Abstract
Transition metal ions are essential micronutrients for all living organisms and exert a wide range of effects on human health. The uptake of transition metal ions occurs primarily in the gastrointestinal tract, which is colonized by trillions of bacterial cells. In recent years, increasing studies have indicated that transition metals have regulatory effects on the gut microbiota. In view of the significant effect of the gut microbiota on human health and involvement in the pathogenesis of a wide range of diseases, in this paper, we provide a comprehensive discussion on the regulatory effects of four kinds of transition metal ions on the gut microbiota. A total of 20 animal model and human studies concerning the regulatory effects of four types of transition metal ions (i.e., iron, copper, zinc, and manganese) on gut microbiota were summarized. Both the deficiency and supplementation of these transition metal ions on the gut microbiota were considered. Furthermore, the potential mechanisms governing the regulatory effects of transition metal ions on the gut microbiota were also discussed.
Key points
• Regulatory effects of iron, copper, zinc, and manganese on gut microbiota were reviewed.
• Both deficiency and supplementation of metal ions on gut microbiota were considered.
• Mechanisms governing effects of metal ions on gut microbiota were discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metals are a series of elements in the d and ds regions of the periodic table, which have unfilled valence layer d tracks and have significant differences in properties from other elements. Transition metal ions exhibit a variety of biological activities, such as redox reactions, oxidative stress, and involvement in the production of energy in respiratory chains, as well as such functions as electronic transfer, oxygen transport, and enzyme activity centers (Lieu et al. 2001; Andreini et al. 2008; Waldron et al. 2009; Andrews 2000).
The gastrointestinal tract of mammals harbors trillions of bacteria, and it has been estimated that in humans, the numbers of gut microbiota may be equal to human body cells (Sender et al. 2016). In the past years, increasing studies indicated that gut microbiota has a wide range of functions, and it was not only a key contributor to the host metabolism but also dysbiosis was associated with a variety of diseases of the gastrointestinal tract and others like diabetes, liver diseases, and neurodegenerative diseases (Illiano et al. 2020; Lavelle and Sokol 2020; Bäckhed et al. 2012; Sekirov et al. 2010; Buret et al. 2019; Shen et al. 2017; Shen et al. 2019; Shen 2020; Sartor and Wu 2017). The availability of transition metals is highly important for many living organisms, particularly microbes. Both exogenous and endogenous environmental factors can alter the gut microbiota, and these changes can affect host health. Both in vitro and animal models have been employed to investigate the effects of transition metal ions, including iron, copper, zinc, and manganese, on gut microbiota and host health. Both deficiency and supplementation of these transition metal ions can affect the species abundance and diversity of the gut microbiota. The gut microbiota also affects the roles played by transition metal ions in humans.
Therefore, it is necessary to discuss the effects of transition metal ions on the gut microbiota, which may deepen the current knowledge about mechanisms underlying the effects of transition metal ions on host health. Herein, we discussed the 20 studies published from 2011 to 2019 regarding the effects of transition metal ions deficiency and supplementation on the gut microbiota.
Iron
Iron is a necessary biological metal and mammalian cells require sufficient iron to meet their metabolic needs. However, iron is also potentially toxic and can catalyze the generation of reactive oxygen species and other highly reactive radicals under aerobic conditions (Wang and Pantopoulos 2011). Iron can also affect on the composition and metabolic activity of the gut microbiota. Several studies have investigated the modulatory effect of iron on the gut microbiota of mice and humans. Information concerning the research subjects, designs, and results were extracted from these studies and summarized in Table 1. Five of these studies reported the effects of iron deficiency on the gut microbiota. As early as 2012, Dostal et al. found an increased relative abundance of Lactobacillus and Enterobacteriaceae and a decreased relative abundance of Roseburia after 26 d of an iron-deficient diet (2.6 mg iron·kg diet-1) in Sprague-Dawley rats (Dostal et al. 2012). Another study by Dostal et al. on human gut microbiota-associated rats demonstrated that an iron-deficient diet (2.9 mg iron·kg diet-1) had little significant effect on the host’s dominant bacterial and gut microbiota metabolic activity (the diet decreased the relative abundance of Bilophila spp., E. hallii, and Coprococcus spp.) (Dostal et al. 2014).
