Abstract
With the only exception of glycine, all amino acids exist in two specular structures which are mirror images of each other, called D-(dextro) and L-(levo) enantiomers. During evolution, L-amino acids were preferred for protein synthesis and main metabolism; however, the D-amino acids (D-AAs) acquired different and specific functions in different organisms (from playing a structural role in the peptidoglycan of the bacterial cell wall to modulating neurotransmission in mammalian brain). With the advent of sophisticated and sensitive analytical techniques, it was established during the past few decades that many foods contain considerable amounts of D-AAs: we consume more than 100 mg of D-AAs every day. D-AAs are present in a variety of foodstuffs, where they fulfill a relevant role in producing differences in taste and flavor and in their antimicrobial and antiaging properties from the corresponding L-enantiomers. In this review, we report on the derivation of D-AAs in foods, mainly originating from the starting materials, fermentation processes, racemization during food processing, or contamination. We then focus on leading-edge methods to identify and quantify D-AAs in foods. Finally, current knowledge concerning the effect of D-AAs on the nutritional state and human health is summarized, highlighting some positive and negative effects. Notwithstanding recent progress in D-AA research, the relationships between presence and nutritional value of D-AAs in foods represent a main scientific issue with interesting economic impact in the near future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
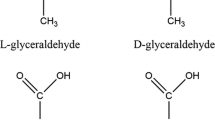
Amino acids have an α-carbon connected to four functional groups: an amine group, a carboxyl group, a hydrogen group, and a side chain. The α-carbon is a stereocenter (or chiral center) of the molecule since, depending on the spatial arrangement of these four different groups, two stereoisomers exist that are mirror images of each other (Fig. 1): levorotatory (L) and dextrorotatory (D). It is likely that more than one physical-chemical event in the primordial environment resulted in the generation of homochirality, favoring L-amino acids (L-AAs): natural proteins are built from L-AAs. Indeed, the D-amino acids (D-AAs) were identified in the past 30 years as natural biomolecules that play interesting and specific roles and that are components of our diet. Interestingly, D-AAs are formed during food processing and originate from microbial sources and from aqueous, soil, and other environments. D-AAs (mainly D-Ala and D-Glu) are key components of the peptidoglycan (PG) in the bacterial cell wall. PG synthesis is carried out in cytosol and periplasm/extra-cytoplasm compartments by a number of enzymes. In most bacteria, racemases convert L-Ala and L-Asp into the corresponding D-enantiomer; a lyase generates the D-alanyl-D-alanine dipeptide; MurA-MurE enzymes generate UDP-muramyl-L-Ala-γ-D-Glu-meso-diaminopimelate, which is then linked to D-Ala-D-Ala to give UDP-MurNAc-pentapeptide and an additional enzyme allows its binding to lipid I yielding lipid II, the lipid-disaccharylpentapeptide. On the extracellular compartment, D,D-transpeptidases link the D-Ala residues at position 4 in one peptide to the meso-diaminopimelate portion of a second peptide. PG is a dynamic structure: periplasmic enzymes edit peptidoglycan by introducing further D-AAs during stationary phase, principally into the terminus of the peptide moiety of muropeptides. For a review, see Cava et al. (2011), Horcajo et al. (2012), and Pidgeon et al. (2015).
D-AAs make the cell wall resistant to most proteases and the presence of alternative D-AAs (i.e., D-Asp or D-Ser) at the terminal position of the stem peptide provides resistance to some antibiotics, such as vancomycin (De Jonge et al. 2002; Reynolds and Courvalin 2005; Marcone and Marinelli 2014). The bacterial flora of the human body and infections constitute an additional permanent, endogenous source of D-AAs.
In mammals, absorption does not constitute a main issue in digesting D-AAs since amino acid transporters have a relatively broad specificity, whereby the preference for D-enantiomer transport is lower than for L-AAs. Two pathways are available for the biological utilization of D-AAs: racemases or epimerases convert D-AAs directly to L-enantiomer; degrading enzymes such as D-amino acid oxidase (DAAO) and D-aspartate oxidase (DASPO or DDO) catalyze the oxidative deamination of D-AAs into α-keto acids (Pollegioni et al. 2007; Pollegioni and Sacchi 2010; Sacchi et al. 2012), which can then be specifically aminated to the L-enantiomer (Brückner and Fujii 2010). In mammals, conversion by the oxidases predominates over the racemases (which occur primarily in bacteria). In the organism, D-AAs are excreted in the urine: the transformation takes place principally in the kidneys.
D-AAs are common constituents of our diet. Grocery stores are selling increasing quantities of foods (fruit juices and pulp, cereals, potatoes, tomato sauces, milk, etc.), which in some cases contain substantial quantities of D-AAs (Csapó et al. 2009). Indeed, during food processing, which is usually done to improve flavor, consistency, or nonperishability, the L-AAs may be racemized to their D-enantiomers (Masters and Friedman 1979; Friedman et al. 1981). D-AAs can also be generated as a consequence of adulteration, as for hydrolyzed proteins added to foodstuffs to hide low nutrient content. Notably, more than half of all dairy products and fermented foods contain D-Ala, D-Asp, and D-Glu. Every day, more than 100 mg D-AAs are likely ingested: for example, consumption of 100 g Emmental cheese corresponds to an intake of 70–80 mg D-AAs and 100 mL of instant coffee to one of > 20 mg.
D-amino acids in foods
In recent years, it has been shown that, in addition to L-AAs normally present in food proteins, foods can also contain D-AAs. D-AAs are found in a free or bound state, in a wide variety of foods and beverages, either naturally (such as in molluscs or in fermented food) (see section “D-amino acids in fermented foods”) or are formed artificially (during food processing or food adulteration) (see section “D-amino acids due to technological processes”). D-AAs have properties that differ from those of L-enantiomers, in terms of taste, flavor, and antimicrobial or anti-aging properties (Mutaguchi et al. 2016). Up to now, little is known about the role of D-AAs in foodstuffs; this is also because it is complicated to detect D-AAs in amino acid racemic mixtures. For this reason, an increasing number of studies have focused on methods to identify and quantify D-AAs in food (see section “Assay of D-amino acids in foods”).
D-AAs are naturally present in some plants, fruits, and vegetables (Brückner and Westhauser 2003). The natural amounts of D-AAs found in fruits and vegetables are usually lower than 3.4% and 0.7%, respectively. The highest amount of individual D-AA found was 3.4% D-Asn and 1.9% D-Asp in grapefruit, 2.7% D-Ala and 1.7% D-Ser in apples, and 1.3% D-Glu in clementines. Apples (Golden Delicious) contain 0.8 μmol/kg of N-malonyl-D-tryptophan (Brückner and Westhauser 1994). In commercially available fruit juices, high amounts of D-Ala (10–42%) can be found as result of bacterial contamination [see section “D-amino acids as a markers of contamination (in unfermented foods)”].
The natural occurrence of D-AAs in higher plants in a free or conjugated form has been widely debated. Such studies highlighted at least three ways that may have contributed to the development of D-AAs in plants, including vegetables and fruits. The first process establishes the involvement of enzymes in the de novo synthesis of free and conjugated D-AAs via different metabolic pathways (Fukuda et al. 1973; Ogawa and Fukuda 1973; Kawasaki et al. 1982; Rekoslavskaya et al. 1999; Rozan et al. 2000). The second, demonstrated by Brückner and coworkers, presupposes that D-AAs were nonenzymatically formed by the reaction of L-AAs and reactive carbonyl compounds (Brückner et al. 2001). The third assumes that D-AAs originate from exogenous sources such as bacteria (i.e., principally due to mycorhizal associations between the roots of plants and fungi), which offer an abundant reservoir of D-AAs in the peptidoglycan-bound form as well as free molecules (Schleifer and Kandler 1972; Brückner et al. 1993). In this latter case, plants readily take up microbial D-AAs (Aldag et al. 1971).
For a list of foods containing D-AAs, see Table 1.
Origin of D-amino acids
D-amino acids in fermented foods
The presence of D-AAs can be considered “natural” in fermented foods. D-AA formation in fermented products can arise from the starting materials or from microbial activity during fermentation. Different fermentation processes are involved in the production of fermented foods (i.e., alcoholic, acetic, and lactic fermentation), and each process could make a different contribution to the formation of D-AAs. A study on D-AAs in fermented foodstuffs produced from the same lot of materials stated that lactic fermentation represents the main means by which D-AAs are generated. For example, lactic fermentation is mainly responsible for producing high levels of D-Asp, D-Ala, and D-Glu in tomatoes and vinegar (Mutaguchi et al. 2013), in kefir, yogurt, curdled milk, and goat fresh cheese, soy sauce, etc., and in particular in beer, wine, and sake (Brückner and Hausch 1990; Erbe and Brückner 2000; Gogami et al. 2011). The behaviors of metabolites such as organic acids and amino acids formed during fermentation are used as indicators of progress during the fermentation process.
