4-HIL, Ile attenuator PilvBNC was used to dynamically downregulate the expression of ilvA, a key gene in Ile synthesis pathway. Finally, in order to reduce the concentration of by-product Lys, the Lys-OFF riboswitch from E. coli MG1655 was used to negatively regulate the expression of dapA, the key gene in Lys synthesis pathway (Fig. 1). The excellent strains with high yield of 4-HIL and no by-products or extremely low by-products content were obtained.
Materials and methods
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli JM109 was used for plasmid construction and propagation. E. coli was cultivated at 37 °C and 200 rpm in Luria–Bertani (LB) medium. C. glutamicum ssp. lactofermentum SN01, an Ile-producing strain, was used for expressing target genes and synthesizing 4-HIL. SN01 was deposited into the China Center for Type Culture Collection (CCTCC) with accession number CCTCC M 2,014,410. The C. glutamicum strain was cultivated at 30 °C and 200 rpm in LBB medium (5 g/L tryptone, 2.5 g/L yeast extract, 5 g/L NaCl, and 18.5 g/L brain heart infusion powder). When necessary, 30 mg/L kanamycin or 10 mg/L chloramphenicol was added to the media.
Construction of dynamic modulation plasmids and strains
All primers used in this study are listed in Table 2. According to previous studies on Ile biosensors, seven inductive promoters of PbrnFEN which can be induced by Ile, i.e., the native PbrnFE (designated as PbrnFE0) and the modified PbrnFE1, PbrnFE5, PbrnFED5, PbrnFE7, PbrnFE9, and PbrnFE13, were designed for expressing target genes (Tan et al. 2020). PbrnFED5 contains two promoter regions, which correspond to PbrnFE5 and the natural PbrnFE0 promoter, respectively. Firstly, the parent plasmid pIL-IU was constructed to dynamically control ido expression. Before construction, the codon-optimized ido gene (idoU) was synthesized. Its sequence was submitted to the GenBank with accession number OM541951. idoU was ligated into pJYW-4 vector (Hu et al. 2014) to form the static expression plasmid pJYW-4-idoU. It was transformed into C. glutamicum SN01, generating the static strain SN01/pJYW-4-idoU, renamed S-I. The lrp-PbrnFEN fragment and idoU gene were amplified, fused, and ligated into the KpnI- and BamHI-digested pIL-I plasmid (Tan et al. 2020), generating seven dynamic plasmids pIL-NIU. These 7 plasmids were transformed into C. glutamicum SN01, generating 7 strains SN01/pIL-NIU, renamed D-NI. Secondly, the plasmids pIL-NIUNONV were constructed to dynamically modulate idoU, odhI, and vgb expression. The 9 PbrnFEN-odhI-PbrnFEN-vgb fragments were amplified from plasmid pIL-7INONV (N = 0, 1, 7) (Tan et al. 2020) and then ligated into the 6 pIL-NIU (N = 0, 1, 5, D5, 7, 9) vectors to form 6 × 9 = 54 pIL-NIUNONV plasmids. These plasmids were transformed into C. glutamicum SN01, generating 54 strains SN01/pIL-NIUNONV, renamed D-NINONV. Thirdly, the plasmids pIL-NIUPA were constructed to positively regulate idoU by Lrp-PbrnFEN and negatively regulate ilvA by PilvBNC. The fragments of PilvBNC and ilvA were amplified from SN01, fused, and ligated into 6 pIL-NIU vectors to form 6 pIL-NIUPA plasmids. These 6 plasmids were transformed into C. glutamicum SN01, generating 6 strains SN01/pIL-NIUPA, renamed D-NIPA. Fourthly, the plasmids pIL-NIUNONVPA were constructed to positively regulate idoU, odhI, and vgb by Lrp-PbrnFEN and negatively regulate ilvA by PilvBNC. The fragment of PilvBNC-ilvA was amplified and ligated into 12 pIL-NIUNONV vectors to form 12 pIL-NIUNONVPA plasmids. However, the pIL-9IU7O7V, pIL-0IU7O7V, and pIL-D5IU7O7V plasmids were failed to be constructed. These 9 plasmids were transformed into C. glutamicum SN01, generating 9 strains SN01/pIL-NIUNONVPA, renamed D-NINONVPA.
At last, the fluorescent reporter plasmid pJYW-4-PilvBNC-egfp was constructed. The fragments of PilvBNC and egfp were amplified from SN01 and pJYW-4-egfp, respectively, fused, and ligated into pJYW-4 vector, resulting in the PilvBNC-regulated reporter plasmid pJYW-4-PilvBNC-egfp. This plasmid was then transformed into C. glutamicum ATCC 13,032, generating the strain 13,032-PilvBNC-egfp.
Integration of Lys-OFF riboswitch in the upstream of dapA gene in chromosome
The Lys-OFF riboswitch was introduced to dynamically control the expression of dapA gene and the synthesis of Lys. Based on pK18mobsacB editing system (Schäfer et al. 1994), the Lys-OFF riboswitch (Zhou and Zeng 2015) was integrated before the start codon of dapA gene in C. glutamicum ssp. lactofermentum SN01 and the synthesis of Lys was negative regulated by intracellular Lys concentration. The upstream and downstream homologous arms of dapA were amplified from C. glutamicum SN01. The Lys-OFF riboswitch was amplified from E. coli MG1655. Then the fragments of dapA-U, Lys-OFF riboswitch, and dapA-D were linked by overlap PCR to obtain dapA-U-LysRS-dapA-D fragment. The dapA-U-LysRS-dapA-D fragment was ligated into the SalI-digested pK18mobsacB vector, resulting in the integrating plasmid pK18mobsacB-PdapA-LysRS. This plasmid was transformed into C. glutamicum ssp. lactofermentum SN01 for two rounds of homologous recombination to get the recombinant strain SN01 PdapA::PdapA-LysRS (designated as D-RS). Finally, the above 6 dynamic regulatory plasmids of pIL-NIUPA were transformed into D-RS, resulting in 6 strains D-RS-NIPA. Meanwhile, the above dynamic regulatory plasmids of pIL-7IU7O1VPA with high yield of 4-HIL were transformed into D-RS for expression and resulted in strain D-RS-7I7O1VPA.
4-HIL fermentation
4-HIL fermentation of statically and dynamically controlled C. glutamicum ssp. lactofermentum strains in shake flasks were conducted as described previously, using optimized fermentation medium (Shi et al. 2019). The cell density, residual glucose, pH, and amino acid and 4-HIL concentrations in the fermentation broth were measured every 24 h by the methods described previously (Shi et al. 2018). The residual glucose concentration was measured by an SBA-40C biosensor (Institute of Biology, Shandong Academy of Science, China). The pH was measured by a pH electrode (Mettler-Toledo, Germany). The amino acids and 4-HIL concentrations were detected by high performance liquid chromatography (HPLC) analysis (Shi et al. 2016). Firstly, protein impurities in the fermentation broth were precipitated with 5% trichloroacetic acid, and then, the precipitate was removed by centrifugation and the supernatant was diluted 20 times for HPLC analysis. The amino acids and 4-HIL concentrations were determined by Agilent 1200 HPLC detector equipped with a Thermo ODS-2 HYPERSIL C18 column (250 mm × 4.6 mm, USA) using the ortho-phthalaldehyde precolumn derivatization method. The conversion ratio of Ile to 4-HIL was calculated as the moles of 4-HIL divided by the total moles of Ile and 4-HIL. To determine the intracellular amino acids’ concentrations, the cell sedimentation was collected by centrifugation, then washed, and disrupted as described previously to isolate the intracellular amino acids (Shi et al. 2016). The intracellular concentration was calculated with the intracellular volume of 1.6 mL/g dry cell weight (DCW). The DCW (g/L) was calculated according to an experimentally determined formula: DCW = 0.6495 × OD562 − 2.7925.