Iron deficiency anemia (IDA) occurs when the body does not have enough iron. Muleviciene et al. revealed microbiota imbalance in infant IDA patients and observed a decrease in the Bifidobacteriaceae/Enterobacteriaceae ratio (Muleviciene et al. 2018). Parmanand et al. investigated the effect of iron on the growth of individual gut microbiota. They confirmed that the low iron availability inhibited the growth of Bifidobacteria in continuous colonic fermentation in vitro (Parmanand et al. 2019). However, Dostal et al. obtained a different result that an increase in Bifidobacteria was observed in continuous colonic fermentation in vitro (Dostal et al. 2013). The two in vitro studies used fecal samples from children and common fresh fecal samples, respectively. Dostal et al. concluded that Bifidobacteria could bind iron to the cell membranes and walls for growth advantage in the iron-deficient environment. In the study of Parmanand et al., the viable counts of Bifidobacteria varied in each sample. They speculated that it may be caused by individual differences of donors, and effects of metabolites and neighboring taxa. In addition, these two studies showed that iron deficiency significantly reduced the growth of E. coli, S. Typhimurium, B. thetaiotaomicron, Eubacterium rectale, Clostridium Cluster IV members, and Bacteroides spp., while this deficiency increased the growth of Lactobacillus spp. (Parmanand et al. 2019; Dostal et al. 2013). A study in Crohn’s disease-like ileitis model mice showed that low concentrations of iron in a sulfate-free diet (<10 mg iron·kg diet-1, 11 weeks) affected the composition of the gut microbiota, and resulted in reduced abundances of Bacteroides and Desulfovibrio spp. and increased abundances of Bifidobacterium, Succinivibrio, Turicibacter, and Clostridium (Werner et al. 2011).
We also searched for five studies of iron supplementation altering the gut microbiota. Dostal et al. found that iron supplementation (35 mg iron·kg diet-1) could significantly improve the metabolic activity of the gut microbiota, especially increased the abundance of Bacteroides spp., which could be attributable to iron-dependent enzymes (Dostal et al. 2014). Although oral iron supplementation can effectively treat IDA, the safety of oral iron supplementation remains controversial in a subset of inflammatory bowel disease (IBD) patients (Ellermann et al. 2020). The imbalance between harmful and protective bacteria, or microbiota imbalance, is the main characteristic of IBD (Kaur et al. 2011). Importantly, dietary iron supplementation may worsen the disease or increase the risk of infection; the reason for this effect may be the alterations of the commensal microbiota (Buret et al. 2019; Paganini and Zimmermann 2017; Mahalhal et al. 2018; Lee et al. 2017), most significantly, the increased abundance of Enterobacteriaceae (Yilmaz and Li 2018). A study by Mahalhal et al. explored the effects of oral iron on C57BL/6 mice model of colitis (Mahalhal et al. 2018). The doubling of iron standards (400 ppm iron) led to major changes in the composition of the microbiome, including an increase in Proteobacteria and a decrease in Firmicutes and Bacteroidetes. Conversely, Bacteroidetes increased moderately in mice receiving a low-iron diet (100 ppm iron). This suggested that the growth of Bacteroidetes was influenced by iron. Iron reduced the growth of many pathogenic bacteria and may change the ratio of pathogenic to protective bacteria (Ng 2016). Jaeggi et al. reported that iron fortification affects the gut microbiome in African infants with diarrhea (Jaeggi et al. 2015). These results showed that the abundance of Enterobacteria, Escherichia/Shigella, and Clostridium spp. and the ratio of Enterobacteria/Bifidobacteria were increased in the iron-fortification groups. The sum of the pathogenic E. coli at the endpoint was higher in the iron groups than in the no-iron groups. Ellermann et al. studied the dietary iron modulating assembly of the gut microbiota in colitis-resistant and colitis-susceptible mice (Ellermann et al. 2020). The results showed that increased oral iron intake increased pathogenic Enterobacteria, including enterotoxigenic Escherichia coli and Salmonella. These findings indicated that the means of supplying iron was also important. Lee et al. evaluated the effect of different methods of iron supplementation on patients with IBD. Compared with intravenous treatment, peroral treatment reduced the abundance of Faecalibacterium prausnitzii, Ruminococcus bromii, Dorea sp., and Collinsella aerofaciens (Lee et al. 2017). Bifidobacteria and Lactobacillus have beneficial effects in maintaining remission in IBD patients. In addition, Bifidobacteriaceae can bind iron to the cell membranes and walls, which subsequently reduce radical formation in the surrounding environment, thereby reducing the risk of colorectal cancer (Saha et al. 2016; Skrypnik and Suliburska 2018).
