Abstract
In recent years, the accumulation of synthetic plastics has led to the development of a serious environmental problem. Nowadays, biodegradable films and coatings have been identified as a new approach to solve this problem by preparing renewable, abundant, low-cost materials. Gums are considered a large group of polysaccharides and polysaccharide derivatives that can easily form viscous solutions at low concentrations. Gums are mainly soluble in water and are composed of sugars like glucose, fructose, and mannose. These compounds are categorized into three groups: plant-origin gums, seaweed-based gums, and microbial gums. Microbial gums are listed as generally recognized as safe (GRAS) by the Food and Drug Administration and have a broad range of physicochemical properties suitable for various pharmacy, medicine, and food applications. In the food industry, they can be used as gelling, viscous, stabilizing, and thickening agents. Among the various materials that can potentially improve the properties of biodegradable packaging films, microbial gums such as gellan, xanthan, pullulan, bacterial cellulose, and curdlan have been the subject of numerous studies. These gums can be extruded into films and coatings with considerable barrier properties against the transport of moisture and oxygen. Microbial gums, due to their microbiological stability, adhesion, cohesion, wettability, solubility, transparency, and mechanical properties, can be used as edible films or coatings. Also, these gums can be applied in combination with bioactive compounds that induce the shelf-life extension of highly perishable products. This review focuses on the properties of films and coatings consisting of xanthan, curdlan, pullulan, gellan, and bacterial cellulose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite the 25-fold boost in the global manufacturing of synthetic plastics over the past decades, less than 5% of these plastics are recycled. In recent years, packaging materials derived from petroleum products have become widely used in food packaging due to their low price, comfortable and extensive access, and desirable characteristics such as brightness, plasticity, and transparency. Nevertheless, the production and accumulation of synthetic plastics related to food packaging have caused many environmental problems since they are resistant to degradation, incompatible with the environment, and the possibility of migration of compounds from packaging to product and the ultimate endangerment of product safety and consumer health exist (Alizadeh-Sani et al. 2018; Espitia et al. 2014; Khalil et al. 2017; Muscat et al. 2012; Sutherland et al. 2015; Tavassoli-Kafrani et al. 2016). Although bioplastics are produced from renewable resources, they are not necessarily biodegradable. Currently, bioplastics comprise approximately one percent of the roughly 320 million tonnes of plastics produced annually. Global bioplastic production capacity is set to increase from around 2.05 million tonnes in 2017 to nearly 2.44 million tonnes in 2022 (Gontard et al. 2018).
Nowadays, a large number of studies have focused on replacing common plastic packaging (food and non-food) with biodegradable, edible, renewable, and low-cost films and coatings (Alves et al. 2011; Espitia et al. 2014; Khezerlou et al. 2018; Moghaddas Kia et al. 2018). Films and coatings with the aforementioned characteristics can be utilized as supplements or substitutes of conventional materials (Cao et al. 2007; Cazon et al. 2017). For this reason, recent investigations have reported the use of edible films and coatings based on proteins (Ramos et al. 2012), polysaccharides such as chitosan (Dutta et al. 2009; Elsabee and Abdou 2013), hemicelluloses (Hansen and Plackett 2008), starch (Jiménez et al. 2012), pectin (Espitia et al. 2014), and lipids (Debeaufort and Voilley 2009). A biodegradable film can be defined as a packaging material or thin layer of edible material placed on or between food components (Espitia et al. 2014; Falguera et al. 2011), while an edible coating (EC) is a thin layer of edible material formed as a coating on a food product (Kang et al. 2013). Edible films have a layer thickness of below 0.3 mm and are wrapped around food products, whereas edible coatings are applied by immersing the food products in liquid solutions of the edible materials (Falguera et al. 2011). Furthermore, the biopolymers that have been used for producing edible films can feature suitable matrices for use as carriers that facilitate the incorporation of additives) such as antimicrobials, antioxidants, nutrients, colors, nanoparticles, and spices). These composite biopolymers are used to improve the properties of the films and prolong the food shelf-life (Ehsani et al. 2017; Espitia et al. 2014; Saha et al. 2016; Sallam 2007).
A group of biodegradable polymers have been referred to as “natural polymers” as they are produced during the growth of living organisms—especially bacteria, fungi, and yeasts. These biopolymers have a wide range of applications in the food and pharmaceutical industries (Freitas et al. 2014; Vijayendra and Shamala 2014). The types of microbial gums employed in various biomedical and pharmaceutical applications are summarized in Table 1. Since most biopolymers derived from microorganisms are carbohydrates in nature, films made of these biopolymers have hydrophilic structures as polysaccharides usually react strongly with water. The moisture content or sorption of water in the film is affected directly by the moisture barrier properties and water vapor permeability (Al-Hassan and Norziah 2012; Bertuzzi et al. 2007). Moreover, increasing the hydration causes an augment in elongation properties but a decrease in mechanical properties (Cuq et al. 1997). In this way, hydrophilic films have good barrier properties to oxygen, carbon dioxide, and lipids, but have poor control of water vapor migration (Prommakool et al. 2011). In regard to their barrier properties, they can act as selective barriers to gases in order to generate modified atmospheres (Dehghani et al. 2018; Ramos et al. 2012; Shit and Shah 2014). Despite the fact that most functions of edible films and coatings are similar to those of traditional packaging, their mechanical properties vary, as do their water and gas permeabilities (Galus and Kadzińska 2015).
In the related literature, a comprehensive review on microbial gums for food packaging and pharmaceutical applications is scarce. Thus, there is a need to provide an insight into this matter using current trends and future projections. The aim of this review is to comprehensively discuss the types and properties of microbial gums and their applications, with special emphasis on pharmaceutical and food packaging applications.
Microbial gums for biodegradable films
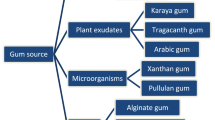
Microbial polysaccharides are high-molecular-weight polymers that are produced by the cell wall–anchored enzymes of microorganisms such as bacteria, molds, and yeasts (Morris 2006). Microbial gums are composed of sugar residues linked by glycosidic linkages, and may be linear or highly branched (Fig. 1). Biopolymers produced by microorganisms, including exopolysaccharides (EPSs), endo-polysaccharides, and polyhydroxyalkanoates, are neutral or acidic in nature and have a wide range of physico-mechanical characteristics (Table 2). These microbial gums are composed primarily of carbohydrate components (glucose, mannose, rhamnose), non-carbohydrate components (acetate, pyruvate, succinate, phosphate), and uronic acid. Microbial polysaccharides are non-toxic compounds produced via batch submerged aerobic fermentation in two forms: EPSs and capsular polysaccharides (CPSs) (Sutherland 2001; Sutherland 2005; Vanhaverbeke et al. 2003).
