Abstract
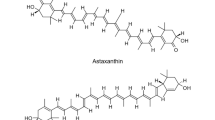
Astaxanthin is a natural pigment, known for its strong antioxidant activity and numerous health benefits to human and animals. Its antioxidant activity is known to be substantially greater than β-carotene and about a thousand times more effective than vitamin E. The potential health benefits have generated a growing commercial interest, and the escalating demand has prompted the exploration of alternative supply chain. Astaxanthin naturally occurs in many sea creatures such as trout, shrimp, and microalgae, some fungi, bacteria, and flowering plants, acting to protect hosts against environmental stress and adverse conditions. Due to the rapid growth and simple growth medium requirement, microbes, such as the microalga, Haematococcus pluvialis, and the fungus Xanthophyllomyces dendrorhous, have been developed to produce astaxanthin. With advances in metabolic engineering, non-carotenogenic microbes, such as Escherichia coli and Saccharomyces cerevisiae, have been purposed to produce astaxanthin and significant progress has been achieved. Here, we review the recent achievements in microbial astaxanthin biosynthesis (with reference to metabolic engineering strategies) and extraction methods, current challenges (technical and regulatory), and commercialization outlook. Due to greenness, sustainability, and dramatic cost reduction, we envision microbial synthesis of astaxanthin offers an alternative means of production (e.g. chemical synthesis) in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astaxanthin, a red xanthophyll or oxygen-containing carotenoid, is known for its potent antioxidant activity, of about 100–1000-fold higher than coenzyme Q10 or vitamin E. Astaxanthin was initially discovered in 1938 and was first used as a pigment for aquaculture. Since 1991, astaxanthin has been approved as a colouring for food supplement. It has diverse biological activities and potential health benefits (e.g. antioxidant, anti-inflammatory, and antitumour activities) to humans and animals and have been extensively investigated (Fakhri et al. 2018; Ng et al. 2020; Nouchi et al. 2020; Yamashita 2015).
In view of potential health benefits of astaxanthin, its demand is increasing rapidly in food industries, medicine, cosmetics, and animal feeds (e.g. salmon and prawns). Its global market is expected to reach $2.57 billion worldwide by 2025 (Park et al. 2018). Hence, multiple routes have been explored to produce astaxanthin. This includes direct extraction from crustacean waste (such as krill, shrimp, and crab); cultivation of natural producers such as Haematococcus pluvialis (microalgae), Paracoccus sp. (bacteria), and Xanthophyllomyces dendrorhous (yeasts); and chemical synthesis. The direct extraction from crustacean wastes is limited by low yields and high costs. Similarly, cultivation of natural producers also has relatively high production costs. For example, the production cost of astaxanthin produced from H. pluvialis is $2500–7000/kg (Bauer and Minceva 2019; Koller et al. 2014; Molino et al. 2018). Hence, the current commercially available astaxanthin is still dominated by chemical synthesis (~ $1000/kg) by various companies (e.g. BASF and DMS) (Khoo et al. 2019). Currently, over 95% of astaxanthin available in the market is produced synthetically. Synthetic astaxanthin was produced by a double Wittig reaction of a 3-methyl-5-(2,6,6-trimethyl-3-oxo-4-hydroxy-1-cyclohexenyl)-2,4-pentadienyltriphenylphosphonium salt (asta-C15-triphenylphosphonium salt) and 2,7-dimethyl-2,4,6-octatrienedial (C10-dial) (Krause et al. 1997).
It was reported that synthetic astaxanthin has a lower antioxidant capacity than its natural counterpart, possibly because synthetic astaxanthin is a racemic mixture, containing three isomers, i.e. 3S,3′S-, 3R,3′S-, and 3R,3′R-astaxanthin in the ratio of 1:2:1, while astaxanthin from H. pluvialis is predominantly 3S,3′S stereoisomer (Koller et al. 2014). In addition, there are safety concerns about the use of synthetic astaxanthin for direct human consumption due to potential carryover of synthesis intermediates and by-products (Shah et al. 2016). To date, synthetic astaxanthin has not been approved for human consumption (Koller et al. 2014; Rajesh et al. 2017). Therefore, the demand for natural astaxanthin is growing more rapidly than that for synthetic astaxanthin in recent years. The growing demand has driven researchers to improve on existing bioproduction methods using natural hosts and explore non-native industrial workhorse microbes (such as Escherichia coli, Saccharomyces cerevisiae, and Yarrowia lipolytica) for astaxanthin production. The main challenge for astaxanthin production in H. pluvialis (microalgae) is the complexity of the bioprocess and difficulty to scale up, as it requires two-stage cultivation with high-intensity light. In addition, as microalga growth rate is slow, the production phase is relatively long (several weeks) and practically more susceptible to contamination. Although the astaxanthin content can reach ~ 50 mg/g DCW in microalgae under optimal conditions (on average, 15–30 mg/g DCW) (Molino et al. 2018), the biomass is relatively low, usually below 15 g/L in about a month of cultivation, hence limiting the rate and titre of astaxanthin production. As a comparison, non-native microbes such as E. coli and some yeasts can readily achieve > 100 g/L biomass within a few days of fermentation. These are ideal industrial chassis microorganisms because of their competitive advantages such as their rapid growth in cost-effective media, ease to reach high cell density, and, more critically, amenability to genetic engineering. The comparison of the advantages and disadvantages of different microbes in astaxanthin biosynthesis is summarized in Table 1. With the advances in metabolic engineering, enzyme engineering, and synthetic biology, the biosynthesis of astaxanthin in aforementioned non-native microbes has achieved exciting progress (e.g. high yields) in the past decade.
This review is an attempt to capture recent progress in microbial astaxanthin biosynthesis and examine the critical issues and challenges when producing astaxanthin in native and non-native microbes, extraction of astaxanthin from microbes, and commercialization opportunities, focusing on metabolically engineered microbes.
Natural biosynthetic pathways of astaxanthin
Before diving into the metabolic engineering strategies for astaxanthin production, we firstly need to have a clear appreciation of the biosynthetic pathways of astaxanthin in natural hosts. In nature, astaxanthin is found in many animals, such as marine animals including salmon, shrimp, lobster, crab, and krill; and birds like flamingos and quails (Zhang 2018). However, these animals do not synthesize astaxanthin but acquire astaxanthin from their diets which contain natural astaxanthin producers. The natural producers of astaxanthin include microalgae (H. pluvialis and Chlorella zofingiensis) (Han et al. 2013), fungi (the basidiomycetous yeast, X. dendrorhous, also known as Phaffia rhodozyma) (Visser et al. 2003), and bacteria (Paracoccus spp. and Brevundimonas spp.) (Ide et al. 2012). In addition, some flowering plants (Adonis aestivalis and Adonis annua) (Cunningham Jr. and Gantt 2011) and protists (Aurantiochytrium sp.) (Watanabe et al. 2018) produce astaxanthin, despite at small amounts.