D-AAs such as D-Ala, D-Glu, D-Asp, D-Leu, and D-Ile are present in wine (Brückner and Hausch 1989; Kato et al. 1995; Jin et al. 1999) and might affect its flavor (i.e., D-Ala is known to have a sweet taste) (see section “D-amino acid taste”) (Schiffman et al. 1981). In some cases, the amount of D-AAs seems to be due to the presence of particular bacterial species: in red and white wine fermentation, an increase in amounts of D-Ala, D-Glu, and D-Lys correlates well with the presence of Gram-positive Oenococcus oeni (Kato et al. 2011), a beneficial bacterium as it performs malolactic fermentation whose end products are responsible for specific flavors. In the past few years, the presence of D-Pro in wine was used as an indicator for age dating (Chiavaro et al. 1998), but more recently, others found no correlation between aging and D-AAs content in wines (Ali et al. 2010). Instead, this study suggested that D-AA formation mainly depends on the bacteria employed for the fermentation: alterations in D-AA composition during fermentation are of great interest for winemakers, especially when related to commercial value and sensory qualities.
In some cases, fermentation is necessary to make a raw material more workable or to acquire characteristics fundamental for an excellent product. For example, sourdough fermentation makes the flour suitable for baking, controls the development of flour components, and inhibits fermentation by undesired bacteria or yeasts. The use of lactic acid bacteria and yeasts in sourdough fermentation before baking produces free D-Ala and D-Glu in the dough (Gobbetti et al. 1994). Large amounts of D-Ala, D-Asp, and D-Glu are also present in well-aged cheeses such as Parmigiano Reggiano and Grana Padano (up to 5 g/kg): the D-AA content varies among cheeses and during their production and ripening (Table 1) (Marchelli et al. 1997; Innocente and Palla 1999; Marchelli et al. 2007).
D-amino acids due to technological processes
The food industry is producing increasing quantities of foods (baked potatoes, fruit juices and fruit pulp, breakfast cereals, tomato sauces, milk, etc.) that can contain substantial quantities of D-AAs. In these foods, the racemization process is mainly responsible for the formation of D-AAs. Although it has been demonstrated that a detectable racemization could appear also at low storage temperature (25 °C) in selected foods, such as Korean kimchi (Taniguchi et al. 2017), the principal factors influencing racemization are usually alkaline or acid pH values, treatment duration, heat treatment, and duration of heating (Palla et al. 1989; Genchi 2017).
The amino acid racemization process can be promoted in the course of preparation of foods that normally contain low quantities of D-AAs, such as milk, meat, and some fruit juices. For example, D-AAs level does not increase in samples following heat treatments at a high temperature for a short time (such as pasteurization and ultra-high temperature processes), while amino acid racemization is apparent in sterilized or powdered milk (Table 1). In those cases, the technological process increased D-Ala level from 3 to 4% (the reference concentration) up to 12% (Gandolfi et al. 1992). In commercially available milk, an increased quantity of D-AAs might represent a marker of inflammatory disease of the cows producing it. Actually, substantial quantities of D-Asp, D-Glu, D-Ala, and D-allo-isoleucine (D-allo-Ile) are present in samples derived from cows with mastitis, and a positive correlation was observed between the D-AA increase and disease severity (Csapò et al. 1995) (see also section “D-amino acids as markers of contamination”).
A recent study concerning protein modification in infant milk formula reported significant D-AAs levels as a consequence of the thermal treatment required for the safety and prolonged shelf life of the products. The analyzed amino acids showed different values of racemization: D-Arg, the most abundant, increased up to 32% of the total amount of Arg (Chen et al. 2019).
D-Met can be chemically modified during food processing and L-Phe and L-Tyr can rapidly racemize into the corresponding D-enantiomer upon alkali or heat treatment (Csapò et al. 2008). The effect of temperature on the D-Ser and D-Thr content of soybean protein was reported by Friedman (1999). Effects of alkali treatment and high temperature on racemization have also been reported in commercial, ripe olives: after heating, D-Ser and D-Ala showed the highest racemization values, reaching 20% and 11%, respectively. In addition, D-Ser, D-Ala, and D-Asn levels increased at high pH (Casado et al. 2007). An interesting study conducted on different food wastes from the agrifood industry reported that a D-Asp increase could be mainly related to the harshness of the process (pH and temperature stress condition) and the percentage of D-Ala might more likely be due to the fermentation process, while D-Glu could be related to both conditions (Prandi et al. 2019). Moreover, elevated temperatures are responsible for a significant increase in D-AAs in honey: samples subjected to high temperatures possessed a greater quantity of D-AAs, where values increased proportionally to the temperature. An increase in D-AA content following roasting and alkaline treatment was also observed in cocoa beans (Pätzold and Brückner 2006). Other processing conditions might also influence D-AA production, such as the percentage of sodium chloride in fermented fish sauce. A study conducted on 60 fish sauce samples, divided into 10%- and 20%-salt preparations, reported that a higher salt concentration could hamper D-AA production (Abe et al. 1999).
Today, the food industry is mindful of the possible risks of treating proteinaceous food harshly: actually, new biotechnological processes are increasingly being adopted to avoid drastic reaction conditions, preferring the use of enzymes working at neutral pH, e.g., soy protein hydrolysates are produced enzymatically nowadays. In some cases, however, such as making gelatins or recovering proteins from cereals, milling by-products or oilseeds, drastic conditions are necessary (Lüpke and Brückner 1998; Seo et al. 2008). Alkali treatments are employed to give foods a special texture (e.g., tofu), to peel fruits and vegetables, and to prepare canned, dried, or frozen fish (Friedman 1999).
Altogether, the presence of D-AAs in foods can be used as a biomarker for excessive thermal and alkaline treatments and of inflammatory disease affecting the animals producing the raw materials. It can also be useful to evaluate protein quality of food wastes generated from the agrifood industry in view of a possible re-use.
D-amino acids as markers of contamination (in unfermented foods)
Dairy products have been reported to contain amounts of D-Ala, D-Asp, and D-Glu (1–3 mg/L) that are generally attributed to bacterial activity rather than processing technologies (Gandolfi et al. 1992): no other D-AAs were detected up to 0.01 ppm. A 3–4% D/(D + L) ratio of the three free D-AAs was detected, a value that could be considered physiological.
The D-Ala content of raw milk samples increases after storage at 4 °C for 1 month: the D/(D + L) ratio reached 50–55% with a D-Ala content of 3–5 mg/L. The increase in D-Ala levels was related to the presence of psychrotrophic bacteria (Gandolfi et al. 1992). Since the amount of D-Ala did not increase from milk treatment (see above), D-Ala can be considered as an indicator of bacterial milk contamination. Actually, microbial contamination contributes to the free D-AA content because of microbial racemase enzymes and cell wall lysis. The D-AA levels were quantified in cow, sheep, and goat milk (Albertini et al. 1996): a higher amount of D-Ala, D-Asp, and D-Lys was detected in goat than in cow and sheep milk samples. Analyses of goat milk detected a ratio of D/(D + L) Ala in the 3.5–36.7% range in comparison to a 1.2–4.5% and 1.2–6.5% range for cow and sheep milk samples, respectively (Albertini et al. 1996). These results can be attributed to a higher microbial contamination of the goat milk, due to less controlled storage conditions.
Significant amounts of D-Ala were also found in fruit juices (Gandolfi et al. 1994). Grapefruit juices stored for 40 days showed an increase in the D-Ala content up to 5–20 mg/L and a bacterial contamination corresponding to a concentration > 107 CFU/mL. As already found in milk samples (Gandolfi et al. 1992), the D-Ala content in fruit juices did not increase with thermal treatments (i.e., pasteurization or sterilization processes) or during the products’ shelf life.
Assay of D-amino acids in foods
Detection of D-AAs in food provides valuable information about quality, authenticity, or microbial contamination. The amino acid assay consists of several steps: the release of the AAs from the food matrix, the separation of the individual AA, detection, and quantification. The established analytical techniques based on high-performance liquid chromatography (HPLC; see Table 2) and gas chromatography (GC) have recently been supplemented by a number of new methods, such as capillary electrophoresis (CE) and ultra-performance HPLC (UPLC) combined with novel derivatization reagents and different detectors, see Table 3. Various methods are continuously being developed that are driven by the need to improve speed of analysis, sensitivity, robustness, and reproducibility.
HPLC and UPLC methods
HPLC is the most frequently used separation technique in bioanalysis. Two main approaches can be followed: (i) an indirect method based on chiral derivatizing reagents to give diasteroisomers separated on an achiral, reversed-phase HPLC column (Buck and Krummen 1987; Brückner et al. 1995; Erbe and Brückner 2000), and (ii) a direct chiral method based on a chiral stationary phase, or achiral, derivatizing reagents followed by separation on a chiral stationary or mobile phase (Guillén-Casla et al. 2010; Konya et al. 2017; Nakano et al. 2017; Hamase et al. 2010). See Table 2.