The advantage of microbial gums over other polymers or synthetics is its potential for production on an industrial scale. On the other hand, it requires high-technology equipment, specific substrates, adequate power and water supplies, and well-trained staff. Among the various types of microbial gums, a number of them are utilized in the preparation of gels (gellan and curdlan), thickening agents (pullulan, xanthan), and film solutions (pullulan, cellulose, and gellan) that are used as packaging materials. Table 3 summarizes the types of microbial gum-based composites for various food packaging and coating applications.
Xanthan
Xanthan gum is a heteropolysaccharide that is produced by Xanthomonas campestris during fermentation (Fitzpatrick et al. 2013). The compound was initially isolated by Kelco-AIL (Keltrol) and, after various experiments, was recognized as a food additive by the FDA in 1969. The molecular structure of xanthan features a backbone like that of cellulose, a linear chain of β-d-glucopyranose with (1 → 4) glycosidic bonds, and side chains having three sugars (β-d-mannose (β → 1,4)-glucuronic acid (α → 1,2)-α-d-mannose) as well as non-carbohydrate substitutes (acetate or pyruvate groups). Xanthan gum is an anionic, water-soluble polymer, stable at a wide range of pH and temperature values. It is commonly utilized in foods (e.g., dairy products, baked products, and beverages), pharmaceuticals, and cosmetics (Mohamed et al. 2013).
García-Betanzos et al. (2016) incorporated solid lipid nanoparticles (SLN) into xanthan-based films and assayed the effect of hot homogenization methods on the properties of the prepared films. The researchers reported improvements in the mechanical properties of these films when 60–75-g/L SLN was incorporated at 4–25 °C under 60–90% relative humidity. The study revealed water vapor permeability (WVP) values of 0.50–0.70 gm−2h−1kPa−1 in xanthan gum–based films incorporated with SLN.
Gellan
Gellan gum is the deacylated form of an EPS produced by Pseudomonas elodea. β-d-Glucose, l-rhamnose, and d-glucuronic acid are the units that form the gellan gum structure (Banik and Santhiagu 2006). Gellan gum has the ability to form 3D gel networks with monovalent or divalent cations (such as Na+ or Ca2+), and gel properties depend on ion-related factors (presence of ions; cation type; ionic strength) and the degree of acylation. Gellan gum is divided into the low-acetyl (brittle gel) and non-deacylated (elastic gel) forms (Bertoni et al. 2006; Fialho et al. 2008). Gellan gum is utilized as a gelling agent in jellies, dairy products, and confectioneries. It is a suitable hydrocolloid for edible film preparations. Initial experiments have shown that gellan films are transparent and have good mechanical properties (Yang and Paulson 2000a).
Composite films have been prepared by the incorporation of gellan/gelatin and different levels of sodium chloride. Results showed that the higher the sodium chloride content, the higher the tensile strength (TS) of the gellan film. When the sodium chloride concentration was 50 mM, the highest tensile elongation (TE) was observed. Also, with increasing gelatin amounts, the TS of the films decreased, while the TE increased (Lee et al. 2004).
Pulsed light (PL) has attracted much interest in recent years as a non-thermal method for the superficial decontamination of fresh foods. In one study, the effect of PL in combination with a gellan gum–based edible coating containing apple fiber on the shelf-life of fresh-cut apples has been investigated. Shelf-life was assessed by evaluating changes in color, firmness, antioxidant capacity, microbial growth, and sensory attributes of a fresh-cut apple stored for 14 days at 4 °C. The combination treatment led to reduced microbiological deterioration, enhanced preservation of antioxidant properties, and reduced softening and browning of apple pieces during storage. These results demonstrated the prebiotic potential and shelf-life extension capabilities of gellan gum–based coatings for fruits such as apples (Moreira et al. 2015).
Yang and Paulson (2000b) evaluated the mechanical properties and water vapor permeability (WVP) of gellan films. Their results showed that increasing the ratio of glycerol to gellan film entailed an increase in WVP and decreases in TS, elastic modulus, and glass transition temperature. Yang and Paulson (2000a) developed an edible gellan film by adding a mixture of lipids (beeswax and stearic–palmitic acid) and showed that adding lipids resulted in significantly improved WVP and mechanical properties. Here, the effect of beeswax on the mentioned properties was greater than that of stearic–palmitic acid. Xiao et al. (2011) investigated the properties of edible gellan membranes prepared under different conditions, namely gellan gum powder concentrations of 0, 0.02, 0.04, 0.06, 0.08, and 0.10% and drying temperatures of 40, 50, 60, 70, and 80 °C. The study demonstrated that the incorporation of 0.08% gellan at drying temperatures of 60–70 °C resulted in significantly better TS and gas barrier and moisture resistance compared with the other experimental conditions.
The mechanical, physical, and antimicrobial properties of a starch/gellan-based film containing emulsified or lecithin-encapsulated thyme (Thymus zygis) essential oil (EO) were studied by Sapper et al. (2018). Their results showed that lecithin improved the film’s water barrier properties and gloss, while recuing film stiffness, resistance to break, and extensibility. This means that incorporating lecithin to the starch–gellan film improved its mechanical and physical properties. The prepared films were also tested against Alternaria alternata (AA) and Botryotinia fuckeliana (BF) to determine their antifungal ability, which was found to be greater against the latter species.
Xu et al. (2007) blended konjac glucomannan with gellan gum and analyzed the mechanical properties of the resulting blend films. The obtained data exhibited that as the konjac glucomannan content in the blend film reached approximately 70%, the TS reached 17.5 MPa.
Ismail et al. (2018) investigated the effects of gellan gum (GG), calcium chloride (CaCl2), and glycerol concentrations on the physical appearance of blend films. Their results showed that while the prepared film was not well dried at 40 °C, the films formed at 70 and 100 °C were easily ruptured due to overheating (Fig. 2). At the highest amount of GG content studied (1.50 g), the film could not be produced due to the formation of a highly viscous and sticky suspension, as observed visually (Fig. 3a) and demonstrated by mildly rubbing between two fingers (Fig. 3b). Under optimized conditions (drying at 50 °C for 24 h), the films were successfully produced with 1.00 g GG, 5 mM CaCl2 cross-linker, and 50% w/w glycerol as the plasticizer.