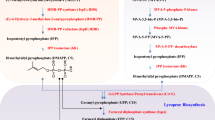
In aforementioned natural producers, astaxanthin (a terpenoid) biosynthesis starts from the two common terpenoid precursors, i.e. isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). IPP and DMAPP are synthesized from two independent biosynthetic pathways: the mevalonate pathway and the 2-C-methyl-d-erythritol 4-phosphate (MEP) pathway, also referred to as the non-mevalonate or the 1-deoxy-d-xylulose 5-phosphate (DXP) pathway. Here we do not elaborate the two terpenoid pathways as they are extensively explained in previous articles (Ajikumar et al. 2008; Chen et al. 2020; Kirby and Keasling 2009). IPP and DMAPP are condensed into geranylgeranyl pyrophosphate (GGPP) by farnesyl pyrophosphate (FPP) and GGPP synthases. GGPP is converted into phytoene by phytoene synthase and further into lycopene by desaturases (Zhang et al. 2013). Lycopene is further transformed into β-carotene by lycopene β-cyclase (Fig. 1) (Cunningham Jr. et al. 1996). Until β-carotene, the biosynthetic pathway is linear and essentially the same in different organisms. However, starting from β-carotene, the biosynthetic routes differ among different organisms, especially between flowering plants and others (Fig. 2). In bacteria, fungi, and algae, the β-ionone ring of β-carotene can be firstly oxidized into 4-keto intermediates or 3-hydroxyl intermediates by β-carotene ketolases and hydroxylases, respectively. In total, there are two hydroxylation and two ketolation reactions. Both hydroxylation and ketolation can occur sequentially (e.g. hydroxylation1-hydroxylation2-ketolation1-ketolation2) or non-sequentially (e.g. hydroxylation1-ketolation1-hydroxylation2-ketolation2). As such, there are 7 intermediates between β-carotene and astaxanthin in bacteria, fungi, and algae: β-cryptoxanthin, zeaxanthin, adonixanthin, echinenone, canthaxanthin, 3-hydroxyechinenone, adonirubin (Figs. 2 and 3) (Zhang et al. 2018b). In bacteria, the β-carotene ketolases and hydrolyases are encoded by genes crtW and crtZ, respectively (Breitenbach et al. 1996; Misawa et al. 1995; Scaife et al. 2009). Similarly, these enzymes are encoded by genes crtO (or bkt) and chyb (or crtR-B) in algae, respectively (Han et al. 2013). In the fungus X. dendrorhous, the β-carotene ketolase and hydrolyase are the same bifunctional cytochrome P450 enzyme, encoded by crtS (Alvarez et al. 2006). It is also known as astaxanthin synthase, where the activity is facilitated by a cytochrome P450 reductase, encoded by the gene crtR.
Biosynthetic pathways of β-carotene. IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; FPPS, farnesyl pyrophosphate synthase; GGPPS, GGPP synthase; crtB, bacterial phytoene synthase; crtI, bacterial phytoene desaturase; PSY, plant 15-cis-phytoene synthase; PDS, plant 15-cis-phytoene desaturase; ZDS, plant ζ-carotene desaturase; crtY, bacterial lycopene β-cyclase; LCYb, plant lycopene β-cyclase
Biosynthetic pathways of astaxanthin from β-carotene in bacteria, fungi, algae, and plants. crtW, bacterial β-carotene ketolase; crtO, alga β-carotene ketolase (bkt); crtZ, bacterial β-carotene hydroxylase; CHYB, alga β-carotene hydroxylase (crtR-B); crtS, yeast astaxanthin synthase (a P450 oxygenase, working with crtR, the cytochrome P450 reductase); HBFD, carotenoid 4-hydroxy-β-ring 4-dehydrogenase (Adketo); CBFD, carotenoid β-ring 4-dehydrogenase (Adkc). Enzymes in yeasts, bacteria, and algae are shown in blue. Enzymes in flowering plants are highlighted in red
In contrast, the astaxanthin biosynthetic pathway is proposed to be linear in the flowering plants A. aestivalis and A. annua (Cunningham Jr. and Gantt 2011). Firstly, β-carotene is converted into a 4-hydroxyl product isocryptoxanthin, isozeaxanthin and successively into 3,4,-tetradehydroisozeaxanthin and further into 3,4,3′,4′-tetradehydroisozeaxanthin, by two carotenoid β-ring 4-dehydrogenases (CBFD) encoded by cbdf1 and cbdf2. The intermediate, 3,4,3′,4′-tetradehydroisozeaxanthin, is further catalyzed into canthaxanthin via 4′-hydroxyechinenone by two carotenoid 4-hydroxy-b-ring 4-dehydrogenases (HBDF), encoded by hbdf1 and hbdf2. Astaxanthin is finally produced from canthaxanthin via adonirubin by CBDF1 and CBDF2, and the two steps are known to converge in other organisms (Fig. 2) (Cunningham Jr. and Gantt 2011). In sum, in flowering plants, there are seven intermediates between β-carotene and astaxanthin. However, five of these intermediates are different in algae, bacteria, and fungi. It is worthy to note that X. dendrorhous naturally produces the 3R,3′R-astaxanthin isomer unlike other organisms where (3S,3′S)-astaxanthin is the product.
Microbial biosynthesis of astaxanthin
In this section, we explain the recent examples and scientific breakthrough of microbial production of astaxanthin. These examples nicely illustrate different strategies in both bioprocess development and metabolic engineering. For better explanation and comparison of these strategies, we classify the microbes into two main classes: native astaxanthin producers and non-native producers. For native producers, targeted genetic engineering is rather challenging; hence, the common strategies are from bioprocess development and untargeted methods (e.g. random mutagenesis). In addition, native producers often produce modified astaxanthin (e.g. astaxanthin esters), which is difficult to be controlled and modified. In contrast, the main strategies for non-native producers are on the basis of rational genetic engineering or metabolic engineering. The challenges for non-natural producers are mainly attributed to inefficiency of heterologous enzymes and pathway complexity (over 10 genes), with the possibility of accumulating many structurally similar intermediate carotenoids (Figs. 2 and 3). Therefore, many novel metabolic engineering and synthetic biology strategies have been developed to address these challenges.
Astaxanthin biosynthesis in native microbes
H. pluvialis, a unicellular freshwater microalga, has been used to produce astaxanthin for many years. The production of astaxanthin in H. pluvialis has been recently reviewed (Khoo et al. 2019; Shah et al. 2016), and we will briefly introduce several recent findings. H. pluvialis cultivation contains two distinct stages: green phase and red phase. In green stage or green motile stage, the biomass increases with vegetative growth without accumulating astaxanthin. In red stage or red non-motile stage, the cells are known as haematocyst, wherein astaxanthin is accumulated in the membrane (Khoo et al. 2019). The transition happens due to stress conditions introduced, which can be nutrient (nitrogen and phosphorus) starvation, high salinity, temperature ramping, or combination of multiple stress factors. The need of this two-stage cultivation approach inevitably prolongs the production period and often results in modest final biomass. Due to the lack of engineering tools, genotype improvement is highly reliant on the more traditional approaches such as random mutagenesis and screening (Khoo et al. 2019). As such, a great deal of effort has instead been focusing on the optimization of abiotic conditions and bioprocess development. Nitrogen limitation (Ding et al. 2018) and high temperature growth conditions (Hong et al. 2015) have been routinely adopted to optimize astaxanthin production. A recent study applied a pattern of combining high illumination at green stage with low illumination and high CO2 levels at the red stage to optimize production. This resulted in a significant improvement (~ 36 mg/g DCW) of about 2–3 times over control conditions (Christian et al. 2018). Another recent study optimized the photoinduction process by tuning the light path and illumination mode to achieve high astaxanthin content in H. pluvialis (5.6%, or 56 mg/g DCW) (Wang et al. 2019b). In addition, ethanol supplementation was recently found to dramatically increase astaxanthin accumulation in microalga cells by upregulating the expression of carotenogenesis genes (Liu et al. 2019). Similarly, iron(II) supplement was found to boost astaxanthin content under outdoor thermal conditions (Hong et al. 2016).