These findings suggested that dietary iron imbalance may disrupt the assembly of the gut microbiota and promote changes in bacterial composition, including an increase in the family Enterobacteriaceae, which is associated with various microbial-driven diseases. Iron availability is a critical signal for the expression of virulence genes by pathogens and hosts. In general, the availability of intestinal iron has a large influence on the virulence of pathogenic microorganisms. Overall, these findings indicate that the gut microbiota can be influenced by oral iron intake in the short term. However, the potential effect of long-term oral iron supplementation on gut microbiota needs to be explored.
Copper
The effect of unbalanced copper intake on the gut microbiota remains unclear (Wei et al. 2015). Five studies revealed the effect of dietary copper supplementation on the gut microbiota, and the main information extracted from these studies was listed in Table 2. Song et al. evaluated the effect of dietary copper on male weanling Sprague-Dawley rats. The results showed that while increased Firmicutes in low-copper-fed (1.5 ppm copper carbonate) rats were predominantly due to the increases in Lachnospiraceae and Peptostreptococcaceae, the increases in Firmicutes in high-copper-fed (60 ppm copper carbonate) rats might be due to the increases in Lactobacillaceae (Lactobacillus), Lachnospiraceae, and Erysipelotrichaceae. The common features of the alterations of the gut microbiota were the depletion of Akkermansia (Song et al. 2018). Meng et al. investigated the effect of waterborne copper exposure on the gut microbiota of juvenile common carp (Cyprinus carpio L.), which showed a decrease in Akkermansia abundance. Moreover, the abundances of small putative short-chain fatty acid-producing bacteria, including Allobaculum, Blautia, Coprococcus, Faecalibacterium, Roseburia, Lactobacillus, Bacillus, and Ruminococcus, decreased significantly, and those of Pseudomonas and Acinetobacter were found to be increased. Copper exposure disturbs the composition of the gut microbiota related to immunity in juvenile common carp, thereby increases the risk of invasion by pathogens (Meng et al. 2018). Ruan et al. investigated the effect of high doses of copper on caecal microbiota in mice. The results showed that compared with the control group, the abundances of bacteria genera Rikenella, Jeotgailcoccus, and Staphylococcus were significantly decreased, whereas the bacteria genus Corynebacterium was significantly increased, in the copper supplementation group (5 mg/kg-bw Copper) (Ruan et al. 2019). Di Giancamillo et al. studied the effects of dietary supplementation with copper sulfate on weaning piglets, which showed that the total bacterial and Enterobacteriaceae bacterial counts were lower in the caecum than was observed in the other groups, and in the colon, Streptococci spp. was lower in both copper sulfate-supplemented groups than in the controls. Copper dietary supplementation may act by restoring gut morphology, improving duodenal structure, and positively modulating the large gut microbiota (Di Giancamillo et al. 2018). Yang et al. studied how exposure to copper affected the gut microbiota in Chinese brown frog (Rana chensinensis). The results showed that the relative abundance of Fusobacteria was significantly decreased, and at the genus level, Flavobacterium had a significantly higher abundance (Yang et al. 2020). Dai et al. investigated the effects of early-life exposure to copper on the gut microbiota in Sprague-Dawley rats. The results showed that copper exposure decreased the ratio of Firmicutes to Bacteroidetes (Dai et al.,2020).
Zinc
As an indispensable metal element for growth and development, imbalance of zinc can also affect gut microbiota. The main information obtained from the studies of the modulation of the gut microbiota by zinc was summarized in Table 3. Clostridium difficile as a nosocomial pathogen exists widely. The gut microbiota is altered by zinc supplementation in the diet and decreases resistance to C. difficile infection (CDI) (Lessa et al. 2015; Zackular et al. 2016; Zackular and Skaar 2018). Zackular et al. investigated the effects of zinc on mice colonized with C. difficile and excess dietary zinc resulted in C. difficile-associated disease exacerbated. The study showed that high dietary zinc generated a dysbiosis that favored expansion of Enterococcus, Porphorymonadaceae, Lachnospiraceae, and Clostridia XI while reduced the population of Turicibacter (Zackular et al. 2016). An impacting effect of excess dietary zinc is the selection for Enterococci in the microbiota (Zackular and Skaar 2018). Enrichment of members of the Enterococci genus has been reported in the gut microbiota in patients with CDI (Poduval et al. 2000). This genus is highly resistant to zinc toxicity (Abrantes et al. 2011).