Gellan gum samples after drying at a room temperature, b 40 °C, c 50 °C, d 70 °C, and e 100 °C for 24 h (With Courtesy to paper by Ismail et al2018)
a Highly viscous and b sticky GG suspension produced using 1.50 g GG (With Courtesy to paper by Ismail et al2018)
Pullulan
Pullulan (Pu) is an extracellular polysaccharide that consists of glucose and is produced by many Aureobasidium species, particularly Aureobasidium pullulans. Pu is composed of (1 → 4) and (1 → 6) α-d-glucopyranose residues. In this compound, the proportion of (1 → 4) to (1 → 6) linkages is about 1:2. The polymer mostly contains maltotriose units interconnected with each other by (1 → 6) bonds as well as scarce units of maltotetraose. The presence of α (1 → 6) bonds in Pu results in enhanced structural flexibility and increased water solubility compared with other linear polysaccharides. The viscosity of Pu solutions is good, but is unsuitable for gel formation. Non-toxic, non-mutagenic, and non-carcinogenic properties have been reported for Pu gum. In recent years, Pu has been surveyed in pharmaceutical (e.g., drug delivery) and biomedical (e.g., smart target delivery, surface modification, nanoparticle fabrication, gene delivery, cancer therapy, and bioimaging) applications. In addition, Pu has displayed dominant film-forming properties. Recent studies have demonstrated that pure Pu films are colorless, transparent, odorless, and highly water permeable, with minor oil and oxygen permeability.
Despite the many potential applications, the extensive usage of Pu has been limited by its high cost. Many researchers have focused on blending Pu with other polysaccharides such as alginate, chitosan, starch, and cellulose derivatives in order to produce films with improved physicochemical and mechanical properties. The developed films are easily dissolved in water, thus having the feature of melting in the mouth as edible food coatings (Singh et al. 2008). Pu film is suitable for the protection of oxidized fats and vitamins in foods due to its resistance to oxygen permeation (Krochta and De Mulder-Johnston 1997). Pu films can be utilized as the coating or packaging material of dried foods including nuts, noodles, confectionaries, vegetables, and meats, and can also replace starch in low-calorie food formulations.
In one study, Pu was successively tested for its ability to generate bionanocomposite films. In that work, montmorillonite nanoparticle (MMT) was used for improving the physicochemical and mechanical properties. The results showed that the MMT improved the TS of the generated films, while decreasing the elongation at break, WVP, moisture content, moisture absorption, and water solubility. Atomic force microscopy (AFM) observation of Pu–whey protein isolate bionanocomposite films showed rough film surfaces. The uniform distribution of MMT into the polymer matrix was confirmed by SEM and X-ray measurements (Hassannia-Kolaee et al. 2016b).
Kanmani and Lim (2013) blended probiotic bacteria and various starches with Pu. It was observed that by increasing the starch content, the relative cell viabilities and mechanical properties decreased, but adding probiotic bacteria did not significantly affect the physical and mechanical properties. The results exhibited that the Pu and Pu/potato starch films were the most suitable carrier matrices, with maximum relative cell viabilities of 70–80% after 2 months of storage at 4 °C. Wu et al. (2013) formulated chitosan and carboxymethyl chitosan (CMCH) into Pu films and observed that the added compounds improved the TS of the prepared films when the CMCH to Pu and chitosan to Pu ratios were 3:1 and 2:2, respectively. Hence, increases in CMCH and chitosan contents resulted in increased TS. The WVP of Pu films was increased by the addition of CMCH and chitosan.
Another useful hydrocolloid mixture was proposed by Tong et al. (2008). The authors blended Pu with alginate and carboxymethyl cellulose (CMC). The results indicated that the solubility of pure Pu films in water was greater compared with that of alginate and CMC films, whereas the WVP and mechanical properties of samples containing Pu < alginate < CMC were reduced in the blend films. Here, the addition of glycerol resulted in enhanced elongation at break and solubilization in water and reduced water barrier properties and TS.
In the study of Zhu et al. (2014), carboxymethyl gellan (CMGe) and Pu films were described and physically characterized. The results demonstrated that elongation at break and barrier properties improved by adding Pu to CMGe, though TS practically decreased.
Lipids have also been used to improve the barrier properties of Pu films, but negatively affect the mechanical properties. For example, the addition of rice wax (up to 46.4%) to Pu film wax significantly decreased the WVP value. Brunauer–Emmet–Teller (BET) and Guggenheim–Anderson–de Boer (GAB) sorption models gave a good fit up to the water activity (aw) of 0.55 for BET and a full range of aw (from 0.12 to 0.95) for GAB (Shih et al. 2011).
Plasticizers are generally required for producing polysaccharide- or protein-based edible films with appropriate mechanical properties. Vuddanda et al. (2017) analyzed the effects of different plasticizers and their concentrations on the physico-mechanical properties of Pu edible films. Films with 20% glycerol displayed the highest elongation. Pattanayaiying et al. (2015a) proposed an optimum combination of lauric alginate (LAE) and nisin Z in Pu film preparations in terms of mechanical properties. The authors reported that the two films with the best mechanical properties were as follows: (i) Pu = 150 g/L, glycerol = 2.5 g/L, LAE = 2 mg/mL, nisin Z = 320 AU/mL, and no locust bean gum; (ii) Pu = 120 g/L, glycerol = 2.5 g/L, LAE = 2 mg/mL, nisin Z = 320 AU/mL, and 1-g/L locust bean gum.
Also, the influence of edible films made from Pu on the physiological responses of coated fruit has been reported in one study. Here, the results exhibited that the use of Pu films effectively maintained the quality and extended the shelf-life of strawberries. Pu films declined the rate of weight loss and improved firmness and color retention (Eroglu et al. 2014).
Curdlan
Curdlan (CL) is an extracellular polysaccharide produced by non-pathogenic and non-toxicogenic bacteria, such as Rhizobiaceae (Alcaligenes faecalis var.). CL has been recognized as a food additive by the FDA, and its linear structure is composed of (1–3) β-d-glycosidic linkages (Grandpierre et al. 2008). This polysaccharide is soluble in alkaline solutions, but cannot be solubilized in water, alcohol, or acid solution. According to the degree of heating and through thermo-reversible processes, CL forms two types of gels, namely high-set gel (above 80 °C) or low-set gel (55 to 60 °C). CL gels are highly elastic and, in contrast with agar gels, can be formed within a wide pH range of 3.0 to 9.5. Due to its unique functions (e.g., its gelling properties), CL cannot be used as a carrier for drug delivery. CL also has bioactive features, such as antitumor and anti-HIV properties. However, its application in food is limited due to its poor mouth-feel effect (Phillips and Williams 2009).