Chromochloris zofingiensis is another promising microalga to produce astaxanthin (Zhang et al. 2019). C. zofingiensis can be cultivated in heterotrophic conditions with glucose as the carbon source. In heterotrophic conditions, it has a higher growth rate and is feasible to attain high cell density. The main limitation of H. pluvialis culture is the low production rate and low biomass where 20 g/L of biomass is already considered as “ultrahigh” (Han et al. 2013).
As compared with microalgae, the yeast X. dendrorhous grows faster and is easier to scale up in bioreactors and relatively amenable to genetic engineering. Similar to microalgae, the media (e.g. carbon sources, C/N ratio, and phosphate) and abiotic factors (e.g. oxygen levels, temperature, and pH) can be optimized to increase astaxanthin production in the yeast (Schmidt et al. 2011). Furthermore, genetic engineering methods have been applied to this yeast. The first review of metabolic engineering of X. dendrorhous for astaxanthin production was more than a decade (Visser et al. 2003). Due to the lack of efficient molecular biology tools, metabolic engineering strategies were mainly limited to the overexpression of the carotenogenesis genes like the bifunctional phytoene synthase/lycopene cyclase (crtYB) and phytoene synthase (crtI) (Verdoes et al. 2003). At that time, the highest astaxanthin content achieved in X. dendrorhous was only about 3–4 mg/g DCW. From 2000 to 2010, several novel strategies were developed, including the use of flow cytometry to screen high producer X. dendrorhous strains based on florescence emission of astaxanthin (Ukibe et al. 2008). Since then, several major steps forward have been made. The combination of classical mutagenesis and genetic engineering (overexpression of 3-hydroxymethyl-3-glutaryl coenzyme A reductase, GGPP synthase, phytoene synthase/lycopene cyclase, and astaxanthin synthase) enabled the astaxanthin content to reach ~ 9 mg/g DCW (Gassel et al. 2014) (Table 2). The overexpression of astaxanthin synthase (crtS) was able to boost astaxanthin production by 33.5% by direct activity and interestingly, indirectly upregulated the carotenoid pathway genes (Chi et al. 2015). Furthermore, the deletion of a C22 sterol desaturase reduced ergosterol biosynthesis and increased astaxanthin production due to the alleviation of negative feedback of ergosterol to the mevalonate pathway genes (Yamamoto et al. 2016). In addition, a recent study identified glutamate feeding can enhance astaxanthin biosynthesis by affecting tricarboxylic acid cycle and increasing the channelling of carbon fluxes into acetyl-CoA, the precursor to terpenoids (Wang et al. 2019a).
In addition to microalgae and fungi, the bacterium Paracoccus sp. has also been used to produce astaxanthin. By random mutagenesis and overexpression of astaxanthin biosynthetic genes, the best strain was able to produce up to 480 mg/L in fed-batch fermentation (Ide et al. 2012).
Often, it is challenging to obtain high purity of free astaxanthin in native hosts as they are produced in esterified or glycosylated forms (Ambati et al. 2014; Liu et al. 2019; Yokoyama et al. 1998), along with many other structurally similar carotenoids, such as adonixanthin, adonirubin, canthaxanthin, torulene, torularhodin, and 3,3′-dihydroxy-β, ψ-carotene-4,4′-dione (Barredo et al. 2017). In contrast, aided by metabolic engineering and synthetic biology strategies, it is relatively easy to obtain free astaxanthin with high purity in non-native microorganisms as demonstrated previously (Jin et al. 2018; Park et al. 2018; Zhang et al. 2018b) in Table 2.
Metabolic engineering of non-native hosts for astaxanthin biosynthesis
In the past decade, metabolic engineering and synthetic biology have contributed remarkably in the use of microbes as biofactories by (1) broadening the use of raw materials which include sugars, lignocellulose (Zhang and Too 2019), food and industrial wastes (Mano et al. 2020), and natural gas and CO2 (Clomburg et al. 2017); (2) expanding the product lines from basic chemicals to specialty chemicals, consumer chemicals (Zhang et al. 2018a), liquid fuels (Liao et al. 2016), and others (Denby et al. 2018); and (3) achieving high TRYs (titres, rates, and yields) more rapidly and pushing the boundary of theoretical yields (Meadows et al. 2016). With these advancements, industrial microbial workhorses that are not carotenoid-producing hosts have been engineered to produce astaxanthin. Particularly, remarkable progress has been achieved recently in the use of three microbes: E. coli, the budding yeast S. cerevisiae, and the oleaginous yeast Y. lipolytica.
As a non-carotenogenic bacterium, E. coli has long been engineered to produce astaxanthin. To our knowledge, the first example was demonstrated as early as in the year 1998, where astaxanthin and its glucosides were produced by overexpression of all the carotenogenesis genes (Yokoyama et al. 1998). From 2000 to 2010, the main strategy for astaxanthin production was to screen for more active β-carotene ketolases, β-carotene hydroxylases, and their use in combinations. It was found that crtW-type ketolase could accept 3-hydroxy-β-ionone ring as the substrate but not for crtO-type ketolase (Choi et al. 2007a). Later, 12 β-carotene ketolases and 4 β-carotene hydroxylases from 5 cyanobacterial species were screened for astaxanthin biosynthesis in E. coli. The best combination enabled over 23.5-fold increase in the production of carotenoids, of which > 90% is astaxanthin and its content reached 1.99 mg/g DCW (Scaife et al. 2009) (Table 2). From 2011 to the present, more strategies have emerged and greater success in production was achieved. Chromosomal integration of carotenoid pathway genes was pioneered by a group in the University of Stuttgart, where the modified strain produced about 1.41 mg/g DCW of astaxanthin (Lemuth et al. 2011). Another team screened for 12 β-carotene hydroxylases from archaea, bacteria, cyanobacteria, and plants and identified the use of hydroxylase from a thermoacidophilic archaeon to be highly efficient and produced up to 0.31 mg/g DCW of astaxanthin (Scaife et al. 2012). Another effective strategy is the use of ribosome-binding sites (RBSs) to balance carotenoid pathway genes. With the RBSs to balance the 7 genes (idi, crtE, crtB, crtI, crtY, crtZ, and crtW), the astaxanthin content in E. coli reached a level of 2.64 mg/g DCW (Zelcbuch et al. 2013).