Ninhydrin, phenyl isothiocyanate (PITC), and o-phthaldialdehyde (OPA) have been used most frequently for the pre- and post-column derivatization of AAs: OPA reacts with primary amines, giving unstable derivatives with Gly and Lys (Bidlingmeyer et al. 1984), and PITC reacts with both primary and secondary amino acids, giving unstable derivatives with Glu and Asp. To avoid these drawbacks, new derivatizing reagents have been developed in the past few years (Thippeswamy et al. 2006): the best solution is represented by a stable and very sensitive reagent that reacts rapidly and at room temperature. The indirect chiral method based on OPA-NAC reaction has been optimized to detect seven free D-AAs (D-Ser, D-Thr, D-Ala, D-Tyr, D-Val, D-Trp, and D-Leu) in milk and oyster samples in 25 min (Rubio-Barroso et al. 2006), the level of detection (LOD, defined as 3 times the signal-to-noise ratio) being between 0.02 and 0.17 ng (Table 2). A method that combined two pre-column derivatization reactions with OPA-NAC and (+)-1-(9-fluorenyl)ethyl chloroformate (FLEC)/1-aminoadamantane (ADAM) (Einarsson et al. 1987) and one post-column derivatization reaction with OPA-NAC (Ishida et al. 1981) was used to detect and quantify D-AAs in 141 different sake samples (Gogami et al. 2011). In particular, Gly and D- and L-forms of Ala, Asp, Glu, Ile, Leu, Phe, Ser, Thr, Trp, and Val were detected by using the OPA-NAC pre-column derivatization method and separated by applying the gradient elution mode; Arg, Asn, Gln, His, Lys, and Pro were analyzed with the FLEC/ADAM pre-column derivatization method and separated by the gradient elution mode (with the exception of Pro separated by isocratic elution mode); Cys was detected by using the OPA-NAC post-column derivatization method (Table 2).
A number of chiral derivatizing reagents are reported in Ilisz et al. (2008). The use of 2,7-dimethyl-3,8-dinitrodipyrazolo[1,5-a:1′,5′-d]pyrazine-4,9-dione (Gioia et al. 2006) or 2,5-dimethyl-1H-pyrrole-3,4-dicarbaldehyde (Gatti et al. 2010), combined with a reversed-phase HPLC system, resulted in a detection limit range of 20–80 pmol and 3–11 pmol, respectively. Higher selectivity and sensibility were achieved using the fluorescent derivatization reagent 2-[2-(7H-dibenzo[a,g]carbazol-7-yl)-ethoxy] ethyl chloroformate: a LOD value of 0.19–1.17 fmol/μL was obtained in a HPLC-fluorimetric detection-tandem MS system (Li et al. 2011).
The fluorescent chiral reagents R(−)- and S(+)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazoles (DBD-PyNCS) react rapidly and quantitatively with both primary and secondary amino acids to give stable derivatives that can be detected with a high sensitivity. The resolution of 17 D,L-AAs was achieved by employing an isocratic (for hydrophilic AAs) or gradient elution (for hydrophobic AAs) method on a reversed-phase HPLC system (Jin et al. 1999). The detection limits were in the 0.16- to 0.75-pmol range (Table 2). The method was also applied to identify D-AAs in milk, cream, fermented dairy products, tomato products, and fermented beverages, obtaining a recovery of the internal standards of 92–94%.
A two-dimensional HPLC (2D-HPLC) method combining a pre-column derivatization with 4-fluoro-7-nitro-2,1,3-benzoxadiazole (NBD-F) followed by a reversed-phase separation (by means of a microbore monolithic ODS column) and a chiral separation (by a narrow-bore enantioselective column), made it possible to detect the enantiomers of all proteinogenic amino acids as well as of allo-Thr and allo-Ile in different Japanese Kurozu vinegar samples (Miyoshi et al. 2014).
A highly sensitive detection method (detection sensitivity = 0.5–3.2 fmol, Table 2) involves using light and heavy L-pyroglutamic acid succinimidyl ester (L-PGA-OSu) reagents combined with a UPLC method and the detection by ESI-MS/MS (Mochizuki et al. 2014). The pairs of 9 amino acids were separated in a single chromatographic run, and this method was applied to detect D-AAs in two yogurt samples (Mochizuki et al. 2014).
A high-throughput analytical method based on UPLC equipped with a circular dichroism (CD) detector was developed to detect the 20 proteinogenic AAs. Interestingly, rapid analysis was performed since the CD detector does not require separation of optical isomers to evaluate the enantiomeric ratio; the analysis time was within 5.5 min with a detection limit of 11–64 pmol/injection using a pre-column derivatization technique with NBD-F. The method was applied to assay AAs in Japanese black vinegars, fermented milk drinks, and yogurt samples (Eto et al. 2011).
A higher sensitivity can be achieved by combining pre-column derivatization methods with MS detection. A specific and sensible quantification of L- and D-AAs, excluding the interference of co-eluting isomers or matrix ions with identical m/z, was achieved by employing a UPLC method coupled to ion mobility high-resolution MS (IM-HRMS) (Tian et al. 2017). Indeed, 18 different chiral AAs were characterized in human, cow, yak, buffalo, goat, and camel milk. Using the (S)-NIFE derivatization method (Visser et al. 2011) to separate L- and D-AAs, the extraction recovery was in the 82–105% range and the LOD values from 0.01 ng/mL for methionine to 6.14 ng/mL for serine (Table 2) (Tian et al. 2017).
Axial chiral derivatizing reagents are of utmost relevance: recently, the complete chiral separation of 19 proteinogenic amino acids was achieved within 11.5 min with Rs > 1.9 using a new axial chiral reagent derived from 6,6′-dimethyl-2,2′-biphenyldiamine ((R)-BiAC) and a LC-MS/MS system (Harada et al. 2019). By employing the optimized method, D-AAs in black vinegar and in a lactic acid bacteria beverage were detected (Table 2).
Derivatization methods are time consuming and often the source of analytical bias when performed simultaneously on different AAs. Actually, real samples often contain more than 100-fold higher L-AA concentrations than the corresponding D-forms: a nonquantitative conversion of the analytes to their derivatives represents a possible source of analytical bias (Harada et al. 2019). Indeed, high temperatures and acidic conditions can result in amino acid racemization and can induce hydrolysis of the amide group of Asn and Gln to Asp and Glu (Waldhier et al. 2010). All these issues can be overcome using direct chiral methods. Here, the simultaneous detection of 18 chiral proteinogenic AAs, combining a chiral column and liquid chromatography time-of-flight mass spectrometry (LC-TOFMS), made it possible to obtain an excellent peak resolution without any derivatization steps (Konya et al. 2017). The analytical separation (run of 10 min) by means of the CROWNPAK CR-I(+) and CR-I(−) chiral columns achieved a LOD value ranging from 1 to 40 nmol/mL (Table 2). The method was validated using water, milk, and vinegar as matrices; the recovery rate of all D-AAs in milk and vinegar samples was 72–90%, the only exception being D,L-Pro (not separated). Using the same chiral columns, a highly sensitive and selective detection of trace amino acids was achieved by using a versatile and quantitative method based on multiple reaction monitoring (MRM) mode applied to tandem mass spectrometry analysis (MS/MS) (Nakano et al. 2017). The simultaneous detection of 18 D-AAs without a derivatization process was obtained; the LOD value ranged from 0.005 to 0.5 nmol/mL (Table 2). The method was used on three vinegar samples.
A comparison between performance, time, and cost of D-AAs analysis by HPLC-based techniques and alternative methods is reported in Table 3.
GC-MS and GC-FID methods
Gas chromatography (GC) was one of the first separation techniques used to detect amino acids. Similar to HPLC methods, direct and indirect chiral analyses can be performed, although direct analysis is the preferred method for D-AA assays. Usually, two different chiral stationary phases are used: the Chirasil-L-Val capillary column and cyclodextrin-based chiral stationary phases. Bruckner and coworkers widely used the capillary column to quantify D-AAs. Capillary GC equipped with a flame ionization detector (GC-FID) assayed free D-AAs in fermented and roasted cocoa beans, cocoa powder, chocolate, and cocoa shells. AAs were isolated using a Dowex cation exchanger, converted into volatile N(O)-pentafluoropropionyl amino acid 2-propyl esters, adding 1% antioxidant 2,6-di-tert-butyl-p-cresol, and analyzed on a Chirasil-L-Val column (Pätzold and Brückner 2006). The same method was used to detect free D-AAs in dairy products (kefir and Gorgonzola cheese), fermented sausages, and vegetable juices, alcoholic beverages (beer, white wine, and sake), milk and sour milk products, coffee, and fruits (Brückner and Hausch 1989; Brückner and Hausch 1990; Brückner and Westhauser 1994). D-AAs were determined in beers and raw materials on a GC instrument equipped with FID or a mass spectrometer (GC-MS) (Erbe and Brückner 2000): the limit of quantification (LOQ) values ranged from 0.57 to 1.49 mg/L and from 0.06 to 0.39 mg/L, using the FID and the MS detection system, respectively (Table 3). Worthy of note is that the same food samples were analyzed by applying the HPLC method, giving LOQ values from 0.04 to 0.68 mg/L. The RSD values ranged from 1.1% to 9.2% using FID to quantify D-AAs and 1.1–7.2% using the MS detector. The method based on GC-MS equipped with the silica capillary column Chirasil-L-Val was also used to detect L- and D-AAs in 26 wines, comprising white, red, and sparkling wines (Ali et al. 2010). The Arg, His, Cys, and Trp content could not be determined by employing derivatization chemistry, nor could Asn and Gln be determined as they are hydrolyzed to Asp and Glu under the acidic derivatization conditions used (Erbe and Brückner 2000).