Fluorescence assays
The fluorescent reporter strain 13,032-PilvBNC-egfp was precultured in LBB medium for 12 h, and the cells were washed and resuspended with 0.9% NaCl solution. Then, the cell resuspension was transferred to the Ile restricted medium (25 g/L glucose·H2O, 1 g/L KH2PO4, 0.5 g/L (NH4)2SO2, 0.75 g/L MgSO4, 1.0 g/L FeSO4, 10 μg/L biotin, 1 mg/L thiamine, and 0–10 mM Ile, pH 7.20) with the initial OD562 of 0.05 and cultured for 24 h. At every 4 h, the cultured cells were collected, washed with, and resuspended in 0.9% NaCl solution. Subsequently, the cell suspension was transferred to a black 96-well plate with a transparent bottom. Green fluorescence was detected by the Cytation5 (BiotTek) with an excitation filter of 479/20 nm and an emission filter of 520/20 nm.
Results
Positive regulation of codon-optimized ido expression by Ile biosensor
Isoleucine dioxygenase (coded by ido) that catalyzes the conversion of Ile to 4-HIL is a key enzyme for de novo synthesis of 4-HIL. The expression level of ido and the enzyme activity of IDO are very important for the synthesis of 4-HIL. In the previous research, the strong promoter PtacM was used to constitutively express ido in plasmid pJYW-4-ido of strain SN02 as well as in other plasmids such as pJYW-4-imi3-vgb of strain SZ05 (Shi et al. 2015, 2019). However, constitutive expression brought metabolic burden to cells, and intracellular resources would be continuously utilized to synthesize IDO. Then Ile biosensor Lrp-PbrnFEN was used to dynamically control the expression of ido, because Ile is the direct precursor of 4-HIL synthesis. PbrnFEN were several modified promoters of native PbrnFE promoter with different strengths. They were obtained from the lrp-PbrnFE mutant library through tetA dual genetic selection, and their − 10 region and spacer between − 35 and − 10 regions were modified (Tan et al. 2020). Although the 4-HIL titer was comparable to that of PtacM-controlled ido expressing strain SN02, the titer was not ideal. Besides the Ile sensitivity and threshold of Lrp-PbrnFEN biosensors as well as their strength after activation, the preference of ido codons and burden expression of other proteins may also influence the synthesis of 4-HIL. So, the codon-optimized ido gene was expressed and dynamically controlled here by several Ile biosensors Lrp-PbrnFEN (Fig. 2A). The resulting seven plasmids pIL-NIU were transferred into SN01 to obtain D-NI strains (N = 0, 1, 5, D5, 7, 9, 13). And the pJYW-4-idoU plasmid was transferred into SN01 strain to obtain SI as static control strain.
Regulation mode and 4-HIL fermentation of strains D-NI. A Diagram of the Lrp-PbrnFEN regulation circuit. Transcription factor Lrp combines with BCAAs. Then Ile-Lrp complex binds to PbrnFEN, thereby activates the transcription of PbrnFEN-controlled ido.U gene, and promotes the conversion of Ile to 4-HIL according to Ile concentration. B Cell growth and glucose consumption. C 4-HIL accumulation. D Ile accumulation. E Other amino acids concentration at 144 h. F Intracellular accumulation of Ile, Val, Met and Leu in D-1I
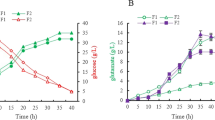
During the whole fermentation process, D-7I grew slightly faster, while other 6 D-NI strains showed similar growth rate to SI. However, the sugar consumption of 7 D-NI strains were similar to each other and were slightly faster than SI (Fig. 2B). Although the 4-HIL titer of D-13I was extremely low, the overall 4-HIL titer of other 6 D-NI strains was high and similar (Fig. 2C). And compared with the ido expression strains ST01–ST06 controlled by the same biosensor (Tan et al. 2020), the 4-HIL production of idoU-expressing strains D-NI improved overall. Finally, D-1I, D-0I, D-D5I, and D-5I accumulated 111.1 ± 0.8, 106.3 ± 7.1, 97.5 ± 5.7 and 92.0 ± 6.7 mM 4-HIL, respectively, which was 23.5%, 18.2%, 8.4%, and 2.3% higher than SI (89.9 ± 2.5 mM) and 57.8%, 51.0%, 38.4%, and 30.7% higher than the best ido-expressing strain ST04 (70.4 ± 8.2 mM) controlled by the same biosensors. While D-7I and D-9I accumulated 84.2 ± 0.7 and 79.5 ± 2.8 mM 4-HIL respectively, which was 6.4% and 11.6% lower than SI and 19.6% and 12.9% higher than the best ido-expressing strain ST04 controlled by the same biosensors. The above results suggested that the expression of codon-optimized idoU gene increased the IDO activity, which enhanced the conversion of Ile to 4-HIL. Dynamically regulating the expression of idoU by Lrp-PbrnFEN biosensors was more conducive to the synthesis of 4-HIL than dynamically regulating the expression of ido by the same biosensors.
In D-1I, Ile accumulated rapidly in 24 h and then reduced gradually to 0 mM during 24–96 h, while in D-D5I and D-7I, Ile was consumed before 48 h (Fig. 2D). The synthesis of 4-HIL may be limited in these three strains due to the insufficient supply of Ile. In D-0I, D-5I, and D-9I, Ile accumulated continuously in 72 h and slightly decreased and fluctuated thereafter (Fig. 2D). Finally, 40–80 mM of Ile was remained and not converted to 4-HIL. Besides the substrate Ile, the α-KG and oxygen are also required in the synthesis of 4-HIL. The 4-HIL titer of these three strains did not increase after 96 h, likely due to the insufficient supply of α-KG and oxygen. However, in the D-13I strain, the yield of 4-HIL was relatively low, and a large amount of Ile was not converted into 4-HIL (Fig. 2C and 2D). This result suggested that the strength of PbrnFE13 was low, which led to the low activity of IDO enzyme, thus triggering the strong inhibition of IDO by excess Ile and the extra accumulation of by-product L-threonine (Thr), the precursor of Ile synthesis. Therefore, this strain was discarded in the subsequent study. In all of the 7 recombinant D-NI strains, the content of by-product Lys was high, while other by-products were almost absent or showed extremely low content (Fig. 2E). Thereby, besides the dynamic control of the ido expression, the supply of various substrates, such as oxygen, α-KG, and Ile, should be appropriately controlled.