Recently, a robust and effective strategy to produce astaxanthin in E. coli is the development of a multidimensional heuristic process (MHP). MHP strategy adopts the use of modular metabolic engineering strategy where the 14 genes in the mevalonate and carotenoid biosynthetic pathways were segmented into four modules, each of which was individually controlled by four different promoters. Furthermore, the balance of intramodular genes was further regulated by different RBSs of varied translational initiation efficiencies. Hence, the MHP method not only does transcriptionally balance the modules including all the pathway genes but also has the flexibility to fine tune the expression of critical genes within a module (e.g. crtZ and crtW). As a result, the best strain produced 15.1 mg/g DCW (> 5-fold higher than previous highest data) and in fed-batch fermentation, it produced up to 320 mg/L of astaxanthin in an unoptimized fermentation process (Zhang et al. 2018b). In addition, astaxanthin was found to be preferentially secreted into media during E. coli fermentation. Therefore, medium absorbance correlating to extracellular astaxanthin concentration was used as a simple means to screen for high astaxanthin producers, which was more accurate and less subjective than colony colour–dependent visual inspection (Zhang et al. 2018b). Extending from this study, efflux pumps for astaxanthin export can be explored which should further improve astaxanthin production in E. coli (Zhang et al. 2016). As a complementary approach, comparable production of astaxanthin of up to 433 mg/L (7.12 mg/g DCW) by in silico flux analysis–based gene overexpression and process optimization has been reported after MHP publication (Park et al. 2018).
In S. cerevisiae, the pioneer work for astaxanthin biosynthesis dates back to year 2009, when a Japanese group provided evidence that both X. dendrorhous astaxanthin pathway genes crtS and crtR (Fig. 2) and bacterial pathways genes crtZ and crtW were able to convert β-carotene to astaxanthin in S. cerevisiae. Although the yield was rather low (detectable level, Table 2), the yeast cells exhibited enhanced oxidative stress tolerance than the β-carotene-accumulating strains, indicative of an antioxidant activity of astaxanthin (Ukibe et al. 2009). Since then, astaxanthin has been produced at significant amounts in S. cerevisiae (Zhou et al. 2019; Zhou et al. 2017; Zhou et al. 2015). Initial studies showed promising results by introducing H. pluvialis β-carotenoid hydroxylase (CHYB) and ketolase (bkt or crtO) genes (Fig. 2) into β-carotene-accumulating yeasts. By controlling gene copy numbers and supplementing iron to the growth media, the strain produced up to 4.7 mg/g DCW of astaxanthin. Thereafter, by protein and metabolic engineering strategies, the content and titre of astaxanthin were further improved to 8.1 mg/g DCW and 47.2 mg/L, respectively (Table 2) (Zhou et al. 2017). Further studies demonstrated the use of a temperature-controlled system which increased the titre to 235 mg/L, although the astaxanthin content was not improved further (Table 2) (Zhou et al. 2019). Similar to these studies, bacterial astaxanthin biosynthetic genes crtZ and crtW have been used in the same host (Fig. 2) (Wang et al. 2017). Through screening of the best combination of crtZ and crtW genes from diverse species and tuning the relative expression ratio between crtZ and crtW, 81 mg/L of astaxanthin was produced (Table 2). The productivity of the strain was further improved by atmospheric and room temperature plasma (ARTP)–based mutagenesis, and the best strain produced up to 217.9 mg/L astaxanthin, with a content of 13.8 mg/g DCW (Table 2) (Jin et al. 2018).
Besides E. coli and baker’s yeast, a few other microbes have recently been engineered to produce astaxanthin. This includes two yeasts Yarrowia lipolytica and Kluyveromyces marxianus, a bacterium Corynebacterium glutamicum, and a fast-growing cyanobacterium Synechococcus sp. In recent years, Yarrowia lipolytica has become a popular microbial host for the production of metabolites including lipids and carotenoids due to its unique capabilities of using diverse substrates, innate de novo lipogenesis, and tolerance to extreme pH values (3–10) (Sekova et al. 2015). Y. lipolytica was initially engineered to produce lycopene and β-carotene by Microbia, Inc. (Grenfell-Lee et al. 2014) and later by other academic groups (Gao et al. 2017; Larroude et al. 2018; Matthaus et al. 2014; Schwartz et al. 2017). As an oleaginous yeast, Y. lipolytica has the advantage of producing hydrophobic compounds including carotenoids as intracellular lipid bodies act as storage reservoirs. Hence, relatively high content of carotenoids, including astaxanthin, has been produced in Y. lipolytica. In the first report, astaxanthin was produced at a content of 3.5 mg/g DCW (54.6 mg/L) by the heterologous expression of β-carotene ketolase (crtW) from Paracoccus sp. N81106 and hydroxylase (crtZ) from Pantoea ananatis (Kildegaard et al. 2017). More recently, the same group re-optimized the biosynthetic pathway to accumulate more β-carotene by further modulating the copy numbers of β-carotene ketolase and hydroxylase. The optimized strain produced 6 mg/g DCW (285 mg/L) of astaxanthin in bioreactors (Table 2) (Tramontin et al. 2019). Repeated genome integration of the genes chyb and bkt from H. pluvialis and engineering of CHYB enzyme enabled K. marxianus strain has produced up to 9.97 mg/g DCW of astaxanthin in a 5-L bioreactor (Lin et al. 2017). Through balancing crtZ and crtW at translational levels and screening for more catalytically efficient crtZ and crtW from different marine bacteria, astaxanthin content has been reported to be about 1.6 mg/g DCW in the gram-positive bacterium C. glutamicum (Henke et al. 2016). Recently, Synechococcus sp. PCC 7002 has been engineered to produce astaxanthin at 3 mg/g DCW (Table 2) using the β-carotene hydroxylase and ketolase from the marine bacterium Brevundimonas sp. (Hasunuma et al. 2019).
From these studies, it is evident that improvement in astaxanthin titre has been achieved by the use of enzymes with high catalytic activities from various sources. Further improvements can be achieved by effectively channelling the global carbon fluxes from central pathways to carotenoid biosynthesis and modulating the enzymatic activities of ketolase and hydroxylase in these promising heterologous hosts (Zhang et al. 2018b).
Extraction of astaxanthin from microbes
The downstream extraction and purification is a critical part that greatly influences the successful commercialization of astaxanthin. For fermentation products, the downstream cost can take 20–90% of the total manufacturing costs (Stanbury et al. 2017). As an intracellular product, astaxanthin is also thermo- and light-sensitive. All these factors tend to increase the difficulties and cost of product recovery. This is mainly because fermentation products have relatively low concentrations or titres with water as the major content (e.g. microbial-derived astaxanthin, ~ 0.4 g/L; Table 2; in contrast, synthetic astaxanthin can reach ~ 60 g/L (Krause et al. 1997)). Hence, to develop a successful commercialization route of astaxanthin, it is imperative to take a holistic view from strain engineering to downstream recovery. The necessity contrasts with the fact that many metabolic engineers and synthetic biologists overlook the importance of recovery processes. To highlight the importance of recovery step, here we briefly introduce the commonly used extraction methods of microbial astaxanthin.