Capillary electrophoresis method
Capillary electrophoresis (CE) is a powerful separation method that combines electromigration and chromatographic techniques. For derivatization, the chiral selectors most widely used are naphthalene dicarboxaldeyde (NDA), NBD-F, and FITC, combined with capillary zone electrophoresis or micellar electrokinetic chromatography (MEKC) separation analyses. The in-capillary derivatization procedure is fully automated and minimizes consumption of sample and derivatizing reagents. A fast in-capillary derivatization method with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate was successfully applied to determine the enantiomers of Arg, Lys, and ornithine in wines and dietary supplement samples (Table 3) (Martínez-Girón et al. 2009). A linear concentration range of 20–400 μM for Arg and Lys and 10–200 μM for ornithine was obtained. By employing a chiral MEKC method, including FITC derivatization and laser-induced fluorescence detection (MEKC-LIF), the L- and D-forms of Arg, Ser, Ala, Glu, and Asp could be separated in 25 min, with LOD values in the nM range (Table 3) (Herrero et al. 2007). The MEKC-LIF method proved to be a fast (run of 20 min) and sensitive method to analyze and quantify enantiomers of Pro, Ala, Arg, Glu, and Asp in vinegars (Carlavilla et al. 2006). With the same procedure, three types of commercial orange juices could be classified, providing the separation of the main 15 L- and D-AAs found in orange juices (Simó et al. 2004).
In capillary zone electrophoresis, cyclodextrins are the most frequently used chiral selectors. In particular, the use of modified 3-monodeoxy-3-monoamino-β-CD cyclodextrin to separate five chiral amino acids (Glu, Asp, Ala, Asn, and Arg) was investigated for the CE-TOF-MS separation technique. The time of analysis was of 19.2 min, with LOD values in the nM range (Table 3). The method was used to detect D- and L-AAs in vinegar and in wild and transgenic soybeans (Giuffrida et al. 2009).
Biosensors
Chromatographic methods require time-consuming sample pretreatment, expensive instruments, and trained personnel. Biosensors overcome these drawbacks, allowing amino acids to be detected simply, fast, and specifically. Actually, biosensors work optimally within the 2 to 900 s and 5.3 to 9.5 pH range and a temperature range of 25–45 °C, with LOD values between 0.02 and 1250 μM and a working potential from −0.05 to 0.45 V (Pundir et al. 2018). A number of D-AA biosensors have been reported based on immobilization of the enzyme DAAO from different sources by applying several immobilization methods, such as adsorption, cross-linking, and covalent immobilization (Table 3) (Sarkar et al. 1999; Sacchi et al. 1998; Compagnone and Trojanowicz 2007; Rosini et al. 2008).
A flow electrochemical device based on DAAO from Rhodotorula gracilis adsorbed on the graphite electrode was used to detect D-AAs in dairy products: this system showed the complete recovery of 1 mM D-Ala when added to milk samples (Sacchi et al. 1998). Samples from different stages of brewing were investigated by a flow injection analysis (FIA) system consisting of a thin-layer enzyme cell made of porcine DAAO immobilized on a membrane: the hydrogen peroxide produced by the enzymatic reaction was determined by the amperometric detector. With an optimal flow rate of 0.7 mL/min, 50–60 samples were measured in 1 h. The working concentration range was between 0.2–3 mM, with a RSD value of 2–2.7% (Table 3) (Varadi et al. 1999). A different FIA system consisting of immobilized porcine DAAO and pyruvate oxidase was developed to detect D-Ala: pyruvic acid formed by DAAO was further oxidized by pyruvate oxidase and the oxygen consumed was proportional to D-Ala concentration (Inaba et al. 2003). A linear response was obtained in the range of 0.1–1 mM D-Ala with a LOD value of 0.05 mM, one assay requiring 12 min (Table 3). The performance of the biosensor was tested on fish sauces samples, yielding values in good agreement with those obtained by conventional methods (Inaba et al. 2003).
To facilitate the hydrogen peroxide oxidation, a screen-printed rhodinized carbon working electrode that used immobilized Crotalus adamateus L-amino acid oxidase (LAAO) and porcine DAAO was developed (Sarkar et al. 1999). The device responded to all amino acids (except Pro), exhibiting stability over 56 days: a linear response was obtained for Gly, L-Leu, and L-Phe with LOD values of 0.47, 0.15, and 0.2 mM, respectively. In 4 min, the bi-enzymatic sensor monitored changes in amino acid content in milk, fruit juice, and urine samples, giving values similar to those obtained by a ninhydrin-based photometric assay (Table 3).
The cross-linking of porcine DAAO and bovine serum albumin with glutaraldehyde on a screen-printed graphite working electrode modified with Prussian Blue and Naflon layers was able to reach LOD values in the range 1–30 μM, with a linear response for D-Ala between 5 and 200 μM; it was used to assay commercial fruit juices (Weislo et al. 2007). A highly sensitive D-AA biosensor (LOD value = 1 μM; Table 3) was obtained by the covalent immobilization of goat DAAO onto polyindole 5-carboxylic acid/zinc sulfide nanoparticles hybrid film electrodeposited on an Au electrode (Lata et al. 2012). The biosensor was used to quantify D-AAs in fruit juices. The same research group developed an improved biosensor by covalent immobilization of the enzyme onto carboxylated multi-walled carbon nanotube/copper nanoparticles/polyaniline hybrid film electrodeposited on a gold electrode, reaching a LOD value of 0.2 μM with a LOQ of 0.03 μM and a linear response on D-Ala in the range 0.001–0.7 mM (Lata et al. 2012).
A quantitative system for the enantiomeric determination of L- and D-AAs was developed by combining the efficiency of HPLC method, enzymatic specificity, and electrochemical sensitivity (Voss and Galensa 2000); different D-AAs were detected in beer, sherry, port wine, wine, and fruit juice without performing an evaporation or derivatization step. In this system, Crotalus durissus venom LAAO and porcine DAAO were immobilized on pore glass activated with glutaraldehyde and filled into reactor cartridges. The oxidative deamination of different amino acids, separated isocratically, produced hydrogen peroxide that was detected electrochemically. The method was optimized for the detection of D-Ala, allowing a very sensitive detection of bacterial contamination: less than 0.1 mg/L D-Ala was detected in 32 fruit concentrates and purees, each analysis requiring 55 min (Voss and Galensa 2000).
Worthy of note is that the biosensor performance depends on the substrate specificity of the employed enzyme and thus the total D-AA content cannot be measured. A low variability of response as a function of the D-AAs composition was achieved by using the Amberzyme-immobilized T60A/Q144R/K152E and M213G variants of DAAO from Rhodotorula gracilis: a limited dependence on the solution composition was apparent when at least 20% of the D-AAs was made up of D-Ala (Rosini et al. 2008). The entire D-AAs content was detected, with a LOD value of 0.25 mM in 10–15 min (Table 3). By using this device, the content of D-AAs in Grana Padano cheese could be quantified.
D-amino acid taste
Work published in 1965 reported that D-His, D-Leu, D-Phe, D-Trp, and D-Tyr have a sweet taste while the corresponding L-enantiomers possess a bitter taste (Solms et al. 1965). Although it is not a general rule, D-AAs frequently taste sweeter than L-enantiomers: this represents a case of correlation between stereochemistry and flavor. In some cases, the sweetening power of D-Val, D-Phe, and D-Trp is higher than that of sucrose (Linden and Lorient 1999). Alitame (L-α-aspartyl-N-(2,2,4,4-tetramethyl-3-thioethanyl)-D-alaninamide; Fig. 2), an artificial dipeptide sweetener containing L-Asp and D-Ala, is of commercial interest because it is about 2000 times sweeter than sucrose, about 10 times sweeter than aspartame, and six times sweeter than saccharin (Chattopadhyay et al. 2014). A principal component analysis of sake taste and D-AA concentrations identified the strong taste as the most important component and the sweet taste as second most important (Okada et al. 2013). The high score for the first component is apparent at a D-Ala concentration > 100 μM, as well as for D-Asp and D-Glu. The origin of these D-AAs is due to lactic acid bacteria during storage.