Lrp responds to Ile and also to Met, L-valine (Val) and Leu. The intracellular or extracellular accumulation of Val, Met, and Leu may also affect the activated level of Lrp-PbrnFEN biosensors and the yield of 4-HIL. Therefore, the intracellular and extracellular contents of Ile, Val, Met, and Leu in the best idoU-expressing strain D-1I were determined. Its extracellular contents of Val, Met, and Leu were very low (data not shown), while its intracellular contents of Ile, Val, Met, and Leu were 19.0–7.3 mM, 10.5–3.2 mM, 8.1–1.7 mM, and 3.4–1.3 mM, respectively (Fig. 2F). Meanwhile, these intracellular contents were relatively high during 24–96 h, but much low thereafter, in accordance with the quick accumulation of 4-HIL during 24–96 h and slow thereafter (Fig. 2C).
In the previous study, PbrnFEN promoters were divided into strong (PbrnFE7), medium (PbrnFE1, PbrnFE5) and weak (PbrnFE0, PbrnFE9, PbrnFE13) groups, and the 4-HIL yield of the ido-expressing strain dynamically controlled by strong promoter PbrnFE7 was the highest (Tan et al. 2020). Here, the 4-HIL yield of the idoU-expressing strain under PbrnFE7 promoter was also high. As expected, the 4-HIL yield of idoU-expressing strains controlled by other PbrnFEN promoters has been improved in greater degrees, which indicated that the IDO activity of codon-optimized idoU-expressing strains is improved, and all the PbrnFE0, PbrnFE1, PbrnFE5, PbrnFED5, PbrnFE7, and PbrnFE9 were strong for expressing idoU. Therefore, the strains D-NI (N = 0, 1, 5, D5, 7, or 9) with high yield of 4-HIL were used for further study.
Positive regulation of ido.U , odhI, and vgb expression by Ile biosensor
Although the synthesis of 4-HIL was improved in the above six D-NI strains, Ile was used up too early in D-D5I and D-7I, while in D-0I, D-5I, and D-9I, Ile was no longer converted to 4-HIL in the later fermentation stage. The substrates required for the synthesis of 4-HIL include not only Ile, but also α-KG and O2. Therefore, odhI and vgb were overexpressed in this section to promote the coordinated supply of the other two substrates, α-KG and O2. The unphosphorylated OdhI can bind to OdhA subunit of ODHC, thus inhibiting ODHC activity and finally intercepting more α-KG for the synthesis of 4-HIL. The vgb gene encodes Vitreoscilla hemoglobin VHb, which can increase the uptake of O2 by cells. However, excessive O2 intake will form free radicals and cause damage to cells. In our previous studies, PbrnFE7 with high strength, PbrnFE1 with medium strength and PbrnFE0 with low strength were used to control odhI and vgb expression in dynamic ido-expression strains, which promoted the titer of 4-HIL to 135.34 mM (Tan et al. 2020). Therefore, in this section, these 3 PbrnFEN promoters were used to positively regulate odhI and vgb expression on the basis of 6 dynamic idoU-expression plasmids pIL-NIU (Fig. 3A). The obtained 54 plasmids of pIL-NIUNONV were transformed into SN01, and 54 tri-gene dynamic regulatory strains named D-NINONV were obtained.
Regulation circuit and 4-HIL production of strains D-NINONV. A Diagram of regulation circuit. Lrp-PbrnFEN positively regulated the expression of ido.U, odhI, and vgb in response to intracellular Ile concentration. B 4-HIL production of D-NINONV. C Intracellular accumulation of Ile, Val, Met, and Leu in D-0I7O7V. D 4-HIL production of D-0I7O7V after adding 10 mM Val, Met, or Leu
In the 9 D-0INONV strains, D-0I0O1V, D-0I1O1V, D-0I1O7V, and D-0I7O7V accumulated 119.4 ± 22.4, 125.3 ± 27.6, 134.5 ± 12.3, and 141.1 ± 15.5 mM 4-HIL, respectively (Fig. 3B), which increased by 12.3%, 17.9%, 26.5%, and 32.7% compared with D-0I (106.3 ± 7.1 mM). While the 4-HIL yield of D-0I0O7V (92.1 ± 18.8 mM) was a little lower than that of D-0I, but it was still 2.4% higher than that of SI (89.9 ± 2.5 mM). In the 9 D-9INONV strains, D-9I0O0V, D-9I0O1V, D-9I0O7V, and D-9I7O7V accumulated 107.3 ± 17.9, 115.5 ± 21.1, 103.2 ± 4.2, and 131.2 ± 4.5 mM 4-HIL, respectively (Fig. 3B), which increased by 35.0%, 45.4%, 29.8%, and 65.1% compared with D-9I (79.5 ± 2.8 mM). The above results indicated that the dynamic supply of α-KG and oxygen could promote the remaining Ile in D-0I and D-9I to convert into 4-HIL and thereby further increase the yield of 4-HIL. In the 9 D-1INONV strains, only D-1I0O7V could produce more 4-HIL (Fig. 3B), but its yield of 4-HIL was lower than those of D-1I and SI, so the D-1I0O7V strain was discarded in the subsequent studies. Perhaps the supply of three substrates in D-1I is relatively coordinated with the synthesis of 4-HIL, making further supply of α-KG and oxygen ineffective in D-1I. In the 9 D-5INONV strains, 9 D-D5INONV strains and 9 D-7INONV strains, the 4-HIL yield of D-5I0O1V (89.9 ± 10.8 mM) and D-D5I7O7V (93.5 ± 12.2 mM) was similar to that of SI, and the 4-HIL yield of D-7I7O1V (132.7 ± 13.3 mM) increased by 57.6% compared with D-7I (84.20 ± 0.7 mM), while the 4-HIL yield of other strains were lower than that of D-5I, D-D5I, D-7I, and SI (Fig. 3B). The scarcely improved 4-HIL production in these strains suggests that other reason such as the insufficient supply of Ile might also be a crucial constraint for 4-HIL synthesis. Thereby, dynamic regulation of Ile synthesis was performed in the next section. In addition, 12 strains of this section, i.e., D-5I0O1V, D-D5I7O7V, D-7I7O1V, D-0I0O1V, D-0I1O1V, D-0I7O7V, D-0I0O7V, D-0I1O7V, D-9I0O0V, D-9I0O1V, D-9I0O7V, and D-9I7O7V with higher 4-HIL yield than SI (89.9 ± 2.5 mM) were selected for further study.
To check the influence of Val, Met, and Leu on Ile biosensor and 4-HIL production, the intracellular and extracellular contents of Ile, Val, Met, and Leu as well as the effect of Val, Met, and Leu addition on 4-HIL fermentation were analyzed in the best tri-gene dynamic strain D-0I7O7V. As shown in Fig. 3C, its intracellular contents of Val, Met, and Leu were a little high at 24 h and then fluctuated around 1–4 mM thereafter, while its extracellular contents could not be detected. These contents were much lower than that of Ile, indicating that the cellular response of Lrp-PbrnFEN biosensors to Val, Met, and Leu might be much lower than that to Ile. When separately adding 10 mM Val, 10 mM Met, or 10 mM Leu, cells grew a little slowly, and 4-HIL titers decreased to 69.5 ± 9.0, 96.0 ± 7.1, and 85.4 ± 0.7 mM, respectively (Fig. 3D), 50.7%, 32.0%, and 39.5% lower than that produced without addition. Thereby, the addition of Val, Met, and Leu could not promote 4-HIL production.