The extraction methods of astaxanthin from either native or non-native microbes are similar, with the exception from microalgae where astaxanthin is found mainly in esterified forms. Hence, an additional saponification step is required to obtain free astaxanthin. For the extraction of astaxanthin, a common workflow is used, with the following sequential steps: (1) cell harvest from bioreactors by centrifugation, sedimentation, or filtration; (2) mechanical cell disruption; (3) drying of cells; (4) solvent extraction or supercritical CO2 extraction (Bauer and Minceva 2019; Choi et al. 2007b; Molino et al. 2018) (Fig. 4). Depending on solvent types, extraction temperature, and cell stages, mechanical cell disruption may not be necessary. For example, when germination of the red H. pluvialis is induced by fresh medium, the flagellated zoospores containing astaxanthin is released and this allows direct liquid-liquid solvent extraction from fermentation broth, negating the need of mechanical disruption which markedly simplifies the extraction processes (Bauer and Minceva 2019). However, to use liquid-liquid extraction method, the solvent has to be carefully chosen; green solvent such as ethanol is not economical due to its miscibility with water and the high stability of ethanol/water system which makes it a challenge to recycle ethanol and water. In addition, the extraction timing has to be carefully determined so as to obtain the highest yield. Furthermore, capital investment in equipment increases greatly when there is a need to process large volumes (fermentation broth volume versus cell pellet volume). In general, microalgae and fungi have thick and rigid cell walls and are more resistant to solvent disruption, and this will require a mechanical cell disruption step. In contrast, bacteria such as E. coli have weak cell membrane and are less resistant to solvent, making extraction from this host more amenable. Hence, step 2 (mechanical cell disruption) is optional for astaxanthin extracted from bacteria. More interestingly, in the case where astaxanthin is secreted into the media, a two-phase fermentation system is used to harvest extracellular astaxanthin with a biocompatible organic solvent (Zhang et al. 2018b). Thus, purifying astaxanthin from the organic layer is as simple as separating solvent from fermentation broth followed by evaporation of the solvent.
The key step in extracting astaxanthin is the choice of solvent used. This can be based on the “Pfizer Solvent Selection Guide”, where “preferred” solvents are green and safe (e.g. ethanol, ethyl acetate), “usable” ones are less green but have no better substitutions (e.g. cyclohexane and n-heptane), and “undesirable” (e.g. n-hexane and dichloromethane) ones are less safe but green (Bauer and Minceva 2019). Alternatively, supercritical CO2 is an attractive method for extraction due to adjustable selectivity, zero solvent residues, ease to be recycled, and greenness of CO2 (Sanzo et al. 2018). However, co-solvents are often required in most cases to improve the extraction efficiency of carotenoids (Krichnavaruk et al. 2008; Nobre et al. 2006). Furthermore, the capital investment in the use of supercritical CO2 is noticeably higher as compared with conventional solvent extraction method.
The operation cost of astaxanthin produced from microalgae is relatively high. This is due to the cost incurred for harvesting the low biomass of microalgae from bioreactors, which accounts for as much as 20–30% of the total production cost (Bauer and Minceva 2019; Panis and Carreon 2016). For production in bacteria and yeasts, such cost can be considerably reduced because of high biomass attainable in fed-batch fermentation.
Challenges and commercialization outlook
The breakthrough in methodologies and the objectives of achieving high “TRY” (titre, rate, and yield) in microbial synthesis of astaxanthin, especially in non-native hosts, together with maturity of extraction technology, have greatly decreased the production cost which is now comparable or even lower, in several cases, than that of chemical synthesis.
Regardless of whether astaxanthin and other carotenoids are produced naturally, synthetically, or otherwise, there are regional legal and regulatory challenges associated with the commercialization as food/feed, nutraceutical, and cosmetic products (Novoveská et al. 2019). One advantage of using native astaxanthin-producing microbes is the greater regulatory acceptance and better consumer reception as products from non-genetically modified organism (GMO) sources. In other words, the main challenge to commercialize astaxanthin produced by non-native microbes is the regulatory hurdles and associated consumers’ perception. However, these non-GMO demands are applicable only for direct human consumption as food additives and nutraceuticals, but not for other applications such as in medicine, animal feed, and cosmetics, where quality and cost are critical factors. To date, only natural astaxanthin has been approved for human consumption as a nutraceutical (Capelli et al. 2013). Furthermore, the GMO regulation rules vary in different countries and regions. Some regulatory parties loosely define non-chemically synthetic substances as natural products. If astaxanthin is purified from biomass to be DNA-free and toxin-free, it is not labelled as a GMO material but a “natural” or “natural identical” product. In these cases, the metabolically engineered microbes hold good potential in terms of lower cost and higher purity.
All the aforementioned microbes in Table 2 have the potential to be developed into biofactories for astaxanthin production, and we have further summarized the unique advantages of each microorganism in Table 1. Briefly, E. coli is the fastest-growing species among all the astaxanthin-producing microbes and is the most studied bacterium. As the first microbe engineered to produce astaxanthin, it has been optimized to achieve the highest titre and production rate (Park et al. 2018) and content (Zhang et al. 2018b). S. cerevisiae is the second microbe engineered to synthesize astaxanthin with high TRY (Jin et al. 2018; Zhou et al. 2019). As S. cerevisiae has been used for over 10,000 years for brewing and baking, it is one of the most recognizable strains and well-accepted in food products. The oleaginous yeast Y. lipolytica naturally accumulates lipid bodies which act as a “sink” for hydrophobic compounds such as astaxanthin. Hence, it has a higher storage capacity of astaxanthin over E. coli and budding yeast.
Other microbes with promising characteristics may also serve as biofactories for astaxanthin production. The gram-positive bacterium C. glutamicum grows rapidly and has native carotenogenesis genes and produces C50 carotenoids such as decaprenoxanthin. K. marxianus is an industrially used ascomycetous yeast capable of co-utilizing lactose and glucose and is thermos-tolerant up to 45 °C (Lane and Morrissey 2010). In addition, this strain of yeast can use industrial whey waste to produce high-value compounds including astaxanthin (Lane and Morrissey 2010). Synechococcus sp. is a fast-growing cyanobacterium and is able to attain high density of growth under stress-free phototrophic conditions. Repurposing the metabolic pathways by modulating the expression of heterologous genes in these organisms may serve as the next generation of useful host for the production of astaxanthin.
Looking forward, biotechnology-inspired microbial synthesis will be a significant competitor to the chemical synthetic approach in production cost and purity. Although microbial-produced astaxanthin has yet to be widely adopted, we envision that this product will become one of the main sources of astaxanthin in the near future.
Conclusion
The health and nutritional benefits of astaxanthin contribute significantly to the recent increase in the demand for astaxanthin. In general, microbe-derived astaxanthin is considered natural and due to consumer preference, it has commanded premium price over synthetic forms. The recent promising results from metabolic engineering of biosynthetic pathways in microbes are beginning to rival the conventional methods to produce astaxanthin in high titre, rate, and yield. This will result in the lowering of cost and the increase in the availability of astaxanthin dramatically. Furthermore, these biosynthetic approaches are green and sustainable and as such, we envision that microbial-produced astaxanthin will gradually replace synthetic and naturally extracted astaxanthin in the near future.