Zinc is usually added to animal feed as an alternative to antibiotics (Bednorz et al. 2013). Shao et al. studied the effects of zinc on the caecal microbial community in broilers. The results showed that zinc regulated the caecal microbial community by increasing the populations of total bacteria and beneficial Lactobacillus bacteria in broilers and decreasing the populations of Salmonella (Shao et al. 2014). It has been indicated that dietary zinc supplementation might help maintain the stability of the gut microbiota, increasing the populations of beneficial bacteria and reducing the chance of S. typhimurium colonization in the caecum (Sommer and Bäckhed 2013). Mayneris-Perxachs et al. investigated the effects of protein- and zinc-deficient diets on the murine microbiome and metabolic phenotype. The results showed that after zinc malnutrition the fecal microbiota exhibited only marginal changes. The abundance of Proteobacteria increased significantly from the age of 36 d. Several species within this phylum may gain a growth advantage by ZnuABC (Mayneris-Perxachs et al. 2016). Reed et al. used the broiler chicken model to explore changes in the gut microbial ecology of the host under zinc deficiency. They observed that zinc deficiency significantly reduced the species richness and diversity of the gut microbiota. Zinc deficiency significantly increased the relative abundance of Enterococcus, Enterobacteriaceae, and Ruminococcaceae and significantly decreased the relative abundance of Clostridiales and Peptostreptococcaceae (Reed et al. 2015).
Manganese
Manganese is an important trace element that is very important for the normal development of the host (Bowman et al. 2011). It has been observed that the effect of manganese exposure on the gut microbiota may be sex-specific. The main information obtained from the studies of the modulation of the gut microbiota by manganese is summarized in Table 4. For example, the abundance of Firmicutes significantly increased in Mn2+-exposed male mice but decreased in Mn2+-exposed female mice (Chi et al. 2017). Chi et al. also observed that the genus Lactobacillus was specifically enriched in Mn2+-exposed female mice (Chi et al. 2017). In fact, despite the prevalence of manganese and the potential risk that it poses to human health, the mechanisms by which manganese exerts its effects on the gut and the gut microbiota have not been elucidated to date (Ghaisas et al. 2016).
Conclusions and remarks
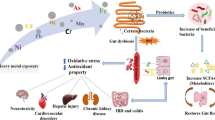
Transition metals are essential for animal and human health. Gut microbiota can affect the content of transition metals in the body, and the availability of transition metals determines the state of the gut microbiota (Skrypnik and Suliburska 2018). Unbalanced metal intake may lead to changes in the gut microbiota. These findings imply that the regulation of the gut microbiota may represent an important action pathway underlying its impact on health. In this article, we reviewed 20 studies on transition metal elements (i.e., iron, copper, zinc, and manganese) in the gut microbiota. On the basis of these studies, a variety of potential mechanisms underlying the effects of transition metals on the gut microbiota were proposed as summarized in Fig. 1.
According to studies reviewed in this paper, intestinal iron concentration directly affects the number of short-chain fatty acid producers (such as F. protococcus XIVa, Roseburia spp., Ruminococcus spp., and Coprococcus spp.), the reason is that the synthesis of short-chain fatty acids is influenced by iron as a key cofactor (Dostal et al. 2012; Dostal et al. 2013; Dostal et al. 2014; Lee et al. 2017). Siderophilic bacteria can steal iron from the host by using aggressive iron removal methods, which further limits the local iron availability of their non-siderophilic counterparts (Ellermann and Arthur 2017). Low iron-demand bacteria gain or lose competitive advantages with iron concentration changes (Parmanand et al. 2019; Dostal et al. 2013; Werner et al. 2011). As an important signal for pathogens and hosts to express virulence genes, iron can promote the replication and virulence of gut enteric pathogens; it is one of the main factors affecting gut microbiota (Yilmaz and Li 2018). In addition, for most intestinal gram-negative bacteria, such as Salmonella, Shigella, and pathogenic E. coli, iron acquisition plays a critical role in virulence and colonization (Dostal et al. 2014; Parmanand et al. 2019; Ellermann et al. 2020).