Bidirectional regulation of Ile synthesis and conversion
Ile is the direct precursor of 4-HIL, which is very important for the synthesis of 4-HIL. Therefore, in this section, the key gene for Ile synthesis, i.e., ilvA was expressed to strengthen the Ile supply. However, the activity of IDO is greatly inhibited by excessive Ile (Shi et al. 2016; Tan et al. 2020), so the synthesis of Ile and the conversion of Ile to 4-HIL shall be carefully balanced. Thereby, the expression of ilvA was negatively regulated here by an Ile attenuator PilvBNC on the basis of positive regulation of idoU by lrp-PbrnFEN in plasmids pIL-NIU (Fig. 4A), in order to balance the upstream supply and downstream conversion of Ile. Therefore, PilvBNC-ilvA was expressed in pIL-NIU, and six pIL-NIUPA plasmids were obtained. These plasmids were transformed into SN01 to obtain 6 strains with bidirectional dynamic regulations of 4-HIL synthesis, named D-NIPA.
Bidirectional regulating circuit and 4-HIL fermentation of strains D-NIPA. A Diagram of the bidirectional regulation circuit. When the concentration of Ile is high, the expression of ido.U is strengthened to enhance the downstream pathway of Ile conversion; meanwhile, the expression of ilvA is weakened to reduce the upstream pathway of Ile synthesis and thereby avoids substrate inhibition by excess Ile. When the concentration of Ile is low, the expression of ilvA is strengthened to enhance the Ile synthesis, thereby providing sufficient Ile for IDO reaction and 4-HIL synthesis. B Cell growth and glucose consumption. C 4-HIL accumulation. D Ile accumulation. E The by-product Lys accumulation. F Relative fluorescence intensity of 13,032-PilvBNC-egfp after adding 0.1–10 mM Ile
In the whole fermentation process, D-1IPA and D-5IPA grew slightly faster than their original strains D-1I and D-5I, while the other D-NIPA strains grew slower than their original strains D-NI. Correspondingly, D-1IPA and D-5IPA consumed sugar similarly to D-1I and D-5I, while other D-NIPA strains consumed sugar slightly slower than their initial strains D-NI (Fig. 4B). Eventually, 4-HIL titer of D-D5IPA and D-7IPA is 118.2 ± 14.2 mM and 123.2 ± 20.5 mM, respectively, which increased by 21.3% and 46.3% compared with D-D5I and D-7I. However, Ile accumulated rapidly to the highest value of about 40 mM before 48 h, declined rapidly to around 10 and 40 mM at 48–96 h, and fluctuated thereafter in D-D5IPA and D-7IPA (Fig. 4C, D). These results suggested that Ile was stably and continuously supplied in these two strains, which led to the continuous synthesis of 4-HIL. 4-HIL yields of D-1IPA and D-5IPA were 109.7 ± 3.7 mM and 118.0 ± 15.0 mM, respectively, which was similar to those of D-1I and D-5I. Meanwhile, the Ile concentration of these two strains increased to around 20 mM at 24 h and decreased to 0 mM at 72 h, but the 4-HIL concentration increased continuously in the middle stage of fermentation (Fig. 4C, D), suggesting that Ile has been continuously synthesized and immediately converted into 4-HIL in these two strains. Therefore, the metabolism between the upstream synthesis pathway and the downstream conversion pathway of Ile is relatively coordinated among the above four strains. However, D-0IPA and D-9IPA accumulated 73.6 ± 16.3 mM and 58.4 ± 9.6 mM 4-HIL, respectively, which decreased by 30.8% and 26.5% compared with D-0I and D-9I. Although their total sum of 4-HIL and Ile was relatively high, a large amount of Ile accumulated could not be converted and 4-HIL was slowly synthesized after 72 h (Fig. 4C, D). These results implied the insufficient supply of α-KG and/or O2 and the excessive accumulation of Ile after PilvBNC-ilvA expression in these two strains. Although the growth of most D-NIPA strains was slower, their synthesis of 4-HIL was improved compared with D-NI in the first 72 h. The results indicated that the bidirectional dynamic regulation strategy alleviated the substrate inhibition and promoted the synthesis of 4-HIL. Therefore, the yield of 4-HIL of most strains increased, suggesting that the bidirectional dynamic regulation strategy had more advantages than the unidirectional dynamic adjustment strategy. However, D-NIPA still accumulated a large amount of by-product Lys (Fig. 4E).
Since Ile secretion of some strains was still very high, the dose response of Ile attenuator PilvBNC was characterized with the fluorescence reporter eGFP. The reporter plasmid pJYW-4-PilvBNC-egfp was constructed and introduced into C. glutamicum ATCC13032 which hardly produced Ile. This reporter strain was cultured in the absence of Ile or presence of 0.1 mM, 1 mM, 5 mM, or 10 mM Ile. Although the growth of this strain in Ile-restricted medium was poor, the variation of its relative fluorescence intensity could be observed. The fluorescence decreased slightly in the presence of 0.1–5 mM Ile but significantly in the presence of 10 mM Ile (Fig. 4F). Therefore, the minimum Ile concentration which PilvBNC attenuator can respond to was 10 mM, and at that concentration, the PilvBNC would attenuate the expression of downstream genes.
Synergistically dynamic control of IDO activity and Ile, α-KG, O 2 supply
The above strains D-1IPA, D-5IPA, D-D5IPA, and D-7IPA possessed the improved 4-HIL synthesis through balancing the supply and hydroxylation of Ile. While in the second section, the 4-HIL production of 12 strains was improved significantly by upregulating the supply of O2 and α-KG. Ile, α-KG, and O2 are the three direct substrates of IDO reaction in the synthesis of 4-HIL. Therefore, in this section, Ile, α-KG, and O2 supply as well as the conversion of Ile to 4-HIL was synergistically controlled by upregulating the expression of idoU, odhI, and vgb through Ile biosensor Lrp-PbrnFEN and downregulating the expression of ilvA through Ile attenuator PilvBNC (Fig. 5A). The PilvBNC-ilvA fragment was directly fused in the plasmids of above 12 strains for expression, and 9 D-NINONVPA strains were obtained.
Regulation circuit and 4-HIL fermentation of strains D-NINONVPA. A Diagram of synergistic regulation circuit. Lrp-PbrnFEN positively regulated the expression of ido.U, odhI, and vgb in response to intracellular Ile concentration, and PilvBNC negatively regulated the expression of ilvA in response to intracellular Ile concentration. B 4-HIL accumulation. C Ile accumulation. D The by-product Lys accumulation
Eventually, D-7I7O1VPA accumulated 127.1 ± 20.2 mM of 4-HIL, which was similar to the original strain D-7I7O1V (131.2 ± 13.3 mM) and D-7IPA (123.2 ± 20.5 mM). However, other strains generated much less 4-HIL than their original strains and SI (Fig. 5B). In these strains, the accumulation of Ile increased continuously in 48 h and then fluctuated thereafter. Finally, 40–90 mM of Ile was remained and not converted to 4-HIL (Fig. 5C). The above results showed that excessive Ile inhibited the enzyme activity of IDO and made Ile unable to convert into 4-HIL, resulting in low titer of 4-HIL. The concentration of Lys in these strains (9.3–26.4 mM) was slightly lower than D-NIPA (16.1–31.8 mM) and SI (34.7 ± 4.5 mM) (Fig. 5D and 4E). In conclusion, except for D-7I7O1VPA, the metabolic flow of other strains was redirected to the Ile or other by-products pathway, and simultaneous supply of Ile, α-KG, and O2 failed to promote 4-HIL synthesis. Therefore, D-7I7O1VPA in this section and D-NIPA strains in the previous section are selected for further study.