References
Ajikumar PK, Tyo K, Carlsen S, Mucha O, Phon TH, Stephanopoulos G (2008) Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol Pharm 5(2):167–190. https://doi.org/10.1021/mp700151b
Alvarez V, Rodriguez-Saiz M, de la Fuente JL, Gudina EJ, Godio RP, Martin JF, Barredo JL (2006) The crtS gene of Xanthophyllomyces dendrorhous encodes a novel cytochrome-P450 hydroxylase involved in the conversion of beta-carotene into astaxanthin and other xanthophylls. Fungal Genet Biol 43(4):261–272. https://doi.org/10.1016/j.fgb.2005.12.004
Ambati RR, Phang SM, Ravi S, Aswathanarayana RG (2014) Astaxanthin: sources, extraction, stability, biological activities and its commercial applications--a review. Mar Drugs 12(1):128–152. https://doi.org/10.3390/md12010128
Barredo JL, García-Estrada C, Kosalkova K, Barreiro C (2017) Biosynthesis of astaxanthin as a main carotenoid in the heterobasidiomycetous yeast Xanthophyllomyces dendrorhous. Journal of Fungi 3(3):44
Bauer A, Minceva M (2019) Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography. RSC Advances 9(40):22779–22789. https://doi.org/10.1039/c9ra03263k
Breitenbach J, Misawa N, Kajiwara S, Sandmann G (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140(2–3):241–246. https://doi.org/10.1016/0378-1097(96)00187-5
Capelli B, Jenkins U, Cysewski GR (2013) Role of astaxanthin in sports nutrition. In: Bagchi D, Nair S, Sen CK (eds) Nutrition and enhanced sports performance. Academic Press, San Diego, pp 465–471
Chen X, Zhang C, Lindley ND (2020) Metabolic engineering strategies for sustainable terpenoid flavor and fragrance synthesis. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.9b06203
Chi S, He Y, Ren J, Su Q, Liu X, Chen Z, Wang M, Li Y, Li J (2015) Overexpression of a bifunctional enzyme, CrtS, enhances astaxanthin synthesis through two pathways in Phaffia rhodozyma. Microb Cell Fact 14:90. https://doi.org/10.1186/s12934-015-0279-4
Choi SK, Harada H, Matsuda S, Misawa N (2007a) Characterization of two beta-carotene ketolases, CrtO and CrtW, by complementation analysis in Escherichia coli. Appl Microbiol Biotechnol 75(6):1335–1341. https://doi.org/10.1007/s00253-007-0967-z
Choi SK, Kim JH, Park YS, Kim YJ, Chang HI (2007b) An efficient method for the extraction of astaxanthin from the red yeast Xanthophyllomyces dendrorhous. J Microbiol Biotechnol 17(5):847–852
Christian D, Zhang J, Sawdon AJ, Peng CA (2018) Enhanced astaxanthin accumulation in Haematococcus pluvialis using high carbon dioxide concentration and light illumination. Bioresour Technol 256:548–551. https://doi.org/10.1016/j.biortech.2018.02.074
Clomburg JM, Crumbley AM, Gonzalez R (2017) Industrial biomanufacturing: the future of chemical production. Science 355(6320):aag0804. https://doi.org/10.1126/science.aag0804
Cunningham FX Jr, Gantt E (2011) Elucidation of the pathway to astaxanthin in the flowers of Adonis aestivalis. The Plant cell 23(8):3055–3069. https://doi.org/10.1105/tpc.111.086827
Cunningham FX Jr, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E (1996) Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant cell 8(9):1613–1626. https://doi.org/10.1105/tpc.8.9.1613
Denby CM, Li RA, Vu VT, Costello Z, Lin W, Chan LJG, Williams J, Donaldson B, Bamforth CW, Petzold CJ, Scheller HV, Martin HG, Keasling JD (2018) Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat Commun 9(1):965. https://doi.org/10.1038/s41467-018-03293-x
Ding W, Zhao Y, Xu JW, Zhao P, Li T, Ma H, Reiter RJ, Yu X (2018) Melatonin: a multifunctional molecule that triggers defense responses against high light and nitrogen starvation stress in Haematococcus pluvialis. J Agric Food Chem 66(29):7701–7711. https://doi.org/10.1021/acs.jafc.8b02178
Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M (2018) Astaxanthin: a mechanistic review on its biological activities and health benefits. Pharmacol Res 136:1–20. https://doi.org/10.1016/j.phrs.2018.08.012
Gao S, Tong Y, Zhu L, Ge M, Zhang Y, Chen D, Jiang Y, Yang S (2017) Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous beta-carotene production. Metab Eng 41:192–201. https://doi.org/10.1016/j.ymben.2017.04.004
Gassel S, Breitenbach J, Sandmann G (2014) Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high-yield mutant. Appl Microbiol Biotechnol 98(1):345–350. https://doi.org/10.1007/s00253-013-5358-z
Grenfell-Lee D, Zeller S, Cardoso R, Pucaj K (2014) The safety of beta-carotene from Yarrowia lipolytica. Food Chem Toxicol 65:1–11. https://doi.org/10.1016/j.fct.2013.12.010
Han D, Li Y, Hu Q (2013) Astaxanthin in microalgae: pathways, functions and biotechnological implications. Algae 28(2):131–147. https://doi.org/10.4490/algae.2013.28.2.131
Hasunuma T, Takaki A, Matsuda M, Kato Y, Vavricka CJ, Kondo A (2019) Single-stage astaxanthin production enhances the nonmevalonate pathway and photosynthetic central metabolism in Synechococcus sp. PCC 7002. ACS Synth Biol 8(12):2701–2709. https://doi.org/10.1021/acssynbio.9b00280
Henke NA, Heider SA, Peters-Wendisch P, Wendisch VF (2016) Production of the marine carotenoid astaxanthin by metabolically engineered Corynebacterium glutamicum. Mar Drugs 14(7):124–121. https://doi.org/10.3390/md14070124
Hong ME, Choi YY, Sim SJ (2016) Effect of red cyst cell inoculation and iron(II) supplementation on autotrophic astaxanthin production by Haematococcus pluvialis under outdoor summer conditions. J Biotechnol 218:25–33. https://doi.org/10.1016/j.jbiotec.2015.11.019
Hong ME, Hwang SK, Chang WS, Kim BW, Lee J, Sim SJ (2015) Enhanced autotrophic astaxanthin production from Haematococcus pluvialis under high temperature via heat stress-driven Haber-Weiss reaction. Appl Microbiol Biotechnol 99(12):5203–5215. https://doi.org/10.1007/s00253-015-6440-5
Ide T, Hoya M, Tanaka T, Harayama S (2012) Enhanced production of astaxanthin in Paracoccus sp. strain N-81106 by using random mutagenesis and genetic engineering. Biochem Eng J 65:37–43. https://doi.org/10.1016/j.bej.2012.03.015
Jin J, Wang Y, Yao M, Gu X, Li B, Liu H, Ding M, Xiao W, Yuan Y (2018) Astaxanthin overproduction in yeast by strain engineering and new gene target uncovering. Biotechnol Biofuels 11(1):230. https://doi.org/10.1186/s13068-018-1227-4
Khoo KS, Lee SY, Ooi CW, Fu X, Miao X, Ling TC, Show PL (2019) Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour Technol 288:121606. https://doi.org/10.1016/j.biortech.2019.121606
Kildegaard KR, Adiego-Perez B, Domenech Belda D, Khangura JK, Holkenbrink C, Borodina I (2017) Engineering of Yarrowia lipolytica for production of astaxanthin. Synth Syst Biotechnol 2(4):287–294. https://doi.org/10.1016/j.synbio.2017.10.002
Kirby J, Keasling JD (2009) Biosynthesis of plant isoprenoids: perspectives for microbial engineering. Annu Rev Plant Biol 60(1):335–355. https://doi.org/10.1146/annurev.arplant.043008.091955
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Research 6:52–63. https://doi.org/10.1016/j.algal.2014.09.002
Krause W, Henrich K, Paust J, Ernst H (1997) Preparation of astaxanthin. Available online at: https://www.google.com/patents/US5654488.