Copper in the diet plays an important role in the destruction of intestinal barrier function. High concentrations of copper ions improve intestinal permeability, and this effect is supported by a significant downregulation of intestinal connexin and a reduction in the number of goblet cells (Song et al. 2018; Dai et al. 2020), leading to the reduction of intestinal barrier-related microorganisms (e.g., Akkermansia, Lactobacillus, Bifidobacterium, and Romboutsia) and the number of core genera producing short-chain fatty acids (such as Allobaculum, Blautia, Faecalibacterium, Roseburia, and Ruminococcus) (Meng et al. 2018; Yang et al. 2020). Excessive zinc shows metallic toxicity, mismatches proteins, and releases metals that have oxidative activity. In mice colonized with C. difficile, excessive dietary zinc not only increases toxin activity but also affects the host immune response, which exacerbates C. difficile-associated disease (Zackular and Skaar 2018). Some bacteria (e.g., Proteobacteria, Enterobacteriaceae, and Enterococcus) can gain a growth advantage by increasing ZnuABC (Zackular et al. 2016; Zackular and Skaar 2018; Mayneris-Perxachs et al. 2016; Reed et al. 2015). Furthermore, due to lower zinc levels, some families of bacteria may gain an advantage by either having less demand for zinc, more sufficient intake mechanisms, or lack of competition through more zinc-sensitive bacteria (Paganini et al. 2016). Overexposure to manganese may affect the gut microbiota by inducing oxidative stress, which may impair nutritional immunity. In addition, high concentrations of manganese interfere with the equilibrium of ions, especially regarding iron homeostasis. The gender differences in the effects of manganese on the gut microbiota may arise from two aspects: manganese transport genes and changes in the quorum-sensing system (Chi et al. 2017).
In summary, as discussed above, four types of transition metal ions, i.e., iron, copper, zinc, and manganese, possess regulatory effects on gut microbiota through multiple pathways, which may be involved in the effects of these metals on host health and disease conditions. Both supplementation and deficiency of these transition metals can affect the gut microbiota. Thus, manipulating the transition metal ion-mediated interactions between the gut microbiota and the host is expected to be an attractive intervention strategy developed in the future. Despite several possible acting mechanisms have been proposed by previous studies, more mechanistic elucidation still need to be conducted as the interactions of these metal with gut microbiota are rather complex. In addition, the availability of transition metals in the gut tract also deserves special attention, and estimating the availability of metals and their effects on gut microbiota can help provide clues to understand how transition metals affect host health and better supplementation use of these transition metals to maintain health.
References
Abrantes MC, Lopes Mde F, Kok J (2011) Impact of manganese, copper and zinc ions on the transcriptome of the nosocomial pathogen Enterococcus faecalis V583. PLoS One 6:e26519
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Andrews NC (2000) Iron homeostasis: insights from genetics and animal models. Nat Rev Genet 1:208–217
Bäckhed F, Fraser CM, Ringel Y (2012) Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe 12:611–622
Bednorz C, Oelgeschläger K, Kinnemann B, Hartmann S, Neumann K, Pieper R, Bethe A, Semmler T, Tedin K, Schierack P, Wieler LH, Guenther S (2013) The broader context of antibiotic resistance: zinc feed supplementation of piglets increases the proportion of multi-resistant Escherichia coli in vivo. Int J Med Microbiol 303:396–403
Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 25:191–203
Buret AG, Motta JP, Allain T, Ferraz J, Wallace JL (2019) Pathobiont release from dysbiotic gut microbiota biofilms in intestinal inflammatory diseases: a role for iron? J Biomed Sci 26:1
Chi L, Gao B, Bian X, Tu P, Ru H, Lu K (2017) Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol 331:142–153