Negative regulation of dapA expression and Lys synthesis by Lys-OFF riboswitch
A large amount of by-product Lys was accumulated in the above strains D-NIPA. However, Lys is very important for cell growth. It is very difficult to eliminate Lys by gene knockout and other strategies (Yu et al. 2021). Therefore, in this section, a Lys OFF riboswitch was used to regulate the expression of dapA, the initial gene of Lys synthetic pathway, and thereby weaken the synthesis of Lys. When the Lys content increases, Lys can bind to the riboswitch to turn off the expression of dapA and weakens the synthesis of Lys. So that the concentration of Lys can be maintained at a level only necessary for cell growth (Fig. 6A). Therefore, the Lys-OFF riboswitch from E. coli MG1655 was integrated between the promoter and start codon of chromosomal dapA gene of SN01 to obtain the strain D-RS. Then, 7 dynamic regulatory plasmids of pIL-NIUPA and pIL-7IU7O1VPA were transformed into D-RS strain, generating strains of D-RS-NIPA and D-RS-7I7O1VPA.
Regulation circuit and 4-HIL fermentation of strains D-RS-NIPA. A Circuit diagram regulated by Lys-OFF riboswitch. When Lys concentration is high, Lys combines with riboswitch to cause structural change of riboswitch. Then, this structure can prevent ribosomes from combining with SD region, thus turn off the transcription of dapA. B Cell growth and glucose consumption. C 4-HIL accumulation. D Ile accumulation. E The by-product Lys accumulation. F Other amino acid concentration at 144 h
During the whole fermentation process, the strain D-RS-7I7O1VPA could not grow normally (data not shown). The strains D-RS-1IPA and D-RS-5IPA grew slightly slower, while the other D-RS-NIPA strains grew similar to their corresponding D-NIPA strains. Except the slightly slower rate of strain D-RS-5IPA, the sugar consumption of other D-RS-NIPA strains was similar to their corresponding D-NIPA strains (Fig. 6B).
In D-RS-5IPA, 4-HIL was synthesized steadily and continuously; finally, 177.3 ± 8.9 mM 4-HIL was accumulated (Fig. 6C). This titer was increased by 50.2% compared with D-5IPA and increased by 97.2% compared with SI. Meanwhile, the concentration of Lys decreased to 6.1 ± 0.6 mM, which was 62.0% lower than D-5IPA (Fig. 6E). Furthermore, this strain hardly produced other by-products (Fig. 6F). In D-RS-D5IPA, the final titer of 4-HIL was similar to that of D-D5IPA, but the concentration of Lys decreased to 6.8 mM, which was 74.0% lower than D-D5IPA. And the Ile accumulation of these two strains was maintained at approximately 14 mM and 60 mM, respectively after 96 h (Fig. 6D). These results indicated that the regulation of dapA by Lys-OFF riboswitch weakened Lys synthesis without damaging the yield of 4-HIL in this two strains. While in D-RS-0IPA and D-RS-9IPA, although the concentration of Lys decreased greatly, the titer of 4-HIL also decreased by 17.6% and 28.6% compared with D-0IPA and D-9IPA, and these titers were even much lower than that of static strain SI (Fig. 6C, E). The above 4 strains grew rapidly but accumulated 4-HIL slowly in 0–48 h, while after 48 h, these strains grew slowly and accumulated 4-HIL rapidly (Fig. 6B, C), suggesting that the regulation of Lys synthesis by Lys-OFF riboswitch can provide basic Lys required for cell growth and thereby well balance the cell growth and 4-HIL synthesis. Strangely, in D-RS-1IPA and D-RS-7IPA strains, the production of 4-HIL and Ile was very low. However, a large amount of Lys was accumulated in D-RS-1IPA (167.6 ± 4.6 mM) and D-RS-7IPA (189.1 ± 4.9 mM) (Fig. 6C, E). The metabolic variation of these two strains will be studied in the future. In conclusion, the combined positive regulation of ido expression by Lrp-PbrnFE5 biosensor, negative regulation of ilvA expression by PilvBNC attenuator, and negative regulation of dapA expression by Lys-OFF riboswitch can better balance the metabolic flow and thereby promote the synthesis of 4-HIL and reduce the by-product Lys concentration greatly compared with other dynamic regulation strategies.
Discussion
Metabolic engineering faces many challenges. Competition for cell resources and sensitivity to fermentation conditions may lead to metabolic burden, imbalance of cofactors, or accumulation of metabolic intermediates to toxic levels, which will interfere with cell production and growth (Hartline et al. 2021). Dynamic metabolic engineering refers that the cell can independently regulate the expression of pathway, reroute metabolic flux, and guide the target metabolic pathway to solve the difficult problems in metabolic engineering, according to the changing intracellular and extracellular environment (Brockman and Prather 2015). Dynamic control strategies include different kinds of switches or devices that can sense light, temperature, cell density, or some specific metabolites. Photogenetic switch can sense the visible light signal and make the responder undergo conformational changes and thereby regulate the expression of the target gene. In E. coli, a photosensor histidine kinase CcaS was used to respond to red light and green light to control the cell growth in the automatic photogenetic feedback control system (Milias-Argeitis et al. 2016). Similarly, a temperature-dependent system was used to dynamically regulate TCA cycle and thereby increase itaconate production in E. coli (Harder et al. 2018). In quorum sensing system, when the cell density exceeds a threshold, specific small molecules will accumulate and can be sensed to induce a specific reaction; thereby, this system can be used to dynamically balance cell growth and target yield (Tan and Prather 2017). For example, through regulating the response of cells to population signals at different cell densities, the conversion of E. coli from growth to production can be realized in different populations to improve the isopropanol yield (Soma and Hanai 2015). Metabolite-responsive biosensors usually sense specific metabolites to trigger conformational change of cognate actuator and regulate the expression of downstream genes. These biosensors can solve the problem of uncoordinated production and growth caused by the accumulation of intermediate metabolites, substrates, cofactors, and products. For example, Farmer et al. constructed a dynamic control switch to regulate lycopene synthesis by using acetyl phosphate responsive TF-promoter (Farmer and Liao 2000). Zhou and Zeng used Lys-responsive riboswitch to regulate gltA expression and Lys synthesis (Zhou and Zeng 2015). The PilvBNC attenuator was used to weaken and regulate the expression of icd and odhA, thus increasing the production of Leu and 4-HIL, respectively (Luo et al. 2021; Zhang et al. 2018). There are fewer metabolite-responsive biosensors for metabolic engineering of C. glutamicum. Fortunately, both the upregulated and downregulated BCAAs-responsive biosensors have been found and explored, especially the Ile upregulated biosensors Lrp-PbrnFEN with different dynamic range (Tan et al. 2020). However, the Ile attenuator PilvBNC and Lys-OFF riboswitch were only preliminarily explored and they have not been applied cooperatively with other biosensors and verified thoroughly. Here, the Lrp-PbrnFEN and PilvBNC were applied coordinately to balance the upstream Ile synthesis and downstream Ile conversion pathways and to attenuate or avoid the substrate inhibition caused by excess Ile. The 4-HIL titer successfully increased to more than 120 mM (Fig. 4D, 5B). Most effectively, after synergistically downregulating Lys synthesis by Lys-OFF riboswitch, the 4-HIL titer increased greatly to more than 170 mM and the main by-product Lys content decreased greatly to about 6 mM (Fig. 6C). Therefore, the combined dynamic regulation of 4-HIL biosynthesis and Lys branch by these three biosensors can effectively promote 4-HIL biosynthesis in C. glutamicum.