Krichnavaruk S, Shotipruk A, Goto M, Pavasant P (2008) Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour Technol 99(13):5556–5560. https://doi.org/10.1016/j.biortech.2007.10.049
Lane MM, Morrissey JP (2010) Kluyveromyces marxianus: a yeast emerging from its sister’s shadow. Fungal Biol Rev 24(1–2):17–26. https://doi.org/10.1016/j.fbr.2010.01.001
Larroude M, Celinska E, Back A, Thomas S, Nicaud JM, Ledesma-Amaro R (2018) A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of beta-carotene. Biotechnol Bioeng 115(2):464–472. https://doi.org/10.1002/bit.26473
Lemuth K, Steuer K, Albermann C (2011) Engineering of a plasmid-free Escherichia coli strain for improved in vivo biosynthesis of astaxanthin. Microb Cell Fact 10(1):29. https://doi.org/10.1186/1475-2859-10-29
Liao JC, Mi L, Pontrelli S, Luo S (2016) Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat Rev Microbiol 14(5):288–304. https://doi.org/10.1038/nrmicro.2016.32
Lin YJ, Chang JJ, Lin HY, Thia C, Kao YY, Huang CC, Li WH (2017) Metabolic engineering a yeast to produce astaxanthin. Bioresour Technol 245(Pt A):899–905. https://doi.org/10.1016/j.biortech.2017.07.116
Liu YH, Alimujiang A, Wang X, Luo SW, Balamurugan S, Yang WD, Liu JS, Zhang L, Li HY (2019) Ethanol induced jasmonate pathway promotes astaxanthin hyperaccumulation in Haematococcus pluvialis. Bioresour Technol 289:121720. https://doi.org/10.1016/j.biortech.2019.121720
Mano J, Liu N, Hammond JH, Currie DH, Stephanopoulos G (2020) Engineering Yarrowia lipolytica for the utilization of acid whey. Metab Eng 57:43–50. https://doi.org/10.1016/j.ymben.2019.09.010
Matthaus F, Ketelhot M, Gatter M, Barth G (2014) Production of lycopene in the non-carotenoid-producing yeast Yarrowia lipolytica. Appl Environ Microbiol 80(5):1660–1669. https://doi.org/10.1128/AEM.03167-13
Meadows AL, Hawkins KM, Tsegaye Y, Antipov E, Kim Y, Raetz L, Dahl RH, Tai A, Mahatdejkul-Meadows T, Xu L, Zhao L, Dasika MS, Murarka A, Lenihan J, Eng D, Leng JS, Liu CL, Wenger JW, Jiang H, Chao L, Westfall P, Lai J, Ganesan S, Jackson P, Mans R, Platt D, Reeves CD, Saija PR, Wichmann G, Holmes VF, Benjamin K, Hill PW, Gardner TS, Tsong AE (2016) Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 537(7622):694–697. https://doi.org/10.1038/nature19769
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177(22):6575–6584. https://doi.org/10.1128/jb.177.22.6575-6584.1995
Molino A, Rimauro J, Casella P, Cerbone A, Larocca V, Chianese S, Karatza D, Mehariya S, Ferraro A, Hristoforou E, Musmarra D (2018) Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J Biotechnol 283:51–61. https://doi.org/10.1016/j.jbiotec.2018.07.010
Ng QX, De Deyn MLZQ, Loke W, Foo NX, Chan HW, Yeo WS (2020) Effects of astaxanthin supplementation on skin health: a systematic review of clinical studies. Journal of Dietary Supplements:1–14 doi:https://doi.org/10.1080/19390211.2020.1739187
Nobre B, Marcelo F, Passos R, Beirão L, Palavra A, Gouveia L, Mendes R (2006) Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur Food Res Technol 223(6):787–790. https://doi.org/10.1007/s00217-006-0270-8
Nouchi R, Suiko T, Kimura E, Takenaka H, Murakoshi M, Uchiyama A, Aono M, Kawashima R (2020) Effects of lutein and astaxanthin intake on the improvement of cognitive functions among healthy adults: a systematic review of randomized controlled trials. Nutrients 12(3). https://doi.org/10.3390/nu12030617
Novoveská L, Ross ME, Stanley MS, Pradelles R, Wasiolek V, Sassi J-F (2019) Microalgal carotenoids: a review of production, current markets, regulations, and future direction. Mar Drugs 17(11):640. https://doi.org/10.3390/md17110640
Panis G, Carreon JR (2016) Commercial astaxanthin production derived by green alga Haematococcus pluvialis: a microalgae process model and a techno-economic assessment all through production line. Algal Research 18:175–190. https://doi.org/10.1016/j.algal.2016.06.007
Park SY, Binkley RM, Kim WJ, Lee MH, Lee SY (2018) Metabolic engineering of Escherichia coli for high-level astaxanthin production with high productivity. Metab Eng 49:105–115. https://doi.org/10.1016/j.ymben.2018.08.002
Rajesh K, Rohit MV, Venkata Mohan S (2017) Chapter 7 - Microalgae-based carotenoids production. In: Rastogi RP, Madamwar D, Pandey A (eds) Algal green chemistry. Elsevier, Amsterdam, pp 139–147
Sanzo GD, Mehariya S, Martino M, Larocca V, Casella P, Chianese S, Musmarra D, Balducchi R, Molino A (2018) Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar Drugs 16(9):334. https://doi.org/10.3390/md16090334
Scaife MA, Burja AM, Wright PC (2009) Characterization of cyanobacterial beta-carotene ketolase and hydroxylase genes in Escherichia coli, and their application for astaxanthin biosynthesis. Biotechnol Bioeng 103(5):944–955. https://doi.org/10.1002/bit.22330
Scaife MA, Ma CA, Ninlayarn T, Wright PC, Armenta RE (2012) Comparative analysis of beta-carotene hydroxylase genes for astaxanthin biosynthesis. J Nat Prod 75(6):1117–1124. https://doi.org/10.1021/np300136t
Schmidt I, Schewe H, Gassel S, Jin C, Buckingham J, Humbelin M, Sandmann G, Schrader J (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89(3):555–571. https://doi.org/10.1007/s00253-010-2976-6
Schwartz C, Frogue K, Misa J, Wheeldon I (2017) Host and pathway engineering for enhanced lycopene biosynthesis in Yarrowia lipolytica. Front Microbiol 8:2233. https://doi.org/10.3389/fmicb.2017.02233
Sekova VY, Isakova EP, Deryabina YI (2015) Biotechnological applications of the extremophilic yeast Yarrowia lipolytica (review). Appl Biochem Microbiol 51(3):278–291. https://doi.org/10.1134/S0003683815030151
Shah MM, Liang Y, Cheng JJ, Daroch M (2016) Astaxanthin-producing green microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front Plant Sci 7:531. https://doi.org/10.3389/fpls.2016.00531
Stanbury PF, Whitaker A, Hall SJ (2017) Chapter 10 - The recovery and purification of fermentation products. In: Stanbury PF, Whitaker A, Hall SJ (eds) Principles of fermentation technology, Third edn. Butterworth-Heinemann, Oxford, pp 619–686
Tramontin LRR, Kildegaard KR, Sudarsan S, Borodina I (2019) Enhancement of astaxanthin biosynthesis in oleaginous yeast Yarrowia lipolytica via microalgal pathway. Microorganisms 7(10):472–417. https://doi.org/10.3390/microorganisms7100472