Ahmad et al. (2015) investigated the effects of different ratios of fish gelatin/CL on the physico-mechanical and thermal properties of blend films. The results showed that the TS of gelatin/curdlan blend films was low, while the elongation at break, WVP, and water solubility were high. Moreover, contact angle and moisture content of blend films improved with higher CL contents. Furthermore, increased CL content in the fish gelatin/CL blend matrix led to decreases in redness (a*) and transparency; however, lightness (L*), yellowness (b*), and DE* values became enhanced. Furthermore, the fish gelatin/CL (8:2) blend film demonstrated more heat stability compared with the other samples, which was demonstrated by its higher heat-stable mass residues. Thus, CL can be used to develop a water barrier capability and thermal stability of fish gelatin films because of its water insoluble and thermo-gelable properties. Wu et al. (2012) studied a composite film composed of konjac glucomannan (KGM) and CL, which was prepared by a solvent-casting method. The results demonstrated that the WVP, tensile properties, and interaction of the blend film improved when the konjac glucomannan content in the composite was up to 70%.
Bacterial cellulose
Cellulose is produced by bacteria as well as by plants and other organisms. Cellulose has several unique properties, such as its high TS and water content. Recently, studies have focused on its potential applications in different fields. Bacterial cellulose (BC) can be produced in substantial amounts via the microbial fermentation of glucose (as a carbon source) by Gluconacetobacter xylinus strains and Gram-negative acetic acid bacteria. BC is well recognized for its greater characteristics compared with other celluloses (Iguchi et al. 2000), such as its higher mechanical strength, crystallinity, hydrophilicity (Rozenberga et al. 2016), purity, and water holding capacity, thereby displaying a decent potential for a variety of applications. The main advantage of BC is that it can be easily processed into microfibrils, nanofibrils, and nanocrystals for use in the production of edible nanocomposite films. In fact, BC has been used in its native form in the preparation of packaging materials (Shi et al. 2014). Recently, BC membranes have been utilized as antimicrobials in active packaging. BC membranes and films can be incorporated with antimicrobial agents to create an active packaging system that maintains antimicrobial activity during food storage. BC also has extensive applications in cosmetics and medicine (Jagannath et al. 2010), acoustic diaphragms (Nishi et al. 1990), ion exchange membranes (Choi et al. 2004), and electronic devices (Nogi and Yano 2008).
Wan et al. (2016) investigated the water resistance properties of multilayer films based on BC–zein, in which the strong BC film formed the outer layer and the electrospun zein fibers formed the inner layer. They found that the incorporation of electrospun zein fibers made multilayer films become rigid; however, the zein interlayers did not have a significant impact on the surface characteristics and thermal stability of the films. Due to the hydrophobicity of the zein protein, the water resistance properties improved. Jipa et al. (2012) prepared mono- and multilayer films based on powdered BC (BCP) and poly(vinyl) alcohol (PVA) containing sorbic acid (SA). The results displayed that sensitivity to water, antimicrobial ability, and release rate impressed with higher SA and BCP concentrations. By adding SA and BCP, the swelling degree increased, while the WVP and water solubility decreased.
Lately, several studies have focused on nanotechnological methods for improving the barrier properties of films. For example, the addition of nanoclays to the polymeric matrix of polysaccharide based–films has been investigated (Chivrac et al. 2009; Eroglu et al. 2014; Hassannia-Kolaee et al. 2016b). Gelatin nanocomposite films containing BC have been studied by George (2012). The formation of networks of cellulose nanocrystals within the gelatin matrix resulted in improved mechanical properties of nanocomposites and significantly reduced the WVP. In another study, Jebel and Almasi (2016) investigated the combination of BC with ZnO NPs in the production of monolayer and multilayer films. The results showed that incorporating ZnO NPs improved the mechanical properties but diminished the WVP and moisture absorption of BC films.
Conclusion
The applications of edible films in food technology open new opportunities for producing innovative biodegradable food packaging as a good alternative for replacing synthetic plastics. This can solve the problem of the accumulation of wastes related to non-biodegradable petroleum-based plastics. Film-forming microbial biopolymers such as gellan, bacterial cellulose, xanthan, pullulan, and curdlan are non-toxic, biocompatible, and biodegradable. They are used in the food industry as edible food films for coating and packing purposes. The films prepared from these polymers are transparent and have good mechanical, moisture, and oxygen barrier properties, but weak water barrier properties. The blending of microbial gums with lipids, hydrocolloids, or reinforcement agents improves the functional properties of edible films. Overall, an interdisciplinary approach for exploring novel applications is essential for preparing new composites of these microbial polymers with other polymers and hydrocolloids.
References
Ahmad N, Amin MCIM, Mahali SM, Ismail I, Chuang VTG (2014) Biocompatible and mucoadhesive bacterial cellulose-g-poly(acrylic acid) hydrogels for oral protein delivery. Mol Pharm 11(11):4130–4142. https://doi.org/10.1021/mp5003015
Ahmad M, Nirmal NP, Chuprom J (2015) Blend film based on fish gelatine/curdlan for packaging applications: spectral, microstructural and thermal characteristics. RSC Adv 5(120):99044–99057
Al-Hassan A, Norziah M (2012) Starch–gelatin edible films: water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocoll 26(1):108–117