4-HIL has potential value in the treatment of diabetes. In our previous study, 4-HIL was de novo synthesized from glucose by expressing the ido gene in C. glutamicum ssp. lactofermentum strain SN01, an Ile producer, and neither Ile nor α-KG was added (Shi et al. 2015). Therefore, the IDO activity is very important for the synthesis of 4-HIL. In the previous studies, the IDO activity was effectively improved by a series of static regulation methods, such as the directed evolution and site-specific mutation of ido, the coexpression of ido and ido3, and the ribosomal binding site (RBS) engineering of ido (Huang and Shi 2018; Shi et al. 2019, 2020). However, constitutive expression of ido may bring great metabolic burden to cells. Then, the Lrp-PbrnFEN biosensors were used to upregulate the expression of ido by sensing the intracellular Ile concentration. The yield of 4-HIL reached to 28.9 − 74.4 mM. However, 60 − 130 mM Ile was still accumulated and not converted into 4-HIL (Tan et al. 2020). In our study here, in order to further improve the IDO activity, the codon of ido was optimized and the Lrp-PbrnFEN biosensors were applied to upregulate the expression of idoU. As expected, the yield of 4-HIL was further increased to 38.7 − 111.1 mM and the growth of the strains were not affected (Fig. 2B, C).
The coordinated supply of α-KG and O2, the co-substrates of IDO reaction, is also very important for the synthesis of 4-HIL. In previous studies, to increase the supply of α-KG, the aceA gene (encoding isocitrate lyase of glyoxylate cycle) was deleted, thereby increasing the 4-HIL titer to 69.5 ± 2.2 mM (Shi et al. 2019). Then, strong promoters PdnaK and PtacM were used to statically control the expression of vgb on the basis of aceA deletion and ido-mqo-ido3 static expression. The yield of 4-HIL (91.2 mM and 88.0 mM) did not change significantly (Shi et al. 2019). Later, RBS with high, medium, and low intensities were used to statically fine-tune the expression of odhI on the basis of ido expression, and the yield of 4-HIL did not increase. Then RBS engineering was applied to fine-tune the expression of both odhI and vgb genes and the resulting supply of α-KG and O2. The highest yield of 4-HIL was increased to 119.3 ± 5.0 mM (Shi et al. 2020). Subsequently, the natural and modified Lrp-PbrnFEN biosensors were used to dynamically upregulate the expression of odhI and vgb, and the highest yield of 4-HIL was increased to 135.3 ± 12.6 mM (Tan et al. 2020). Therefore, dynamic regulation may be more effective than static regulation in 4-HIL biosynthesis. In our study here, these three lrp-PbrnFEN biosensors with different strengths were utilized to coordinately upregulate the expression of odhI and vgb, and the highest yield of 4-HIL was increased to 141.1 ± 15.5 mM (Fig. 3). Previously, the natural Lrp-PbrnFE biosensor was used alone to dynamically regulate the expression of odhI and the resulting supply of α-KG, and the yield of 4-HIL was increased only by 8.3% (Zhang et al. 2018). Therefore, the modified Lrp-PbrnFEN biosensors with appropriate strength or dynamic range were more effective to regulate the supply of α-KG and O2 and thereby enhanced the synthesis of 4-HIL significantly.
The synthesis of Ile and the conversion of Ile to 4-HIL need to be carefully balanced. In previous studies, constitutive expression of ilvA, lysC, or POS5 led to excessive accumulation of Ile. However, the accumulated Ile could not be completely converted into 4-HIL and thus the 4-HIL titer decreased due to the inhibition of IDO activity by excessive Ile (Shi et al. 2016, 2018). Thereby, Ile supply shall be carefully modulated, but there is no research on the balanced Ile supply and 4-HIL synthesis. In our study here, Ile attenuator PilvBNC was used to dynamically control the synthesis of Ile and thus balance the upstream Ile supply and downstream Ile conversion pathways. The 4-HIL yield of one resulting bidirectional regulation strain D-7IPA increased to 123.2 ± 20.5 mM (Fig. 4C). Therefore, bidirectional dynamic control of the Ile supply and conversion can effectively enhance the synthesis of 4-HIL. However, the dynamic range and threshold of Ile attenuator PilvBNC were not modified and only the natural PilvBNC was applied here. Moreover, in 9 D-NINONVPA strains, 8 strains (except D-7I7O1VPA) generated much less 4-HIL than their original high-producing D-NINONV strains. It is speculated that in these high-producing D-NINONVPA strains, the metabolic flux has been well balanced, and the further expression of PilvBNC-controlled ilvA may destroy the existing balanced state of these strains. These results also indicate the fragility of metabolic balance and the necessity of dynamic regulation of 4-HIL synthesis. Even under the dynamic regulation manner, the upstream, downstream, and co-substrates supplying pathways should be optimized.