By using a cellular model overexpressing sweet and bitter receptors, nine amino acids were tested (Bassoli et al. 2014). This study reported that TAS1R2/TAS1R3 sweet receptors show a stereoselectivity with a preference for binding the D-AAs. Concerning the TASR2 bitter receptors, the TAS2R4 and TAS2R39 variants are activated by both enantiomers of tryptophan while the TAS2R43 and TAS2R49 receptors are activated by the L-enantiomer only. Indeed, a stereoselectivity was apparent for TAS3R8 and TAS2R4 receptors and phenylalanine.
Recently, it was reported that the clinically relevant respiratory Gram-positive Staphylococcus aureus and Staphylococcus epidermidis strains produce D-AAs that activate TAS1R/2 sweet taste receptors in solitary chemosensory cells and inhibit antimicrobial peptide secretion (Lee et al. 2017). This information is relevant because these nonpathogenic bacteria, which play a role in chronic rhinosinusitis, suppress P. aeruginosa virulence.
Are D-amino acids beneficial for human health?
The metabolic fate of D-AAs in humans is still controversial. In mammals, the flavoenzymes DAAO and DASPO convert the D-AAs (via oxidative deamination) to α-keto acids. DAAO is active on a number of D-AAs and it is largely expressed in liver and kidneys. Then, α-keto acids are catabolyzed or transaminated to L-AAs. In the human organism, racemization is restricted to few amino acids (D-Ser and, probably, D-Asp) and selected tissues. D-AA metabolism in mammals is largely due to gut microbiota.
Mice fed a synthetic all-amino acid diet were used to evaluate the nutritional value of D-enantiomers of amino acids (Friedman 2007; Friedman and Levin 2012). The nutritional power of essential D-AAs depends on the form of administration (as free amino acids or as protein components), on the amino acid composition (the use of any D-AA may be affected by other D-AAs present in the diet), the digestibility, and utilization of protein-released amino acids. Actually, peptide bonds with D-L, L-D, or D-D configurations are resistant to proteolytic enzymes, thus inducing a reduced digestibility and eventually the formation of oligopeptides with unknown biological activity. No toxic effects were found in trials with parenteral nutrition of adults and children with free D,L-amino acids (Marchelli et al. 2007). Very recently, an investigation reported a decrease in metabolic activity of 3T3-L1 preadipocytes when the cell medium contained specific D-AAs in place of the L-enantiomer (Chen et al. 2019). While D-Lys showed a stronger inhibitory effect, no depression by D-Ser was apparent. Treatment with 10–200 μM D-AAs of Caco-2 cells, a model of the cells lining the gastrointestinal tract, did not affect the cell growth.
Reduced digestibility of dietary proteins containing D-AAs may in certain cases prove advantageous for nutrition, promoting considerable weight loss when consumed for a few days. Food products formulated to induce weight loss contain 50% D-Ser, 37% D-Asp, and 26% D-Phe; these high D-AA quantities might cause a risk if consumed as the sole source of dietary proteins (Finley 1985). D-AAs have been also classified among the anti-nutritional factors (Gilani et al. 2005; Gilani et al. 2012). Considering the single D-AA, D-Met is poorly used by humans when consumed orally or in parenteral nutrition, and D-Tyr significantly decreases growth in mice, D-Met and D-Lys as well (Friedman and Levin 2012). Actually, in the presence of high levels of free D-AAs, the DAAO degradation system may become saturated and, indeed, the proteins containing D-AAs are hydrolyzed at a slower rate than proteins containing L-AAs only and may generate D-D, D-L, or L-D peptides that are poorly degraded by proteases and that can compete with normal peptides for binding to the active site of the proteolytic enzymes. The ensuing slower adsorption, compared to L-AAs, may contribute to the decrease in protein digestibility.
Concerning a potential therapeutic application of D-AAs, D-Ser was proposed for the therapy of post-traumatic stress disorder (Heresco-Levy et al. 2009) and D-Phe to induce analgesia (Balagot et al. 1981): oral administration of D-Phe (750–1000 mg daily) should result in carboxypeptidase inhibition, which is involved in breaking down opioid pentapeptide in the brain and spinal cord and related to an increase in brain encephalin levels. Some D-AAs inhibit tumor growth in rats: D-Val administration impaired the nutritional status and inhibited the tumor growth of hepatoma-bearing rats compared to control diets (Sasamura et al. 1998). Furthermore, D-Ser supplementation has being investigated in the treatment of schizophrenia (MacKay et al. 2019). Some D-AAs are present in selected antibiotic peptides: it is also conceivable that proteolytic cleavage of D-AAs containing dietary proteins generates peptides with antibiotic properties.
The presence of various D-AAs in the human stratum corneum was reported by the Shiseido company, which also demonstrated that as levels of D-Asp decline during aging, collagen production decreases. After a trial of 2 months of D-Asp ingestion, the thickness of the skin was clearly higher. Accordingly, the first functional food enriched with D-AAs called “kireinosusume” was produced (Mutaguchi et al. 2016). Based on the evidence that D-Ala improves laminin-5 production in the basement membrane of skin and levels are restored in aged people, the same company intends to commercialize cosmetics supplemented with D-Ala as an anti-aging factor. Regarding the aging process, long-term administration of D-AAs to mice was recently reported to improve spatial reference memory (a test for cognitive function) and to increase the levels in the cerebral cortex (but not in plasma) of 5-hydroxytryptamine, various L-AAs (Ala, Ser, Val and Ile), and D-Ser (Kawase and Furuse 2019). The latter molecule was suggested as being responsible for improving the spatial reference memory by acting on NMDA receptors: accordingly, bacteria-derived molecules might affect central nervous system function.
Altered levels of D-AAs were reported to induce serious injury, especially to rodent kidneys. D-Ser has been reported to enlarge rat kidney cells (cytomegaly): sodium benzoate (Williams and Lock 2004), protein-deficient diets (Levine and Saltzman 2003), and α-aminoisobutyric acid (Krug et al. 2007) attenuated D-Ser nephrotoxicity in rats.
Conclusions
The presence of the “wrong enantiomers” of amino acids (the D-AAs) in foods is now well established. However, standardized methods designed to systematically ascertain D-AAs level in foodstuffs and their role in nutrition still represent a priority.
In order to clarify the effect of D-AAs on human health, we identified some unresolved questions:
- Do free and protein-associated D-AAs bind and modulate the proteolytic enzymes in the digestive tract?
- What is/are the biological effect(s) of D-AAs, depending on whether they are consumed in the free state or as food protein components?
- Do poorly digestible food proteins containing D-AAs serve as dietary fiber?
- Do metabolic interactions among free and protein bound D-AAs occur in vivo?
Notwithstanding amazing recent novel findings in D-AA research, this field is expected to grow considerably in the near future and we greatly anticipate the new scientific findings.