Alizadeh-Sani M, Khezerlou A, Ehsani A (2018) Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind Crop Prod 124:300–315
Alvarado-González J, Chanona-Pérez J, Welti-Chanes J, Calderón-Domínguez G, Arzate-Vázquez I, Pacheco-Alcalá S, Garibay-Febles V, Gutiérrez-López G (2012) Optical, microstructural, functional and nanomechanical properties of Aloe vera gel/gellan gum edible films. Rev Mex Ing Quim 11(2):193–210
Alves VD, Ferreira AR, Costa N, Freitas F, Reis MA, Coelhoso IM (2011) Characterization of biodegradable films from the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr Polym 83(4):1582–1590
Amin M, Abadi AG, Ahmad N, Katas H, Jamal JA (2012) Bacterial cellulose film coating as drug delivery system: physicochemical, thermal and drug release properties. Sains Malays 41(5):561–568
Arismendi C, Chillo S, Conte A, Del Nobile MA, Flores S, Gerschenson LN (2013) Optimization of physical properties of xanthan gum/tapioca starch edible matrices containing potassium sorbate and evaluation of its antimicrobial effectiveness. LWT Food Sci Technol 53(1):290–296. https://doi.org/10.1016/j.lwt.2013.01.022
Azarakhsh N, Osman A, Ghazali H, Tan C, Mohd Adzahan N (2012) Optimization of alginate and gellan-based edible coating formulations for fresh-cut pineapples. Int Food Res J 19(1)
Azarakhsh N, Osman A, Ghazali HM, Tan CP, Mohd Adzahan N (2014) Effects of gellan-based edible coating on the quality of fresh-cut pineapple during cold storage. Food Bioprocess Technol 7(7):2144–2151. https://doi.org/10.1007/s11947-014-1261-6
Bae S, Sugano Y, Ohi K, Shoda M (2004) Features of bacterial cellulose synthesis in a mutant generated by disruption of the diguanylate cyclase 1 gene of Acetobacter xylinum BPR 2001. Appl Microbiol Biotechnol 65(3):315–322
Bajaj IB, Survase SA, Saudagar PS, Singhal RS (2007) Gellan gum: fermentative production, downstream processing and applications. Food Technol Biotechnol 45(4):341
Banik R, Santhiagu A (2006) Improvement in production and quality of gellan gum by Sphingomonas paucimobilis under high dissolved oxygen tension levels. Biotechnol Lett 28(17):1347–1350
Bera H, Kumar S, Maiti S (2018) Facile synthesis and characterization of tailor-made pectin-gellan gum-bionanofiller composites as intragastric drug delivery shuttles. Int J Biol Macromol 118:149–159. https://doi.org/10.1016/j.ijbiomac.2018.06.085
Bertoni F, Barbani N, Giusti P, Ciardelli G (2006) Transglutaminase reactivity with gelatine: perspective applications in tissue engineering. Biotechnol Lett 28(10):697–702
Bertuzzi M, Vidaurre EC, Armada M, Gottifredi J (2007) Water vapor permeability of edible starch based films. J Food Eng 80(3):972–978
Brodnjak UV (2017) Experimental investigation of novel curdlan/chitosan coatings on packaging paper. Prog Org Coat 112:86–92. https://doi.org/10.1016/j.porgcoat.2017.06.030
Caddeo C, Nácher A, Díez-Sales O, Merino-Sanjuán M, Fadda AM, Manconi M (2014) Chitosan–xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J Microencapsul 31(7):694–699. https://doi.org/10.3109/02652048.2014.913726
Cao N, Fu Y, He J (2007) Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll 21(7):1153–1162
Cazon P, Velazquez G, Ramírez JA, Vázquez M (2017) Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocoll 68:136–148
Cevher E, Salomon SK, Makrakis A, Li XW, Brocchini S, Alpar HO (2015) Development of chitosan–pullulan composite nanoparticles for nasal delivery of vaccines: optimisation and cellular studies. J Microencapsul 32(8):755–768. https://doi.org/10.3109/02652048.2015.1073392
Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotechnol 47(2)
Chivrac F, Pollet E, Averous L (2009) Progress in nano-biocomposites based on polysaccharides and nanoclays. Mater Sci Eng R Rep 67(1):1–17
Choi YJ, Ahn Y, Kang MS, Jun HK, Kim IS, Moon SH (2004) Preparation and characterization of acrylic acid-treated bacterial cellulose cation-exchange membrane. J Chem Technol Biotechnol 79(1):79–84
Criado P, Fraschini C, Salmieri S, Becher D, Safrany A, Lacroix M (2016) Free radical grafting of gallic acid (GA) on cellulose nanocrystals (CNCS) and evaluation of antioxidant reinforced gellan gum films. Radiat Phys Chem 118:61–69. https://doi.org/10.1016/j.radphyschem.2015.05.030
Cuq B, Gontard N, Aymard C, Guilbert S (1997) Relative humidity and temperature effects on mechanical and water vapor barrier properties of myofibrillar protein-based films. Polymer Gels and Networks 5(1):1–15
Dahiya S, Rani R, Kumar S, Dhingra D, Dilbaghi N (2017) Chitosan-gellan gum bipolymeric nanohydrogels—a potential nanocarrier for the delivery of epigallocatechin gallate. BioNanoScience 7(3):508–520. https://doi.org/10.1007/s12668-017-0416-0
Danalache F, Carvalho CY, Alves VD, Moldão-Martins M, Mata P (2016) Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int J Biol Macromol 84:43–53. https://doi.org/10.1016/j.ijbiomac.2015.11.079
Debeaufort F, Voilley A (2009) Lipid-based edible films and coatings Edible films and coatings for food applications. Springer, pp 135–168
Dehghani S, Hosseini SV, Regenstein JM (2018) Edible films and coatings in seafood preservation: a review. Food Chem 240:505–513
Dutta P, Tripathi S, Mehrotra G, Dutta J (2009) Perspectives for chitosan based antimicrobial films in food applications. Food Chem 114(4):1173–1182
Ehsani A, Paktarmani M, Yousefi M (2017) Efficiency of dietary sodium alginate coating incorporated with lycopene in preserving rainbow trout. Food Sci Biotechnol; FSB 26(3):557–562
Elsabee MZ, Abdou ES (2013) Chitosan based edible films and coatings: a review. Mater Sci Eng C 33(4):1819–1841
Eroglu E, Torun M, Dincer C, Topuz A (2014) Influence of pullulan-based edible coating on some quality properties of strawberry during cold storage. Packag Technol Sci 27(10):831–838
Espitia PJP, Du W-X, de Jesús Avena-Bustillos R, Soares NFF, McHugh TH (2014) Edible films from pectin: Physical-mechanical and antimicrobial properties-a review. Food Hydrocoll 35:287–296
Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A (2011) Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol 22(6):292–303