Lys is the main by-products in the synthesis of 4-HIL, but it is very important for the growth of cells (Wehrmann et al. 1998). In previous studies, a large amount of Lys was accumulated during the 4-HIL fermentation (Shi et al. 2019, 2020). Then, the strategy of programming adaptive laboratory evolution driven by Lys biosensor was exploited to weaken the synthesis of Lys, but the concentration of Lys did not decrease (Yu et al. 2021). In our study here, the Lys-OFF riboswitch was used to weaken Lys synthesis. The concentration of Lys successfully decreased to about 6 mM in most D-RS-NIPA strains. Among them, the 4-HIL titer of D-RS-5IPA strain (26.1 g/L) reached the highest level in shake flask fermentation according to current reports. However, there are still some shortcomings in this research. The sensitivity and basal expression of Lys-OFF riboswitch has not been optimized. The natural Lys-OFF riboswitch was reported to be very sensitive to Lys. It can strongly sense Lys low to 0.1 mM and thus directly turn off the synthesis of Lys (Zhou and Zeng 2015), but here, D-RS-NIPA strains could accumulate up to 6 mM Lys. Thereupon, the intracellular accumulation of Lys in D-5IPA and D-RS-5IPA was determined. As shown in Fig. 7, the intracellular Lys content of D-5IPA and D-RS-5IPA was 10–15 mM and 5–6 mM, respectively, similar to their extracellular Lys content. This content was much higher than the reported threshold of Lys-OFF riboswitch. Such discrepancy may be caused by the differences between our engineering strain derived from C. glutamicum SN01 and the strain C. glutamicum ATCC 13,032 used by Zhou and Zeng (2015). However, we failed to characterize the response of Lys-OFF riboswitch to Lys and determine its threshold in our engineering strain by fluorescence assay, because our engineering strain could not grow in Lys restricted medium at all. In addition, Lys-OFF riboswitch cannot gradually downregulate gene expression and Lys synthesis. However, prematurely turning off the Lys synthesis will affect cell growth. Therefore, the modified Lys-OFF riboswitch with higher threshold still needs to be exploited in the future. A recent review also suggests that fine-tuning biosensors is required to adjust the threshold concentrations required to switch a TF or riboswitch to the requirements of strain development (Wendisch 2020). Here, the 4-HIL titer of the best strain D-RS-5IPA (26.1 g/L) is 51.7% and 26.7% higher than that of previously reported static control strains SZ05opt (17.2 g/L) (Shi et al. 2019) and SF12 (20.6 g/L) (Shi et al. 2020), respectively (Table 3). This titer is also 31.2% higher than that of previously reported dynamic control strain ST17 regulated by single-functional biosensor (19.9 g/L) (Tan et al. 2020). Therefore, multi-functional dynamic control system would be more effective for 4-HIL biosynthesis than static metabolic engineering and single functional dynamic control system. D-RS-5IPA is a promising candidate for producing 4-HIL. However, its 4-HIL titer in shake flasks was lower than that of HIL18 achieved by 7 steps of static and 1 step of dynamic metabolic engineering in the bioreactor (34.2 g/L) (Zhang et al. 2018). The fed-batch fermentation of D-RS-5IPA will be considered in the future.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Anesiadis N, Cluett WR, Mahadevan R (2008) Dynamic metabolic engineering for increasing bioprocess productivity. Metab Eng 10(5):255–266. https://doi.org/10.1016/j.ymben.2008.06.004
Becker J, Rohles CM, Wittmann C (2018) Metabolically engineered Corynebacterium glutamicum for bio-based production of chemicals, fuels, materials, and healthcare products. Metab Eng 50:122–141. https://doi.org/10.1016/j.ymben.2018.07.008
Brockman IM, Prather KLJ (2015) Dynamic metabolic engineering: New strategies for developing responsive cell factories. Biotechnol J 10(9):1360–1369. https://doi.org/10.1002/biot.201400422
Farmer WR, Liao JC (2000) Improving lycopene production in Escherichia coli by engineering metabolic control. Nat Biotechnol 18(5):533–537. https://doi.org/10.1038/75398
Harder BJ, Bettenbrock K, Klamt S (2018) Temperature-dependent dynamic control of the TCA cycle increases volumetric productivity of itaconic acid production by Escherichia coli. Biotechnol Bioeng 115:156–164. https://doi.org/10.1002/bit.26446
Hartline CJ, Schmitz AC, Han Y, Zhang F (2021) Dynamic control in metabolic engineering: theories, tools, and applications. Metab Eng 63:126–140. https://doi.org/10.1016/j.ymben.2020.08.015
Holtz WJ, Keasling JD (2010) Engineering static and dynamic control of synthetic pathways. Cell 140(1):19–23. https://doi.org/10.1016/j.cell.2009.12.029
Hu J, Li Y, Zhang H, Tan Y, Wang X (2014) Construction of a novel expression system for use in Corynebacterium glutamicum. Plasmid 75:18–26. https://doi.org/10.1016/j.plasmid.2014.07.005
Huang S, Shi F (2018) Directed evolution and site-specific mutagenesis of L-isoleucine dioxygenase derived from Bacillus weihenstephanensis. Biotechnol Lett 40(8):1227–1235. https://doi.org/10.1007/s10529-018-2566-8
Jin LQ, Jin WR, Ma ZC, Shen Q, Cai X, Liu ZQ, Zheng YG (2019) Promoter engineering strategies for the overproduction of valuable metabolites in microbes. Appl Microbiol Biotechnol 103(21–22):8725–8736. https://doi.org/10.1007/s00253-019-10172-y
Jones KL, Kim SW, Keasling JD (2000) Low-copy plasmids can perform as well as or better than high-copy plasmids for metabolic engineering of bacteria. Metab Eng 2(4):328–338. https://doi.org/10.1006/mben.2000.0161
Jones CM, Hernández Lozada NJ, Pfleger BF (2015) Efflux systems in bacteria and their metabolic engineering applications. Appl Microbiol Biotechnol 99(22):9381–9393. https://doi.org/10.1007/s00253-015-6963-9
Kortmann M, Mack C, Baumgart M, Bott M (2019) Pyruvate carboxylase variants enabling improved Lysine production from glucose identified by biosensor-based high-throughput fluorescence-activated cell sorting screening. ACS Synth Biol 8(2):274–281. https://doi.org/10.1021/acssynbio.8b00510
Liu Y, Shin HD, Li J, Liu L (2015) Toward metabolic engineering in the context of system biology and synthetic biology: advances and prospects. Appl Microbiol Biotechnol 99(3):1109–1118. https://doi.org/10.1007/s00253-014-6298-y
Liu C, Zhang B, Liu YM, Yang KQ, Liu SJ (2018) New intracellular shikimic acid biosensor for monitoring shikimate synthesis in Corynebacterium glutamicum. ACS Synth Biol 7(2):591–601. https://doi.org/10.1021/acssynbio.7b00339
Liu H, Shi F, Tan S, Yu X, Lai W, Li Y (2021) Engineering a bifunctional ComQXPA-PsrfA quorum-sensing circuit for dynamic control of gene expression in Corynebacterium glutamicum. ACS Synth Biol 10(7):1761–1774. https://doi.org/10.1021/acssynbio.1c00149
Luo G, Zhao N, Jiang S, Zheng S (2021) Application of RecET-Cre/loxP system in Corynebacterium glutamicum ATCC14067 for L-leucine production. Biotechnol Lett 43(1):297–306. https://doi.org/10.1007/s10529-020-03000-1
Milias-Argeitis A, Rullan M, Aoki SK, Buchmann P, Khammash M (2016) Automated optogenetic feedback control for precise and robust regulation of gene expression and cell growth. Nat Commun 7:12546. https://doi.org/10.1038/ncomms12546