Ukibe K, Hashida K, Yoshida N, Takagi H (2009) Metabolic engineering of Saccharomyces cerevisiae for astaxanthin production and oxidative stress tolerance. Appl Environ Microbiol 75(22):7205–7211. https://doi.org/10.1128/AEM.01249-09
Ukibe K, Katsuragi T, Tani Y, Takagi H (2008) Efficient screening for astaxanthin-overproducing mutants of the yeast Xanthophyllomyces dendrorhous by flow cytometry. FEMS Microbiol Lett 286(2):241–248. https://doi.org/10.1111/j.1574-6968.2008.01278.x
Verdoes JC, Sandmann G, Visser H, Diaz M, van Mossel M, van Ooyen AJ (2003) Metabolic engineering of the carotenoid biosynthetic pathway in the yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Appl Environ Microbiol 69(7):3728–3738. https://doi.org/10.1128/aem.69.7.3728-3738.2003
Visser H, van Ooyen AJ, Verdoes JC (2003) Metabolic engineering of the astaxanthin-biosynthetic pathway of Xanthophyllomyces dendrorhous. FEMS Yeast Res 4(3):221–231. https://doi.org/10.1016/S1567-1356(03)00158-2
Wang B, Pan X, Jia J, Xiong W, Manirafasha E, Ling X, Yinghua L (2019a) Strategy and regulatory mechanisms of glutamate feeding to enhance astaxanthin yield in Xanthophyllomyces dendrorhous. Enzyme Microb Technol 125:45–52. https://doi.org/10.1016/j.enzmictec.2019.02.010
Wang F, Gao B, Wu M, Huang L, Zhang C (2019b) A novel strategy for the hyper-production of astaxanthin from the newly isolated microalga Haematococcus pluvialis JNU35. Algal Research 39. https://doi.org/10.1016/j.algal.2019.101466
Wang R, Gu X, Yao M, Pan C, Liu H, Xiao W, Wang Y, Yuan Y (2017) Engineering of β-carotene hydroxylase and ketolase for astaxanthin overproduction in Saccharomyces cerevisiae. Front Chem Sci Eng 11(1):89–99. https://doi.org/10.1007/s11705-017-1628-0
Watanabe K, Arafiles KHV, Higashi R, Okamura Y, Tajima T, Matsumura Y, Nakashimada Y, Matsuyama K, Aki T (2018) Isolation of high carotenoid-producing Aurantiochytrium sp. mutants and improvement of astaxanthin productivity using metabolic information. Journal of oleo science 67(5):571–578. https://doi.org/10.5650/jos.ess17230
Yamamoto K, Hara KY, Morita T, Nishimura A, Sasaki D, Ishii J, Ogino C, Kizaki N, Kondo A (2016) Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microb Cell Fact 15(1):155. https://doi.org/10.1186/s12934-016-0556-x
Yamashita E (2015) Let astaxanthin be thy medicine. PharmaNutrition 3(4):115–122. https://doi.org/10.1016/j.phanu.2015.09.001
Yokoyama A, Shizuri Y, Misawa N (1998) Production of new carotenoids, astaxanthin glucosides, by Escherichia coli transformants carrying carotenoid biosynthesis genes. Tetrahedron Lett 39(22):3709–3712. https://doi.org/10.1016/s0040-4039(98)00542-5
Zelcbuch L, Antonovsky N, Bar-Even A, Levin-Karp A, Barenholz U, Dayagi M, Liebermeister W, Flamholz A, Noor E, Amram S, Brandis A, Bareia T, Yofe I, Jubran H, Milo R (2013) Spanning high-dimensional expression space using ribosome-binding site combinatorics. Nucleic Acids Res 41(9):e98. https://doi.org/10.1093/nar/gkt151
Zhang C (2018) Biosynthesis of carotenoids and apocarotenoids by microorganisms and their industrial potential. Progress in Carotenoid Research:85
Zhang C, Chen X, Lindley ND, Too HP (2018a) A “plug-n-play” modular metabolic system for the production of apocarotenoids. Biotechnol Bioeng 115(1):174–183. https://doi.org/10.1002/bit.26462
Zhang C, Chen X, Stephanopoulos G, Too HP (2016) Efflux transporter engineering markedly improves amorphadiene production in Escherichia coli. Biotechnol Bioeng 113(8):1755–1763. https://doi.org/10.1002/bit.25943
Zhang C, Chen X, Zou R, Zhou K, Stephanopoulos G, Too HP (2013) Combining genotype improvement and statistical media optimization for isoprenoid production in E. coli. PLoS ONE 8(10):e75164. https://doi.org/10.1371/journal.pone.0075164
Zhang C, Seow VY, Chen X, Too HP (2018b) Multidimensional heuristic process for high-yield production of astaxanthin and fragrance molecules in Escherichia coli. Nat Commun 9(1):1858. https://doi.org/10.1038/s41467-018-04211-x
Zhang C, Too HP (2019) Revalorizing lignocellulose for the production of natural pharmaceuticals and other high value bioproducts. Curr Med Chem 26(14):2475–2484. https://doi.org/10.2174/0929867324666170912095755
Zhang Z, Sun D, Zhang Y, Chen F (2019) Glucose triggers cell structure changes and regulates astaxanthin biosynthesis in Chromochloris zofingiensis. Algal Research 39:101455. https://doi.org/10.1016/j.algal.2019.101455
Zhou P, Li M, Shen B, Yao Z, Bian Q, Ye L, Yu H (2019) Directed coevolution of beta-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin. J Agric Food Chem 67(4):1072–1080. https://doi.org/10.1021/acs.jafc.8b05003
Zhou P, Xie W, Li A, Wang F, Yao Z, Bian Q, Zhu Y, Yu H, Ye L (2017) Alleviation of metabolic bottleneck by combinatorial engineering enhanced astaxanthin synthesis in Saccharomyces cerevisiae. Enzyme Microb Technol 100:28–36. https://doi.org/10.1016/j.enzmictec.2017.02.006
Zhou P, Ye L, Xie W, Lv X, Yu H (2015) Highly efficient biosynthesis of astaxanthin in Saccharomyces cerevisiae by integration and tuning of algal crtZ and bkt. Appl Microbiol Biotechnol 99(20):8419–8428. https://doi.org/10.1007/s00253-015-6791-y
Author information
Authors and Affiliations
Contributions
C.Z. and X.C. proposed the initial outline of the manuscript. C.Z. and H.P.T. wrote and reviewed the manuscript. All authors contributed to the discussion and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This mini-review does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
• Growing commercial interests and demand of astaxanthin due to its health benefits
• Recent achievements in astaxanthin biosynthesis and extraction in microbes
• A summary of metabolic engineering strategies in astaxanthin biosynthesis
• Discussion about current challenges and outlook of microbial-derived astaxanthin
Rights and permissions
About this article
Cite this article
Zhang, C., Chen, X. & Too, HP. Microbial astaxanthin biosynthesis: recent achievements, challenges, and commercialization outlook. Appl Microbiol Biotechnol 104, 5725–5737 (2020). https://doi.org/10.1007/s00253-020-10648-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10648-2