Farina V, Gentile C, Sortino G, Gianguzzi G, D¿ Asaro A, Saletta F, Piva G, Inglese P, Liguori G (2016) Effects of gellan-based coating application on litchi fruit quality traits. In: VIII International Postharvest Symposium: Enhancing Supply Chain and Consumer Benefits-Ethical and Technological Issues 1194. p 335–342
Feng Y, Zhang X, Shen Y, Yoshino K, Feng W (2012) A mechanically strong, flexible and conductive film based on bacterial cellulose/graphene nanocomposite. Carbohydr Polym 87(1):644–649. https://doi.org/10.1016/j.carbpol.2011.08.039
Fialho AM, Moreira LM, Granja AT, Popescu AO, Hoffmann K, Sá-Correia I (2008) Occurrence, production, and applications of gellan: current state and perspectives. Appl Microbiol Biotechnol 79(6):889–900
Fitzpatrick P, Meadows J, Ratcliffe I, Williams PA (2013) Control of the properties of xanthan/glucomannan mixed gels by varying xanthan fine structure. Carbohydr Polym 92(2):1018–1025
Freitas F, Alves VD, Reis MA, Crespo JG, Coelhoso IM (2014) Microbial polysaccharide-based membranes: current and future applications. J Appl Polym Sci 131(6)
Galindo-Pérez MJ, Quintanar-Guerrero D, Mercado-Silva E, Real-Sandoval SA, Zambrano-Zaragoza ML (2015) The effects of tocopherol nanocapsules/xanthan gum coatings on the preservation of fresh-cut apples: evaluation of phenol metabolism. Food Bioprocess Technol 8(8):1791–1799. https://doi.org/10.1007/s11947-015-1523-y
Galus S, Kadzińska J (2015) Food applications of emulsion-based edible films and coatings. Trends Food Sci Technol 45(2):273–283
García-Betanzos CI, Hernández-Sánchez H, Quintanar-Guerrero D, Alicia Del Real L, de la Luz Zambrano-Zaragoza M (2016) The evaluation of mechanical, thermal, optical and microstructural properties of edible films with solid lipid nanoparticles-xanthan gum stored at different temperatures and relative humidities. Food Bioprocess Technol 9(10):1756–1768
George J (2012) High performance edible nanocomposite films containing bacterial cellulose nanocrystals. Carbohydr Polym 87(3):2031–2037
Gniewosz M, Synowiec A, Kraśniewska K, Przybył JL, Bączek K, Węglarz Z (2014) The antimicrobial activity of pullulan film incorporated with meadowsweet flower extracts (Filipendulae ulmariae flos) on postharvest quality of apples. Food Control 37:351–361. https://doi.org/10.1016/j.foodcont.2013.09.049
Gontard N, Sonesson U, Birkved M, Majone M, Bolzonella D, Celli A, Angellier-Coussy H, Jang G-W, Verniquet A, Broeze J (2018) A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit Rev Environ Sci Technol 48(6):614–654
Grandpierre C, Janssen H-G, Laroche C, Michaud P, Warrand J (2008) Enzymatic and chemical degradation of curdlan targeting the production of β-(1 → 3) oligoglucans. Carbohydr Polym 71(2):277–286
Hansen NM, Plackett D (2008) Sustainable films and coatings from hemicelluloses: a review. Biomacromolecules 9(6):1493–1505
Hassannia-Kolaee M, Khodaiyan F, Pourahmad R, Shahabi-Ghahfarrokhi I (2016a) Development of ecofriendly bionanocomposite: whey protein isolate/pullulan films with nano-SiO2. Int J Biol Macromol 86:139–144. https://doi.org/10.1016/j.ijbiomac.2016.01.032
Hassannia-Kolaee M, Khodaiyan F, Shahabi-Ghahfarrokhi I (2016b) Modification of functional properties of pullulan–whey protein bionanocomposite films with nanoclay. J Food Sci Technol 53(2):1294–1302. https://doi.org/10.1007/s13197-015-1778-3
Iguchi M, Yamanaka S, Budhiono A (2000) Bacterial cellulose—a masterpiece of nature’s arts. J Mater Sci 35(2):261–270
Imeson A (2011) Food stabilisers, thickeners and gelling agents. John Wiley & Sons
Indrarti L, Indriyati, Syampurwadi A, Pujiastuti S (2016) Physical and mechanical properties of modified bacterial cellulose composite films. In: AIP Conference Proceedings. vol 1711. AIP Publishing, p 050007
Ismail NA, Amin KAM, Razali MH (2018) Preparation of gellan gum (GG) film: the effect of GG, calcium chloride (CaCl2), glycerol concentration and heat treatment. IOP Conference Series: Mater Sci Eng 440:012006 doi:https://doi.org/10.1088/1757-899x/440/1/012006
Jagannath A, Raju P, Bawa A (2010) Comparative evaluation of bacterial cellulose (nata) as a cryoprotectant and carrier support during the freeze drying process of probiotic lactic acid bacteria. LWT Food Sci Technol 43(8):1197–1203
Javanmard M (2012) Shelf-Life of apples coated with whey protein concentrate- gellan gum edible coatings. J Food Bioprocess Tech 01 (JFBT (Vol. 1)).
Jebel FS, Almasi H (2016) Morphological, physical, antimicrobial and release properties of ZnO nanoparticles-loaded bacterial cellulose films. Carbohydr Polym 149:8–19. https://doi.org/10.1016/j.carbpol.2016.04.089
Jiménez A, Fabra MJ, Talens P, Chiralt A (2012) Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll 26(1):302–310
Jipa IM, Stoica-Guzun A, Stroescu M (2012) Controlled release of sorbic acid from bacterial cellulose based mono and multilayer antimicrobial films. LWT Food Sci Technol 47(2):400–406
Kang H-J, Kim S-J, You Y-S, Lacroix M, Han J (2013) Inhibitory effect of soy protein coating formulations on walnut (Juglans regia L.) kernels against lipid oxidation. LWT Food Sci Technol 51(1):393–396
Kanmani P, Lim ST (2013) Development and characterization of novel probiotic-residing pullulan/starch edible films. Food Chem 141(2):1041–1049
Khalaf H, Sharoba A, El-Tanahi H, Morsy M (2013) Stability of antimicrobial activity of pullulan edible films incorporated with nanoparticles and essential oils and their impact on turkey deli meat quality. Mansoura J Food Dairy Sci 4(11):557–573
Khalid A, Ullah H, Ul-Islam M, Khan R, Khan S, Ahmad F, Khan T, Wahid F (2017) Bacterial cellulose–TiO 2 nanocomposites promote healing and tissue regeneration in burn mice model. RSC Adv 7(75):47662–47668
Khalil HA, Saurabh CK, Tye Y, Lai T, Easa A, Rosamah E, Fazita M, Syakir M, Adnan A, Fizree H (2017) Seaweed based sustainable films and composites for food and pharmaceutical applications: a review. Renew Sust Energ Rev 77:353–362