Morbach S, Junger C, Sahm H, Eggeling L (2000) Attenuation gontrol of ilvBNC in Corynebacterium glutamicum: evidence of leader peptide formation without the presence of a ribosome binding site. J Biosci Bioeng 90(5):501–507. https://doi.org/10.1016/S1389-1723(01)80030-X
Mustafi N, Grünberger A, Kohlheyer D, Bott M, Frunzke J (2012) The development and application of a single-cell biosensor for the detection of L-methionine and branched-chain amino acids. Metab Eng 14(4):449–457. https://doi.org/10.1016/j.ymben.2012.02.002
Nielsen J, Keasling JD (2016) Engineering cellular metabolism. Cell 164(6):1185–1197. https://doi.org/10.1016/j.cell.2016.02.004
Nowroozi FF, Baidoo EE, Ermakov S, Redding-Johanson AM, Batth TS, Petzold CJ, Keasling JD (2014) Metabolic pathway optimization using ribosome binding site variants and combinatorial gene assembly. Appl Microbiol Biotechnol 98(4):1567–1581. https://doi.org/10.1007/s00253-013-5361-4
Renicke C, Taxis C (2016) Biophotography: concepts, applications and perspectives. Appl Microbiol Biotechnol 100(8):3415–3420. https://doi.org/10.1007/s00253-016-7384-0
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27(10):946–950. https://doi.org/10.1038/nbt.1568
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145(1):69–73. https://doi.org/10.1016/0378-1119(94)90324-7
Shi F, Niu T, Fang H (2015) 4-Hydroxyisoleucine production of recombinant Corynebacterium glutamicum ssp lactofermentum under optimal corn steep liquor limitation. Appl Microbiol Biotechnol 99(9):3851–3863. https://doi.org/10.1007/s00253-015-6481-9
Shi F, Fang H, Niu T, Lu Z (2016) Overexpression of ppc and lysC to improve the production of 4-hydroxyisoleucine and its precursor L-isoleucine in recombinant Corynebacterium glutamicum ssp. lactofermentum. Enzyme Microb Technol 87–88:79–85. https://doi.org/10.1016/j.enzmictec.2016.04.008
Shi F, Zhang M, Li Y, Fang H (2018) Sufficient NADPH supply and pknG deletion improve 4-hydroxyisoleucine production in recombinant Corynebacterium glutamicum. Enzyme Microb Technol 115:1–8. https://doi.org/10.1016/j.enzmictec.2018.04.003
Shi F, Zhang S, Li Y, Lu Z (2019) Enhancement of substrate supply and ido expression to improve 4-hydroxyisoleucine production in recombinant Corynebacterium glutamicum ssp lactofermentum. Appl Microbiol Biotechnol 103(10):4113–4124. https://doi.org/10.1007/s00253-019-09791-2
Shi F, Fan Z, Zhang S, Wang Y, Tan S, Li Y (2020) Optimization of ribosomal binding site sequences for gene expression and 4-hydroxyisoleucine biosynthesis in recombinant corynebacteriumglutamicum. Enzyme Microb Technol 140:109622. https://doi.org/10.1016/j.enzmictec.2020.109622
Smirnov SV, Kodera T, Samsonova NN, Kotlyarova VA, Rushkevich NY, Kivero AD, Sokolov PM, Hibi M, Ogawa J, Shimizu S (2010) Metabolic engineering of Escherichia coli to produce (2S, 3R, 4S)-4-hydroxyisoleucine. Appl Microbiol Biotechnol 88(3):719–726. https://doi.org/10.1007/s00253-010-2772-3
Soma Y, Hanai T (2015) Self-induced metabolic state switching by a tunable cell density sensor for microbial isopropanol production. Metab Eng 30:7–15. https://doi.org/10.1016/j.ymben.2015.04.005
Tan SZ, Prather KL (2017) Dynamic pathway regulation: recent advances and methods of construction. Curr Opin Chem Biol 41:28–35. https://doi.org/10.1016/j.cbpa.2017.10.004
Tan S, Shi F, Liu H, Yu X, Wei S, Fan Z, Li Y (2020) Dynamic control of 4-hydroxyisoleucine biosynthesis by modified L-isoleucine biosensor in recombinant Corynebacterium glutamicum. ACS Synth Biol 9(9):2378–2389. https://doi.org/10.1021/acssynbio.0c00127
Venayak N, Anesiadis N, Cluett WR, Mahadevan R (2015) Engineering metabolism through dynamic control. Curr Opin Biotechnol 34:142–152. https://doi.org/10.1016/j.copbio.2014.12.022
Wehrmann A, Phillipp B, Sahm H, Eggeling L (1998) Different modes of diaminopimelate synthesis and their role in cell wall integrity: a study with Corynebacterium glutamicum. J Bacteriol 180(12):3159–3165. https://doi.org/10.1128/JB.180.12.3159-3165.1998
Wendisch VF (2020) Metabolic engineering advances and prospects for amino acid production. Metab Eng 58:17–34. https://doi.org/10.1016/j.ymben.2019.03.008
Winkler WC, Breaker RR (2005) Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59:487–517. https://doi.org/10.1146/annurev.micro.59.030804.121336
Yang Y, Lin Y, Wang J, Wu Y, Zhang R, Cheng M, Shen X, Wang J, Chen Z, Li C, Yuan Q, Yan Y (2018) Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis. Nat Commun 9(1):3043. https://doi.org/10.1038/s41467-018-05466-0
Yang J, Ran Y, Yang Y, Song S, Wu Y, Qi Y, Gao Y, Li G (2020) 4-Hydroxyisoleucine alleviates macrophage-related chronic inflammation and metabolic syndrome in mice fed a high-fat diet. Front Pharmacol 11:606514. https://doi.org/10.3389/fphar.2020.606514
Yu X, Shi F, Liu H, Tan S, Li Y (2021) Programming adaptive laboratory evolution of 4-hydroxyisoleucine production driven by a lysine biosensor in Corynebacterium glutamicum. AMB Express 11(1):66. https://doi.org/10.1186/s13568-021-01227-3
Zafar MI, Gao F (2016) 4-Hydroxyisoleucine: a potential new treatment for type 2 diabetes mellitus. BioDrugs 30(4):255–262. https://doi.org/10.1007/s40259-016-0177-2
Zhang F, Carothers JM, Keasling JD (2012) Design of a dynamic sensor-regulator system for production of chemicals and fuels derived from fatty acids. Nat Biotechnol 30(4):354–359. https://doi.org/10.1038/nbt.2149
Zhang C, Li Y, Ma J, Liu Y, He J, Li Y, Zhu F, Meng J, Zhan J, Li Z, Zhao L, Ma Q, Fan X, Xu Q, Xie X, Chen N (2018) High production of 4-hydroxyisoleucine in Corynebacterium glutamicum by multistep metabolic engineering. Metab Eng 49:287–298. https://doi.org/10.1016/j.ymben.2018.09.008
Zhou LB, Zeng AP (2015) Exploring lysine riboswitch for metabolic flux control and improvement of L-lysine synthesis in Corynebacterium glutamicum. ACS Synth Biol 4(6):729–734. https://doi.org/10.1021/sb500332c
Zhou P, Xie W, Yao Z, Zhu Y, Ye L, Yu H (2018) Development of a temperature-responsive yeast cell factory using engineered Gal4 as a protein switch. Biotechnol Bioeng 115(2):1321–1330. https://doi.org/10.1002/bit.26544
Zhou L, Ren J, Li Z, Nie J, Wang C, Zeng AP (2019) Characterization and engineering of a clostridium glycine riboswitch and its use to control a novel metabolic pathway for 5-aminolevulinic acid production in Escherichia coli. ACS Synth Biol 8(10):2327–2335. https://doi.org/10.1021/acssynbio.9b00137
Funding
This work was supported by the program of State Key Laboratory of Food Science and Technology (SKLF-ZZA-201904).
Author information
Authors and Affiliations
Contributions
FS conceived and designed the research. WL, ST, HL, and YX conducted the experiments. WL, FS, and ST analyzed the data. FS, WL, and YL wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, W., Shi, F., Tan, S. et al. Dynamic control of 4-hydroxyisoleucine biosynthesis by multi-biosensor in Corynebacterium glutamicum. Appl Microbiol Biotechnol 106, 5105–5121 (2022). https://doi.org/10.1007/s00253-022-12034-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12034-6