Dai J, Yang X, Yuan Y, Jia Y, Liu G, Lin N, Xiao H, Zhang L, Chen J (2020) Toxicity, gut microbiota and metabolome effects after copper exposure during early life in SD rats. Toxicology 433-434:152395
Di Giancamillo A, Rossi R, Martino PA, Aidos L, Maghin F, Domeneghini C, Corino C (2018) Copper sulphate forms in piglet diets: microbiota, intestinal morphology and enteric nervous system glial cells. Anim Sci J 89:616–624
Dostal A, Chassard C, Hilty FM, Zimmermann MB, Jaeggi T, Rossi S, Lacroix C (2012) Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. J Nutr 142:271–277
Dostal A, Fehlbaum S, Chassard C, Zimmermann MB, Lacroix C (2013) Low iron availability in continuous in vitro colonic fermentations induces strong dysbiosis of the child gut microbial consortium and a decrease in main metabolites. FEMS Microbiol Ecol 83:161–175
Dostal A, Lacroix C, Pham VT, Zimmermann MB, Del’homme C, Bernalier-Donadille A, Chassard C (2014) Iron supplementation promotes gut microbiota metabolic activity but not colitis markers in human gut microbiota-associated rats. Br J Nutr 111:2135–2145
Ellermann M, Arthur JC (2017) Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med 105:68–78
Ellermann M, Gharaibeh RZ, Maharshak N, Perez-Chanona E, Jobin C, Carroll IM, Arthur JC, Plevy SE, Fodor AA, Brouwer CR, Sartor RB (2020) Dietary iron variably modulates assembly of the intestinal microbiota in colitis-resistant and colitis-susceptible mice. Gut Microbes 11:32–50
Ghaisas S, Maher J, Kanthasamy A (2016) Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther 158:52–62
Illiano P, Brambilla R, Parolini C (2020) The mutual interplay of gut microbiota, diet and human disease. FEBS J 287:833–855
Jaeggi T, Kortman GA, Moretti D (2015) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut 64:731–742
Kaur N, Chen CC, Luther J, Kao JY (2011) Intestinal dysbiosis in inflammatory bowel disease. Gut Microbes 2:211–216
Lavelle A, Sokol H (2020) Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17:223–237
Lee T, Clavel T, Smirnov K, Schmidt A, Lagkouvardos I, Walker A, Lucio M, Michalke B, Schmitt-Kopplin P, Fedorak R, Haller D (2017) Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 66:863–871
Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC (2015) Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834
Lieu PT, Heiskala M, Peterson PA, Yang Y (2001) The roles of iron in health and disease. Mol Asp Med 22:1–87
Mahalhal A, Williams JM, Johnson S (2018) Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS One 13:e0202460
Mayneris-Perxachs J, Bolick DT, Leng J, Medlock GL, Kolling GL, Papin JA, Swann JR, Guerrant RL (2016) Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am J Clin Nutr 104:1253–1262
Meng XL, Li S, Qin CB, Zhu ZX, Hu WP, Yang LP, Lu RH, Li WJ, Nie GX (2018) Intestinal microbiota and lipid metabolism responses in the common carp (Cyprinus carpio L.) following copper exposure. Ecotoxicol Environ Saf 160:257–264
Muleviciene A, D’Amico F, Turroni S, Candela M, Jankauskiene A (2018) Iron deficiency anemia-related gut microbiota dysbiosis in infants and young children: a pilot study. Acta Microbiol Immunol Hung 65:551–564
Ng O (2016) Iron, microbiota and colorectal cancer. Wien Med Wochenschr 166:431–436
Paganini D, Zimmermann MB (2017) The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: a review. Am J Clin Nutr 106:1688S–1693S
Paganini D, Uyoga MA, Zimmermann MB (2016) Iron fortification of foods for infants and children in low-income countries: effects on the gut microbiome, gut inflammation, and diarrhea. Nutrients 8:494
Parmanand BA, Kellingray L, Le Gall G, Basit AW, Fairweather-Tait S, Narbad A (2019) A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J Nutr Biochem 67:20–27
Poduval RD, Kamath RP, Corpuz M, Norkus EP, Pitchumoni CS (2000) Clostridium difficile and vancomycin-resistant Enterococcus: the new nosocomial alliance. Am J Gastroenterol 95:3513–3515
Reed S, Neuman H, Moscovich S, Glahn RP, Koren O, Tako E (2015) Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 7:9768–9784