Khezerlou A, Ehsani A, Tabibiazar M, Moghaddas Kia E (2018) Development and characterization of a Persian gum–sodium caseinate biocomposite film accompanied by Zingiber officinale extract. J Appl Polym Sci:47215
Kraśniewska K, Gniewosz M, Synowiec A, Przybył JL, Bączek K, Węglarz Z (2014) The use of pullulan coating enriched with plant extracts from Satureja hortensis L. to maintain pepper and apple quality and safety. Postharvest Biol Technol 90:63–72. https://doi.org/10.1016/j.postharvbio.2013.12.010
Krochta J, De Mulder-Johnston C (1997) Scientific Status Summary-edible and biodegradable polymer films. Food Technol 51(2):61–74
Lee KY, Shim J, Lee HG (2004) Mechanical properties of gellan and gelatin composite films. Carbohydr Polym 56(2):251–254
Mirón-Mérida VA, Yáñez-Fernández J, Montañez-Barragán B, Barragán Huerta BE (2019) Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT Food Sci Technol 101:167–174. https://doi.org/10.1016/j.lwt.2018.11.013
Moghaddas Kia E, Ghasempour Z, Alizadeh M (2018) Fabrication of an eco-friendly antioxidant biocomposite: Zedo gum/sodium caseinate film by incorporating microalgae (Spirulina platensis). J Appl Polym Sci 135(13):46024
Mohamed A, Aboul-Anean H, Amal M (2013) Utilization of edible coating in extending the shelf life of minimally processed prickly pear. J Appl Sci Res 9(2):1202–1208
Moreira MR, Tomadoni B, Martín-Belloso O, Soliva-Fortuny R (2015) Preservation of fresh-cut apple quality attributes by pulsed light in combination with gellan gum-based prebiotic edible coatings. LWT Food Sci Technol 64(2):1130–1137. https://doi.org/10.1016/j.lwt.2015.07.002
Morris V (2006) 12 Bacterial Polysaccharides. Food polysaccharides and their applications: https://doi.org/10.1002/9780813823621, 413
Morsy MK, Khalaf HH, Sharoba AM, El-Tanahi HH, Cutter CN (2014) Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J Food Sci 79(4):M675–M684. https://doi.org/10.1111/1750-3841.12400
Morsy MK, Sharoba AM, Khalaf HH, El-Tanahy HH, Cutter CN (2015) Efficacy of antimicrobial pullulan-based coating to improve internal quality and shelf-life of chicken eggs during storage. J Food Sci 80(5):M1066–M1074
Muscat D, Adhikari B, Adhikari R, Chaudhary D (2012) Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J Food Eng 109(2):189–201
Nishi Y, Uryu M, Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S (1990) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 25(6):2997–3001
Nogi M, Yano H (2008) Transparent nanocomposites based on cellulose produced by bacteria offer potential innovation in the electronics device industry. Adv Mater 20(10):1849–1852
Othman LZ, Nawi M, Sufian M, Abul Bashar Mohammed H, Elsayed TMA (2017) Combination of xanthan gum and HPMC as retardants in sustained release gliclazide formulation.
Padrão J, Gonçalves S, Silva JP, Sencadas V, Lanceros-Méndez S, Pinheiro AC, Vicente AA, Rodrigues LR, Dourado F (2016) Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll 58:126–140. https://doi.org/10.1016/j.foodhyd.2016.02.019
Pattanayaiying R, Aran H, Cutter CN (2015a) Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. Int J Food Microbiol 207:77–82
Pattanayaiying R, H-Kittikun A, Cutter CN (2015b) Optimization of formulations for pullulan films containing lauric arginate and nisin Z. LWT Food Sci Technol 63(2):1110–1120. https://doi.org/10.1016/j.lwt.2015.04.016
Phillips GO, Williams PA (2009) Handbook of hydrocolloids. Elsevier
Popescu RA, Magyari K, Taulescu M, Vulpoi A, Berce C, Bogdan S, Lelescu C, Dreancă A, Tudoran O, Papuc I, Baia L (2018) New alginate–pullulan–bioactive glass composites with copper oxide for bone tissue regeneration trials. J Tissue Eng Regen Med 12(10):2112–2121. https://doi.org/10.1002/term.2746
Prajapati VD, Jani GK, Khanda SM (2013) Pullulan: an exopolysaccharide and its various applications. Carbohydr Polym 95(1):540–549
Prezotti FG, Cury BSF, Evangelista RC (2014) Mucoadhesive beads of gellan gum/pectin intended to controlled delivery of drugs. Carbohydr Polym 113:286–295. https://doi.org/10.1016/j.carbpol.2014.07.021
Prommakool A, Sajjaanantakul T, Janjarasskul T, Krochta JM (2011) Whey protein–okra polysaccharide fraction blend edible films: tensile properties, water vapor permeability and oxygen permeability. J Sci Food Agric 91(2):362–369
Ramos ÓL, Fernandes JC, Silva SI, Pintado ME, Malcata FX (2012) Edible films and coatings from whey proteins: a review on formulation, and on mechanical and bioactive properties. Crit Rev Food Sci Nutr 52(6):533–552
Raschip IE, Panainte AD, Pamfil D, Profire L, Vasile C (2015) In vitro testing of xanthan/lignin hydrogels as carriers for controlled delivery of bisoprolol fumarate. The Medical-Surgical J 119(4):1189–1194
Rehm BH (2009) Microbial production of biopolymers and polymer precursors: applications and perspectives. Horizon Scientific Press
Rozenberga L, Skute M, Belkova L, Sable I, Vikele L, Semjonovs P, Saka M, Ruklisha M, Paegle L (2016) Characterisation of films and nanopaper obtained from cellulose synthesised by acetic acid bacteria. Carbohydr Polym 144:33–40
Saha NR, Sarkar G, Roy I, Rana D, Bhattacharyya A, Adhikari A, Mukhopadhyay A, Chattopadhyay D (2016) Studies on methylcellulose/pectin/montmorillonite nanocomposite films and their application possibilities. Carbohydr Polym 136:1218–1227
Sallam KI (2007) Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 18(5):566–575
Sapper M, Wilcaso P, Santamarina MP, Roselló J, Chiralt A (2018) Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 92:505–515. https://doi.org/10.1016/j.foodcont.2018.05.004
Sharma S, Rao TVR (2015) Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci Technol 62(1, Part 2):791–800. https://doi.org/10.1016/j.lwt.2014.11.050
Shi Z, Zhang Y, Phillips GO, Yang G (2014) Utilization of bacterial cellulose in food. Food Hydrocoll 35:539–545
Shih F, Daigle K, Champagne E (2011) Effect of rice wax on water vapour permeability and sorption properties of edible pullulan films. Food Chem 127(1):118–121
Shit SC, Shah PM (2014) Edible polymers: challenges and opportunities. J Polym 2014. https://doi.org/10.1155/2014/427259.