References
Abe H, Park JN, Fukumoto Y, Fujita E, Tanaka T, Washio T, Otsuka S, Shimizu T, Watanabe K (1999) Occurrence of D-amino acids in fish sauces and other fermented fish products. Fisheries Sci 65:637–641. https://doi.org/10.2331/fishsci.65.637
Albertini A, Mentasti T, Moretti VM, Bellagamba F, Luzzana U, Valfre F (1996) Occurrence of free D-amino acids in milk from food-producing animals. Milchwissenschaft 51:127–129
Aldag RW, Young JL, Yamamoto M (1971) An enzymic chromatographic procedure for the determination of D-amino acids in plant and soil extracts. Phytochemistry 10:267–274. https://doi.org/10.1016/S0031-9422(00)94039-1
Ali HS, Pätzold R, Brückner H (2010) Gas chromatographic determination of amino acid enantiomers in bottled and aged wines. Amino Acids 38:951–958. https://doi.org/10.1007/s00726-009-0304-1
Balagot RC, Ehrenpreis S, Kubota K, Greenberg J (1981) Analgesia in mice and humans by D-phenylalanine: relation to inhibition of enkephalin degradation and enkephalin levels. Pain 11:S21. https://doi.org/10.1016/0304-3959(81)90228-1
Bassoli A, Borgonovo G, Caremoli F, Mancuso G (2014) The taste of D- and L-amino acids: in vitro binding assays with cloned human bitter (TAS2Rs) and sweet (TAS1R2/TAS1R3) receptors. Food Chem 150:27–33
Bidlingmeyer BA, Cohen SA, Tarvin TL (1984) Rapid analysis of amino acids using pre-column derivatization. J Chromatogr 336:93–104. https://doi.org/10.1016/S0378-4347(00)85133-6
Brückner H, Fujii N (2010) D-amino acids in chemistry, life sciences, and biotechnology. Wiley-VCH, Weinheim
Brückner H, Hausch M (1989) Detection of free D-amino acids in food by chiral phase capillary gas chromatography. J High Res Chromatog 12:680–684. https://doi.org/10.1002/jhrc.1240121012
Brückner H, Hausch M (1990) D-amino acids in dairy products: detection, origin and nutritional aspects. I. Milk, fermented milk, fresh cheese and acid curd cheese. Milchwissenschaft 45:357–360
Brückner H, Westhauser T (1994) Chromatographic determination of D-amino acids as native constituents of vegetables and fruits. Chromatographia 39:419–426. https://doi.org/10.1007/BF02278756
Brückner H, Westhauser T (2003) Chromatographic determination of L- and D-amino acids in plants. Amino Acids 24:43–55. https://doi.org/10.1007/s00726-002-0322-8
Brückner H, Becker D, Lüpke M (1993) Chirality of amino acids of microorganisms used in food biotechnology. Chirality 5:385–392. https://doi.org/10.1002/chir.530050521
Brückner H, Westhauser T, Godel H (1995) Liquid chromatographic determination of D-and L-amino acids by derivatization with o-phthaldialdehyde and N-isobutyryl-L-cysteine. Applications with reference to the analysis of peptidic antibiotics, toxins, drugs and pharmaceutically used amino acids. J Chromatogr A 711:201–215. https://doi.org/10.1016/0021-9673(95)00158-j
Brückner H, Justus J, Kirschbaum J (2001) Saccharide induced racemization of amino acids in the course of the Maillard reaction. Amino Acids 21:429–433. https://doi.org/10.1007/s0072601700
Buck RH, Krummen K (1987) High-performance liquid chromatographic determination of enantiomeric amino acids and amino alcohols after derivatization with o-phthaldialdehyde and various chiral mercaptans: Application to peptide hydrolysates. J Chromatogr 387:255–265. https://doi.org/10.1016/S0021-9673(01)94529-7
Bunjapamai S, Mahoney RR, Fagerson IS (1982) Determination of D-amino acids in some processed foods and effect of racemization on in vitro digestibility of casein. J Food Sci 47:1229–1234. https://doi.org/10.1111/j.1365-2621.1982.tb07654.x
Carlavilla D, Moreno-Arribas MV, Fanali S, Cifuentes A (2006) Chiral MEKC-LIF of amino acids in foods: analysis of vinegars. Electrophoresis 27(13):2551–2557. https://doi.org/10.1002/elps.200500909
Casado FJ, Sánchez AH, Rejano L, Montaño A (2007) D-amino acid formation in sterilized alkali-treated olives. J Agric Food Chem 55:3503–3507. https://doi.org/10.1021/jf0701685
Cava F, de Pedro MA, Lam H, Davis BM, Waldor MK (2011) Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J 30:3442–3453. https://doi.org/10.1038/emboj.2011.246
Chattopadhyay S, Raychaudhuri U, Chakraborty R (2014) Artificial sweeteners—a review. J Food Sci Technol 51:611–621. https://doi.org/10.1007/s13197-011-0571-1
Chen Z, Kondrashina A, Greco I, Gamon LF, Lund MN, Giblin L, Davies MJ (2019) Effects of protein-derived amino acid modification products present in infant formula on metabolic function, oxidative stress, and intestinal permeability in cell models. J Agric Food Chem 67:5634–5646. https://doi.org/10.1021/acs.jafc.9b01324
Chiavaro E, Caligiani A, Palla G (1998) Chiral indicators of ageing in balsamic vinegars of Modena. Int J Food Sci 10:329–337
Csapò J, Csapò-Kiss Z, Stefler J (1995) Influence of mastitis on D-amino acid content of milk. J Dairy Sci 78:2375–2381. https://doi.org/10.3168/jds.S0022-0302(95)76865-5
Csapò J, Varga-Visi E, Loki K, Albert CS, Salamon SZ (2008) The influence of extrusion on loss and racemization of amino acids. Amino Acids 34:287–292. https://doi.org/10.1007/s00726-006-0484-x
Csapó J, Albert CS, Csapó-Kiss ZS. (2009) The D-amino acid content of foodstuffs (a review). Acta Univ. Sapientiae, Alimentaria, 2, 1: 5–30
De Jonge BL, Gage D, Xu N (2002) The carboxyl terminus of peptidoglycan stem peptides is a determinant for methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 46:3151–3155. https://doi.org/10.1128/AAC.46.10.3151
Einarsson S, Josefsson B, Möller P, Sanchez D (1987) Separation of amino acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid chromatography. Anal Chem 59:1191–1195. https://doi.org/10.1021/ac00135a025
Erbe T, Brückner H (2000) Chromatographic determination of amino acid enantiomers in beers and raw materials used for their manufacture. J Chromatogr A 881:81–91. https://doi.org/10.1016/s0021-9673(00)00255-7
Eto S, Yamaguchi M, Bounoshita M, Mizukoshi T, Miyano H (2011) High-throughput comprehensive analysis of D- and L-amino acids using ultra-high performance liquid chromatography with a circular dichroism (CD) detector and its application to food samples. J Chromatogr B Analyt Technol Biomed Life Sci. 879:3317–3325. https://doi.org/10.1016/j.jchromb.2011.07.025
Finley JW (1985) Environmental effects of protein quality. In: Chemical changes in food during processing. Inst. Food Technologists Basic Symp. Ser., AVI Publ. Westport Conn.. 443–482
Friedman M (1999) Chemistry, nutrition, and microbiology of D-amino acids. J Agr Food Chem 47:3457–3479. https://doi.org/10.1021/jf990080u
Friedman M (2007) Nutritional evaluation of D-amino acids. In: Konno R et al (eds) D-amino acids: a new frontier in amino acid and protein research. Nova Science Publishers, New York, pp 285–298
Friedman M, Levin CE (2012) Nutritional and medicinal aspects of D-amino acids. Amino Acids 42:1553–1582. https://doi.org/10.1007/s00726-011-0915-1
Friedman M, Liardon R (1985) Racemization kinetics of amino acid residues in alkali-treated soybean proteins. J Agric Food Chem 33:666–672. https://doi.org/10.1021/jf00064a025
Friedman M, Zahnley JC, Masters PM (1981) Relationship between in vitro digestibility of casein and its content of lysinoalanine and D-amino acids. J Food Sci 46:127–134. https://doi.org/10.1111/j.1365-2621.1981.tb14545.x
Fukuda M, Tokumura A, Ogawa T (1973) D-alanine in germinating Pisum sativum seedlings. Phytochem 12:2593–2595. https://doi.org/10.1016/0031-9422(73)85061-7
Gandolfi I, Palla G, Delprato L, Nisco F, Marchelli R, Salvadori C (1992) D-amino acids in milk as related to heat treatments and bacterial activity. J Food Sci 57:377–379. https://doi.org/10.1111/j.1365-2621.1992.tb05498.x
Gandolfi I, Palla G, Marchelli R, Dossena A, Puelli S, Salvadori C (1994) D-alanine in fruit juices: a molecular marker of bacterial activity, heat treatments and shelf-life. J Food Sci 59:152–154. https://doi.org/10.1111/j.1365-2621.1994.tb06921.x
Gatti R, Gioia MG, Leoni A, Andreani A (2010) 2,5-dimethyl-1H-pyrrole-3,4-dicarbaldehyde as a precolumn derivatization reagent for HPLC/UV detection of amino acids. J Pharm Biomed Anal 53:207–211. https://doi.org/10.1016/j.jpba.2009.12.031
Genchi G (2017) An overview on D-amino acids. Amino Acids 49:1521–1533. https://doi.org/10.1007/s00726-017-2459-5
Gilani GS, Cockell KA, Sepehr E (2005) Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J AOAC Int 88:967–987
Gilani GS, Xiao CW, Cockell KA (2012) Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br J Nutr 108:S315–S332. https://doi.org/10.1017/S0007114512002371