Ruan Y, Wu C, Guo X, Xu Z, Xing C, Cao H, Zhang C, Hu G, Liu P (2019) High doses of copper and mercury changed cecal microbiota in female mice. Biol Trace Elem Res 189:134–144
Saha P, Yeoh BS, Singh R, Chandrasekar B, Vemula PK, Haribabu B, Vijay-Kumar M, Jala Venkatakrishna R (2016) Gut microbiota conversion of dietary ellagic acid into bioactive phytoceutical urolithin A inhibits heme peroxidases. PLoS One 11:e0156811
Sartor RB, Wu GD (2017) Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology 152:327–339 e324
Sekirov I, Russell SL, Antunes LC, Finlay BB (2010) Gut microbiota in health and disease. Physiol Rev 90:859–904
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533
Shao Y, Lei Z, Yuan J, Yang Y, Guo Y, Zhang B (2014) Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhimurium. J Microbiol 52:1002–1011
Shen L (2020) Gut, oral and nasal microbiota and Parkinson’s disease. Microb Cell Factories 19:50
Shen L, Liu L, Ji HF (2017) Alzheimer’s disease histological and behavioral manifestations in transgenic mice correlate with specific gut microbiome state. J Alzheimers Dis 56:385–390
Shen L, Liu L, Li XY, Ji HF (2019) Regulation of gut microbiota in Alzheimer’s disease mice by silibinin and silymarin and their pharmacological implications. Appl Microbiol Biotechnol 103:7141–7149
Skrypnik K, Suliburska J (2018) Association between the gut microbiota and mineral metabolism. J Sci Food Agric 98:2449–2460
Sommer F, Bäckhed F (2013) The gut microbiota--masters of host development and physiology. Nat Rev Microbiol 11:227–238
Song M, Li X, Zhang X, Shi H, Vos MB, Wei X, Wang Y, Gao H, Rouchka EC, Yin X, Zhou Z, Prough RA, Cave MC, McClain CJ (2018) Dietary copper-fructose interactions alter gut microbial activity in male rats. Am J Physiol Gastrointest Liver Physiol 314:G119–G130
Waldron KJ, Rutherford JC, Ford D, Robinson NJ (2009) Metalloproteins and metal sensing. Nature 460:823–830
Wang J, Pantopoulos K (2011) Regulation of cellular iron metabolism. Biochem J 434:365–381
Wei X, Song M, Yin X, Schuschke DA, Koo I, McClain CJ, Zhang X (2015) Effects of dietary different doses of copper and high fructose feeding on rat fecal metabolome. J Proteome Res 14:4050–4058
Werner T, Wagner SJ, Martínez I, Walter J, Chang JS, Clavel T, Kisling S, Schuemann K, Haller D (2011) Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 60:325–333
Yang Y, Song X, Chen A, Wang H, Chai L (2020) Exposure to copper altered the intestinal microbiota in Chinese brown frog (Rana chensinensis). Environ Sci Pollut Res Int 27:13855–13865
Yilmaz B, Li H (2018) Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals (Basel) 11:98
Zackular JP, Skaar EP (2018) The role of zinc and nutritional immunity in Clostridium difficile infection. Gut Microbes 9:469–476
Zackular JP, Moore JL, Jordan AT, Juttukonda LJ, Noto MJ, Nicholson MR, Crews JD, Semler MW, Zhang Y, Ware LB, Washington MK, Chazin WJ, Caprioli RM, Skaar EP (2016) Dietary zinc alters the microbiota and decreases resistance to Clostridium difficile infection. Nat Med 22:1330–1334
Funding
This work was supported by the Shandong Provincial Natural Science Foundation (Grant No. ZR2019MH020), University Youth Innovation Team of Shandong Province (Grant No. 2019KJK017) and Talent Program of Zibo.
Author information
Authors and Affiliations
Contributions
HFJ and LS conceived and designed research. CYL and XYL collected data. CYL, XYL, HFJ and LS performed analysis. XYL, CYL, HFJ, and LS wrote paper. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
CYL declares that he has no conflict of interest. XYL declares that he has no conflict of interest. HFJ declares that she has no conflict of interest. LS declares that he has no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, CY., Li, XY., Shen, L. et al. Regulatory effects of transition metals supplementation/deficiency on the gut microbiota. Appl Microbiol Biotechnol 105, 1007–1015 (2021). https://doi.org/10.1007/s00253-021-11096-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11096-2