Singh RS, Saini GK, Kennedy JF (2008) Pullulan: microbial sources, production and applications. Carbohydr Polym 73(4):515–531
Singh RS, Kaur N, Rana V, Kennedy JF (2017) Pullulan: a novel molecule for biomedical applications. Carbohydr Polym 171:102–121
Sutherland IW (2001) Microbial polysaccharides from Gram-negative bacteria. Int Dairy J 11(9):663–674
Sutherland IW (2005) 09 Biotechnology of microbial polysaccharides in food Food Biotech, Second Edition. CRC Press, pp 221–248
Sutherland WJ, Clout M, Depledge M, Dicks LV, Dinsdale J, Entwistle AC, Fleishman E, Gibbons DW, Keim B, Lickorish FA (2015) A horizon scan of global conservation issues for 2015. Trends Ecol Evol 30(1):17–24
Synowiec A, Gniewosz M, Kraśniewska K, Chlebowska-Śmigiel A, Przybył J, Bączek K, Węglarz Z (2014a) Effect of Meadowsweet flower extract-pullulan coatings on Rhizopus rot development and postharvest quality of cold-stored red peppers. Molecules 19(9):12925–12939
Synowiec A, Gniewosz M, Kraśniewska K, Przybył JL, Bączek K, Węglarz Z (2014b) Antimicrobial and antioxidant properties of pullulan film containing sweet basil extract and an evaluation of coating effectiveness in the prolongation of the shelf life of apples stored in refrigeration conditions. Innovative Food Sci Emerg Technol 23:171–181. https://doi.org/10.1016/j.ifset.2014.03.006
Tavassoli-Kafrani E, Shekarchizadeh H, Masoudpour-Behabadi M (2016) Development of edible films and coatings from alginates and carrageenans. Carbohydr Polym 137:360–374
Tong Q, Xiao Q, Lim L-T (2008) Preparation and properties of pullulan–alginate–carboxymethylcellulose blend films. Food Res Int 41(10):1007–1014
Trovatti E, Fernandes SCM, Rubatat L, Freire CSR, Silvestre AJD, Neto CP (2012) Sustainable nanocomposite films based on bacterial cellulose and pullulan. Cellulose 19(3):729–737. https://doi.org/10.1007/s10570-012-9673-9
Tukulula M, Hayeshi R, Fonteh P, Meyer D, Ndamase A, Madziva MT, Khumalo V, Lubuschagne P, Naicker B, Swai H, Dube A (2015) Curdlan-conjugated PLGA nanoparticles possess macrophage stimulant activity and drug delivery capabilities. Pharm Res 32(8):2713–2726. https://doi.org/10.1007/s11095-015-1655-9
Ul-Islam M, Khan T, Khattak WA, Park JK (2013) Bacterial cellulose-MMTs nanoreinforced composite films: novel wound dressing material with antibacterial properties. Cellulose 20(2):589–596. https://doi.org/10.1007/s10570-012-9849-3
Ullrich M (2009) Bacterial polysaccharides: current innovations and future trends. Horizon Scientific Press
Vanhaverbeke C, Heyraud A, Mazeau K (2003) Conformational analysis of the exopolysaccharide from Burkholderia caribensis strain MWAP71: impact on the interaction with soils. Biopolymers 69(4):480–497
Viana RM, Sá NMSM, Barros MO, Borges MF, Azeredo HMC (2018) Nanofibrillated bacterial cellulose and pectin edible films added with fruit purees. Carbohydr Polym 196:27–32. https://doi.org/10.1016/j.carbpol.2018.05.017
Vijayendra S, Shamala T (2014) Film forming microbial biopolymers for commercial applications—a review. Crit Rev Biotechnol 34(4):338–357
Vuddanda PR, Montenegro-Nicolini M, Morales JO, Velaga S (2017) Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur J Pharm Sci 96:290–298. https://doi.org/10.1016/j.ejps.2016.09.011
Wan Z, Wang L, Yang X, Guo J, Yin S (2016) Enhanced water resistance properties of bacterial cellulose multilayer films by incorporating interlayers of electrospun zein fibers. Food Hydrocoll 61:269–276
Wei Y-C, Cheng C-H, Ho Y-C, Tsai M-L, Mi F-L (2017) Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll 69:491–502. https://doi.org/10.1016/j.foodhyd.2017.03.010
Wu C, Peng S, Wen C, Wang X, Fan L, Deng R, Pang J (2012) Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr Polym 89(2):497–503
Wu J, Zhong F, Li Y, Shoemaker C, Xia W (2013) Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll 30(1):82–91
Xiao G, Zhu Y, Wang L, You Q, Huo P, You Y (2011) Production and storage of edible film using gellan gum. Procedia Environ Sci 8:756–763
Xiao Q, Lim L-T, Tong Q (2012) Properties of pullulan-based blend films as affected by alginate content and relative humidity. Carbohydr Polym 87(1):227–234
Xu X, Li B, Kennedy J, Xie B, Huang M (2007) Characterization of konjac glucomannan–gellan gum blend films and their suitability for release of nisin incorporated therein. Carbohydr Polym 70(2):192–197
Yang S-T (2007) Bioprocessing–from biotechnology to biorefinery Bioprocessing for value-added products from renewable resources. Elsevier, pp 1–24
Yang S-T (2011) Bioprocessing for value-added products from renewable resources: new technologies and applications. Elsevier
Yang L, Paulson A (2000a) Effects of lipids on mechanical and moisture barrier properties of edible gellan film. Food Res Int 33(7):571–578
Yang L, Paulson A (2000b) Mechanical and water vapour barrier properties of edible gellan films. Food Res Int 33(7):563–570
Yang L, Paulson AT, Nickerson MT (2010) Mechanical and physical properties of calcium-treated gellan films. Food Res Int 43(5):1439–1443. https://doi.org/10.1016/j.foodres.2010.04.010
Zambrano-Zaragoza ML, Mercado-Silva E, Del Real L A, Gutiérrez-Cortez E, Cornejo-Villegas MA, Quintanar-Guerrero D (2014) The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “Red Delicious” apples. Innov Food Sci Emerg Technol 22:188–196. https://doi.org/10.1016/j.ifset.2013.09.008
Zhu G, Sheng L, Li J, Tong Q (2013) Preparation and characterisation of gellan/pullulan composite blend films. Int J Food Sci Technol 48(12):2683–2687
Zhu G, Sheng L, Tong Q (2014) Preparation and characterization of carboxymethyl-gellan and pullulan blend films. Food Hydrocoll 35:341–347
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical statement/conflict of interest
The authors, whose names appear on the submission, declare that they have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results. This manuscript has not been published or presented elsewhere in part or in entirety, and is not under consideration by another journal. All the authors have approved the manuscript and agree with its submission to your esteemed journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alizadeh-Sani, M., Ehsani, A., Moghaddas Kia, E. et al. Microbial gums: introducing a novel functional component of edible coatings and packaging. Appl Microbiol Biotechnol 103, 6853–6866 (2019). https://doi.org/10.1007/s00253-019-09966-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-09966-x