Gioia MG, Cacciari B, Leoni A, Gatti R (2006) 2,7-dimethyl-3,8-dinitrodipyrazolo[1,5-a:1′,5′-d]pyrazine-4,9-dione: a new labelling reagent for liquid chromatographic analysis of amino acids. Anal Chim Acta 579:152–157. https://doi.org/10.1016/j.aca.2006.07.035
Giuffrida A, León C, García-Cañas V, Cucinotta V, Cifuentes A (2009) Modified cyclodextrins for fast and sensitive chiral-capillary electrophoresis-mass spectrometry. Electrophoresis 30:1734–1742. https://doi.org/10.1002/elps.200800333
Gobbetti M, Simonetti MS, Rossi J, Cossignani L, Corsetti A, Damiani P (1994) Free D- and L-amino acid evolution during sourdough fermentation and baking. J Food Sci 59:881–884. https://doi.org/10.1111/j.1365-2621.1994.tb08149.x
Gogami Y, Okada K, Oikawa T (2011) High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake. J Chromatogr B Analyt Technol Biomed Life Sci 879:3259–3267. https://doi.org/10.1016/j.jchromb.2011.04.006
Guillén-Casla V, León-González ME, Pérez-Arribas LV, Polo-Díez LM (2010) Direct chiral determination of free amino acid enantiomers by two-dimensional liquid chromatography: application to control transformations in E-beam irradiated foodstuffs. Anal Bioanal Chem 397:63–75. https://doi.org/10.1007/s00216-009-3376-6
Hamase K, Miyoshi Y, Ueno K, Han H, Hirano J, Morikawa A, Mita M, Kaneko T, Lindner W, Zaitsu K (2010) Simultaneous determination of hydrophilic amino acid enantiomers in mammalian tissues and physiological fluids applying a fully automated micro-two-dimensional high-performance liquid chromatographic concept. J Chromatogr A 1217:1056–1062. https://doi.org/10.1016/j.chroma.2009.09.002
Harada M, Karakawa S, Yamada N, Miyano H, Shimbo K (2019) Biaryl axially chiral derivatizing agent for simultaneous separation and sensitive detection of proteinogenic amino acid enantiomers using liquid chromatography-tandem mass spectrometry. J Chromatogr A 1593:91–101. https://doi.org/10.1016/j.chroma.2019.01.075
Hayase F, Kato H, Fujimaki M (1973) Racemization of amino acid residues in protein during roasting. Agric Biol Chem 37:191–192
Heresco-Levy U, Vass A, Bloch B, Wolosker H, Dumin E, Balan L, Deutsch L, Kremer I (2009) Pilot controlled trial of D-serine for the treatment of post-traumatic stress disorder. Int J Neuropsychopharmacol 12:1275–1282. https://doi.org/10.1017/S1461145709000339
Herrero M, Ibáñez E, Martín-Alvarez PJ, Cifuentes A (2007) Analysis of chiral amino acids in conventional and transgenic maize. Anal Chem 79:5071–5077. https://doi.org/10.1021/ac070454f
Horcajo P, de Pedro MA, Cava F (2012) Peptidoglycan plasticity in bacteria: stress-induced peptidoglycan editing by noncanonical D-amino acids. Microb Drug Resist 18:306–313. https://doi.org/10.1089/mdr.2012.0009
Ilisz I, Berkecz R, Péter A (2008) Application of chiral derivatizing agents in the high-performance liquid chromatographic separation of amino acid enantiomers: a review. J Pharm Biomed Anal 47:1–15. https://doi.org/10.1016/j.jpba.2007.12.013
Inaba Y, Mizukami K, Hamada-Sato N, Kobayashi T, Imada C, Watanabe E (2003) Development of a D-alanine sensor for the monitoring of a fermentation using the improved selectivity by the combination of D-amino acid oxidase and pyruvate oxidase. Biosens Bioelectron 19:423–431. https://doi.org/10.1016/S0956-5663(03)00200-8
Innocente N, Palla G (1999) Occurrence of D-amino acids in a typical semi-hard cheese. J Dairy Res 66:633–637. https://doi.org/10.1017/S0022029999003829
Ishida Y, Fujita T, Asai K (1981) New detection and separation method for amino acids by high-performance liquid chromatography. J Chromatogr 204:143–148. https://doi.org/10.1016/s0021-9673(00)81650-7
Jin D, Miyahara T, Oe T, Toyo'oka T (1999) Determination of D-amino acids labeled with fluorescent chiral reagents, R(−)- and S(+)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazoles, in biological and food samples by liquid chromatography. Anal Biochem 269:124–132. https://doi.org/10.1006/abio.1998.3090
Kato M, Fukushima T, Santa T, Homma H, Imai K (1995) Determination of D-amino acids, derivatized with 4-fluoro-7-nitro-2, 1, 3-benzoxadiazole (NBD-F), in wine samples by high-performance liquid chromatography. Biomed Chromatogr 9:193–194. https://doi.org/10.1002/bmc.1130090409
Kato S, Ishihara T, Hemmi H, Kobayashi H, Yoshimura T (2011) Alterations in D-amino acid concentrations and microbial community structures during the fermentation of red and white wines. J Biosci Bioeng 111:104–108. https://doi.org/10.1016/j.jbiosc.2010.08.019
Kawasaki Y, Ogawa T, Sasaoka K (1982) Two pathways for formation of D-amino acid conjugates in pea seedlings. Agric Biol Chem 46:1–5. https://doi.org/10.1271/bbb1961.46.1
Kawase T, Furuse M (2019) Long-term administration of yoghurt improves spatial memory in mice. J Pet Anim Nutr 22(1):1–13
Konya Y, Taniguchi M, Fukusaki E (2017) Novel high-throughput and widely-targeted liquid chromatography-time of flight mass spectrometry method for D-amino acids in foods. J Biosci Bioeng 123:126–133. https://doi.org/10.1016/j.jbiosc.2016.07.009
Krug AW, Völker K, Dantzler WH, Silbernagl S (2007) Why is D-serine nephrotoxic and alpha-aminoisobutyric acid protective? Am J Physiol Renal Physiol 293:F382–F390. https://doi.org/10.1152/ajprenal.00441.2006
Lata S, Batra B, Pundir CS (2012) Construction of D-amino acid biosensor based on D-amino acid oxidase immobilized onto poly (indole-5-carboxylic acid)/zinc sulfide nanoparticles hybrid film. Process Biochem 47:2131–2138. https://doi.org/10.1016/j.ab.2013.01.030
Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Jiang P, Margolskee RF, Cohen NA (2017) Bacterial D-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal 10:eaam7703. https://doi.org/10.1126/scisignal.aam7703
Levine S, Saltzman A (2003) Acute uremia produced in rats by nephrotoxic chemicals is alleviated by protein deficient diet. Ren Fail 25:517–523. https://doi.org/10.1081/jdi-120022542
Li G, Cui Y, You J, Zhao X, Sun Z, Xia L, Suo Y, Wang X (2011) Determination of trace amino acids in human serum by a selective and sensitive pre-column derivatization method using HPLC-FLD-MS/MS and derivatization optimization by response surface methodology. Amino Acids 40:1185–1193. https://doi.org/10.1007/s00726-010-0742-9
Liardon R, Hurrel RF (1983) Amino acid racemization in heated and alkali-treated proteins. J Agric Food Chem 31:432–437. https://doi.org/10.1021/jf00116a062
Linden G, Lorient D (1999) In: G. Linden and D. Lorient, (ed). New ingredients in food processing: biochemistry and agriculture. CRC press, Boca Raton, pp 184–210
Lüpke M, Brückner H (1998) Gas chromatographic evaluation of amino acid epimerisation in the course of gelatin manufacturing and processing. Z Lebensm Unters Forsch 206:323–328. https://doi.org/10.1007/s0021700502
MacKay MB, Paylor JW, Wong JTF, Winship IR, Baker GB, Dursun SM (2019) Multidimensional connectomics and treatment-resistant schizophrenia: linking phenotypic circuits to targeted therapeutics. Front Psychiatry 9:537–556. https://doi.org/10.3389/fpsyt.2018.00537
Marchelli R, Palla G, Dossena A, Galaverna G, Corradini R, Clementi S (1997) D-amino acids: molecular markers of ageing and authenticity for Parmigiano Reggiano and Grana Padano cheese. Sci Tecn Latt-Cas 48:21–32
Marchelli R, Galaverna G, Dossena A, Palla G, Bobbio A, Santaguida S, Grozeva K, Corradini R, Sforza S (2007) In: Konno R et al (eds) In: D-amino acids: a new frontier in amino acid and protein research. Nova Science Publishers, New York, pp 299–315
Marcone GL, Marinelli F (2014) Glycopeptides: an old but up to date successful antibiotic class. In: Marinelli F, Genilloud O (eds) Antimicrobials. Springer-Verlag, Berlin, pp 85–107
Martínez-Girón AB, García-Ruiz C, Crego AL, Marina ML (2009) Development of an in-capillary derivatization method by CE for the determination of chiral amino acids in dietary supplements and wines. Electrophoresis 30:696–704. https://doi.org/10.1002/elps.200800481
Masters PM, Friedman M (1979) Racemization of amino acids in alkali-treated food proteins. J Agric Food Chem 27:507–511
Miyoshi Y, Nagano M, Ishigo S, Ito Y, Hashiguchi K, Hishida N, Mita M, Lindner W, Hamase K (2014) Chiral amino acid analysis of Japanese traditional Kurozu and the developmental changes during earthenware jar fermentation processes. J Chromatogr B Anal Technol Biomed Life Sci 966:187–192. https://doi.org/10.1016/j.jchromb.2014.01.034
Mochizuki T, Todoroki K, Inoue K, Min JZ, Toyo'oka T (2014) Isotopic variants of light and heavy L-pyroglutamic acid succinimidyl esters as the derivatization reagents for DL-amino acid chiral metabolomics identification by liquid chromatography and electrospray ionization mass spectrometry. Anal Chim Acta 811:51–59. https://doi.org/10.1016/j.aca.2013.12.016
Mutaguchi Y, Ohmori T, Akano H, Doi K, Ohshima T (2013) Distribution of D-amino acids in vinegars and involvement of lactic acid bacteria in the production of D-amino acids. Springerplus 2:691–699. https://doi.org/10.1186/2193-1801-2-691
Mutaguchi Y, Kobayashi J, Oikawa T, Ohshima T (2016) D-amino acids in fermentative foods. In: Yoshimura T et al (eds) D-amino acids. Springer Tokyo, pp 341–357
Nakano Y, Konya Y, Taniguchi M, Fukusaki E (2017) Development of a liquid chromatography-tandem mass spectrometry method for quantitative analysis of trace D-amino acids. J Biosci Bioeng 123:134–138. https://doi.org/10.1016/j.jbiosc.2016.07.008
Ogawa T, Fukuda M (1973) Occurrence of D-amino acid aminotransferase in pea seedlings. Biochem Biophys Res Commun 52:998–1002. https://doi.org/10.1016/0006-291x(73)91036-x
Okada K, Gogami Y, Oikawa T (2013) Principal component analysis of the relationship between the D-amino acid concentrations and the taste of the sake. Amino Acids 44:489–498. https://doi.org/10.1007/s00726-012-1359-y
Opstvedt J, Miller R, Hardy RW, Spinelli J (1984) Heat-induced changes in sulfhydryl groups and disulfide bonds in fish protein and their effect on protein and amino acid digestibility in rainbow trout (Salmo gairdneri). J Agric Food Chem 32:929–935. https://doi.org/10.1021/jf00124a056
Palla G, Marchelli R, Dossena A, Casnati G (1989) Occurrence of D-amino acids in food: detection by capillary gas chromatography and by reversed-phase high-performance liquid chromatography with L-phenylalaninamides as chiral selectors. J Chrom A 475:45–53. https://doi.org/10.1016/S0021-9673(00)91414-6
Pätzold R, Brückner H (2006) Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell. Amino Acids 31:63–72. https://doi.org/10.1007/s00726-006-0330-1
Pawloska M, Armstrong DW (1994) Evaluation of enantiomeric purity of selected amino acids in honey. Chirality. 6:270–276. https://doi.org/10.1002/chir.530060409
Pidgeon SE, Fura JM, Leon W, Birabaharan M, Vezenov D, Pires MM (2015) Metabolic profiling of bacteria by unnatural C-terminated D-amino acids. Angew Chem Int Ed Eng 54:6158–6162. https://doi.org/10.1002/anie.201409927
Pollegioni L, Sacchi S (2010) Metabolism of the neuromodulator D-serine. Cell Mol Life Sci 67:2387–2404. https://doi.org/10.1007/s00018-010-0307-9
Pollegioni L, Piubelli L, Sacchi S, Pilone MS, Molla G (2007) Physiological functions of D-amino acid oxidases: from yeast to humans. Cell Mol Life Sci 64:1373–1394. https://doi.org/10.1007/s00018-007-6558-4
Prandi B, Faccini A, Lambertini F, Bencivenni M, Jorba M, Van Droogenbroek B, Bruggeman G, Schöber J, Petrusan J, Elst K, Sforza S (2019) Food wastes from agrifood industry as possible sources of proteins: a detailed molecular view on the composition of the nitrogen fraction, amino acid profile and racemisation degree of 39 food waste streams. Food Chem 286:567–575. https://doi.org/10.1016/j.foodchem.2019.01.166
Pundir CS, Lata S, Narwal V (2018) Biosensors for determination of D and L- amino acids: a review. Biosens Bioelectron 117:373–384. https://doi.org/10.1016/j.bios.2018.06.033
Rekoslavskaya NI, Yurjeva OV, Salyaev RK, Mapelli S, Kopytina TV (1999) D-tryptophan as IAA source during wheat germination. Bulg J Plant Physiol 25:39–49
Reynolds PE, Courvalin P (2005) Vancomycin resistance in enterococci due to synthesis of precursors terminating in D-alanyl-D-serine. Antimicrob Agents Chemother 49:20–25. https://doi.org/10.1128/AAC.49.1.21
Rosini E, Molla G, Rossetti C, Pilone MS, Pollegioni L, Sacchi S (2008) A biosensor for all D-amino acids using evolved D-amino acid oxidase. J Biotechnol 135:377–384. https://doi.org/10.1016/j.jbiotec.2008.06.001
Rozan P, Kuo YH, Lambein F (2000) Free amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumers. J Agric Food Chem 48:716–723. https://doi.org/10.1021/jf990729v
Rubio-Barroso S, Santosjj-Delgado MJ, Martín-Olivar C, Polo-Díez LM (2006) Indirect chiral HPLC determination and fluorimetric detection of D-amino acids in milk and oyster samples. J Dairy Sci 89:82–89. https://doi.org/10.3168/jds.S0022-0302(06)72071-9
Sacchi S, Pollegioni L, Pilone MS, Rossetti C (1998) Determination of D-amino acids using a D-amino acid oxidase biosensor with spectrophotometric and potentiometric detection. Biotechnol Tech 12:149–153
Sacchi S, Rosini E, Caldinelli L, Pollegioni L (2012) Biosensors for D-amino acid detection. Methods Mol Biol 794:313–324. https://doi.org/10.1007/978-1-61779-331-8_21
Sarkar P, Tothill IE, Setford SJ, Turner AP (1999) Screen-printed amperometric biosensors for the rapid measurement of L- and D-amino acids. Analyst. 124:865–870. https://doi.org/10.1039/A901404G
Sasamura T, Matsuda A, Kokuba Y (1998) Tumor growth inhibition and nutritional effect of D-amino acid solution in AH109A hepatoma-bearing rats. J Nutr Sci Vitaminol (Tokyo) 44:79–87. https://doi.org/10.1271/bbb.62.2418
Schiffman SS, Sennewald K, Gagnon J (1981) Comparison of taste qualities and thresholds of D- and L-amino acids. Physiol Behav 27:51–59. https://doi.org/10.1016/0031-9384(81)90298-5
Schleifer KH, Kandler O (1972) Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev 36:407–477
Seo WH, Lee HG, Baek HH (2008) Evaluation of bitterness in enzymatic hydrolysates of soy protein isolate by taste dilution analysis. J Food Sci 73:41–43. https://doi.org/10.1111/j.1750-3841.2007.00610.x
Simó C, Martín-Alvarez PJ, Barbas C, Cifuentes A (2004) Application of stepwise discriminant analysis to classify commercial orange juices using chiral micellar electrokinetic chromatography-laser induced fluorescence data of amino acids. Electrophoresis 25:2885–2891
Solms J, Vuataz L, Egli RH (1965) The taste of L- and D-amino acids. Experentia XXI/12: 692-693
Taniguchi M, Konya Y, Nakano Y, Fukusaki E (2017) Investigation of storage time-dependent alterations of enenatioselective amino acid profiles in kimchi using liquid chromatography-time of flight mass spectrometry. J Biosci Bioeng 124:414–418. https://doi.org/10.1016/j.jbiosc.2017.04.019
Thippeswamy R, Gouda KG, Rao DH, Martin A, Gowda LR (2006) Determination of theanine in commercial tea by liquid chromatography with fluorescence and diode array ultraviolet detection. J Agric Food Chem 54:7014–7019
Tian H, Zheng N, Li S, Zhang Y, Zhao S, Wen F, Wang J (2017) Characterization of chiral amino acids from different milk origins using ultra-performance liquid chromatography coupled to ion-mobility mass spectrometry. Sci Rep 7:46289–46297. https://doi.org/10.1038/srep46289
Váradi M, Adányi N, Szabó EE, Trummer N (1999) Determination of the ratio of D- and L-amino acids in brewing by an immobilised amino acid oxidase enzyme reactor coupled to amperometric detection. Biosens Bioelectron 14:335–340. https://doi.org/10.1016/S0956-5663(98)00130-4
Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, de Koning TJ (2011) A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. J Chromatogr A 1218:7130–7136. https://doi.org/10.1016/j.chroma.2011.07.087
Voss K, Galensa R (2000) Determination of L- and D-amino acids in foodstuffs by coupling of high-performance liquid chromatography with enzyme reactors. Amino Acids 18:339–352
Waldhier MC, Dettmer K, Gruber MA, Oefner PJ (2010) Comparison of derivatization and chromatographic methods for GC-MS analysis of amino acid enantiomers in physiological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 878:1103–1112. https://doi.org/10.1016/j.jchromb.2010.03.021
Weisło M, Compagnone D, Trojanowicz M (2007) Enantioselective screen-printed amperometric biosensor for the determination of D-amino acids. Bioelectrochemistry. 71:91–98. https://doi.org/10.1016/j.bioelechem.2006.09.001
Williams RE, Lock EA (2004) D-serine-induced nephrotoxicity: possible interaction with tyrosine metabolism. Toxicology 201:231–238. https://doi.org/10.1016/j.tox.2004.05.001
Funding
This work was supported by Fondazione Cariplo (Grant 2018-2786).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GLM declares that she has no conflict of interest. ER declares that she has no conflict of interest. EC declares that she has no conflict of interest. LP declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Marcone, G.L., Rosini, E., Crespi, E. et al. D-amino acids in foods. Appl Microbiol Biotechnol 104, 555–574 (2020). https://doi.org/10.1007/s00253-019-10264-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10264-9