Abstract
Biosurfactants are surface-active molecules that are synthesised non-ribosomally by a wide range of microorganisms including bacteria, yeast and filamentous fungi. The bacterial genus Serratia is gaining international interest, as biosurfactants produced by this genus have emerged as a promising source of antimicrobial, antifouling and antitumour compounds that possess emulsification and surface activity. Various species of Serratia have been identified as biosurfactant producers, including Serratia marcescens, Serratia rubidaea and Serratia surfactantfaciens. Members of the Serratia genus have been reported to principally produce two classes of biosurfactants, namely lipopeptides and glycolipids. Lipopeptides produced by Serratia species include serrawettins and stephensiolides, while identified glycolipids include rubiwettins and rhamnolipids. This review will primarily focus on the classification of biosurfactants produced by Serratia species and the genes and mechanisms involved in the biosynthesis of these biosurfactant compounds. Thereafter, an indication of the primary growth conditions and nutrient composition required for the optimum production of biosurfactants by this genus will be outlined. An overview of the latest advances and potential applications of the biosurfactants produced by Serratia in the medical, pharmaceutical, agricultural and petroleum industries is also provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biosurfactants play important physiological roles in cellular metabolism, motion and the defence mechanisms of microorganisms (Banat et al. 2014). Accordingly, various genera, such as Acinetobacter, Alcanivorax, Arthrobacter, Bacillus, Candida, Corynebacterium, Flavobacterium, Lactobacillus, Mycobacterium, Nocardia, Pseudomonas, Rhodococcus, Rhodotorula, Serratia, Streptomyces and Thiobacillus, amongst others, secrete various classes of biosurfactants as secondary metabolites (Rahman and Gakpe 2008; Zhang et al. 2012; Santos et al. 2016). The production of biosurfactants by microorganisms is often triggered by the presence of hydrophobic substrates and aids in the survival of these microorganisms in nutrient poor or highly contaminated environments (Bodour et al. 2003). This is achieved by increasing the bioavailability of nutrients, thus promoting the uptake and metabolism of less soluble substrates (Fiechter 1992). Furthermore, it has been hypothesised that anionic biosurfactants are able to protect microbial cells by forming complexes with positively charged toxic heavy metals present in the environment (Ron and Rosenberg 2001). Therefore, biosurfactant-producing microorganisms are routinely isolated from highly contaminated sites, such as metal- or hydrocarbon-contaminated soil and water environments, and wastewater treatment plants (Bodour et al. 2003; Ndlovu et al. 2016).
Of the numerous biosurfactant-producing genera isolated from different environments, biosurfactants produced by members of the Serratia genus are gaining increased scientific interest as they have been shown to display emulsification, surface, antifouling, antitumor and antimicrobial activity (Escobar-Díaz et al. 2005; Dusane et al. 2011; Nalini and Parthasarathi 2014; Su et al. 2016). They are also able to modify the hydrophobicity of the cell surface, which plays an important role in the adhesion of these bacteria to various surfaces and contributes to enhancing the surface spreading of bacteria in nutrient-poor environments (Bar-Ness et al. 1988; Wei et al. 2004; Matsuyama et al. 2011; Su et al. 2016). Various biosurfactant-producing S. marcescens, S. rubidaea and S. surfactantfaciens strains as well as Serratia liquefaciens (recently reclassified as a S. marcescens strain) have subsequently been isolated from hydrocarbon-contaminated soil and rhizosphere soil, surface water, marine environments and wastewater treatment plants (Matsuyama et al. 1985, 1990; Lindum et al. 1998; Anyanwu et al. 2010; Dusane et al. 2011; Nalini and Parthasarathi 2013, 2014; Ndlovu et al. 2016; Su et al. 2016; Almansoory et al. 2017).
Serratia species predominantly produce various glycolipids and lipopeptides (Desai and Banat 1997; Banat et al. 2010; Santos et al. 2016), which have been reported to display antibacterial, antifungal and antiprotozoal activities (Kadouri and Shanks 2013; Su et al. 2016; Ganley et al. 2018). In addition, glycolipids produced by Serratia species display biofilm disrupting and antiadhesive activity against bacterial and fungal strains (Dusane et al. 2011). The glycolipids and lipopeptides produced by this genus are thus of interest due to their potential biomedical and therapeutic applications (Cameotra and Makkar 2004). Moreover, the robustness and environmentally friendly nature of biosurfactant compounds in general allows for several potential applications in a number of different industrial fields, such as the petroleum, agricultural, food, cosmetic and pharmaceutical industries (Aparna et al. 2012).
The genus Serratia
Serratia species are Gram-negative, facultative anaerobic bacteria that belong to the Enterobacteriaceae family (Su et al. 2016). The genus is comprised of 18 species, including the type strain Serratia marcescens which is used as a biological marker because of its easily distinguishable red colonies (Khanna et al. 2013; Su et al. 2016). Although S. marcescens was first assumed to be non-pathogenic, it was later found to be an opportunistic pathogen associated with nosocomial infections, such as urinary tract, respiratory tract, surgical wound and blood stream infections (Khanna et al. 2013). Other species within the Serratia genus such as Serratia plymuthica, S. rubidaea and S. nematodiphila are also capable of producing the non-diffusible red pigment, prodigiosin, during secondary metabolism (Su et al. 2016). Prodigiosin has been reported to display antimalarial, antibacterial, antifungal, antiprotozoal, antitumour and immunosuppressant activities (Stankovic et al. 2014). In addition to prodigiosin, various members of the genus Serratia are known to produce other secondary metabolites, such as biosurfactants, oocydin A, carbapenem, althiomycin, bacteriocins and serratin (Foulds 1972; Srobel et al. 1999; Matsuyama et al. 2011; Gerc et al. 2012; Wilf and Salmond 2012; Luna et al. 2013). Although Serratia species produce a number of bioactive secondary metabolites, this review will focus on the glycolipid and lipopeptide biosurfactant compounds produced by members of this genus.
The primary lipopeptides produced by Serratia species include serrawettins (W1, W2 and W3) and stephensiolides (A to K) (Matsuyama et al. 1985; Dwivedi et al. 2008; Su et al. 2016; Ganley et al. 2018). Serratia species have also been reported to produce the glycolipids, rubiwettins (R1 and RG1) and rhamnolipids (Matsuyama et al. 1990; Nalini and Parthasarathi 2014). A few additional glycolipids, including a sucrose lipid, an arabinolipid and a glycolipid composed of a glucose attached to a palmitic acid, have also been detected (Pruthi and Cameotra 2000; Bidlan et al. 2007; Dusane et al. 2011). In addition, a study conducted by Ndlovu et al. (2016) isolated S. marcescens ST29 from a wastewater treatment plant sample and found the strain to contain genes encoding for the biosynthesis of both surfactin and iturin. However, the chemical characterisation of the biosurfactant compounds produced by this strain was not investigated.
Lipopeptides produced by Serratia species
Lipopeptides represent a class of low molecular weight compounds composed of a hydrophilic peptide attached to a hydrophobic lipid or fatty acid (Banat et al. 2014). A wide range of lipopeptide structures have been identified which display variation in the length and conformation of the lipid moiety resulting in different homologues, while analogues exist due to variation in amino acid composition within the peptide moiety (Banat et al. 2014). As indicated, lipopeptides produced by Serratia species include serrawettin W1 (initially referred to as serratamolide A) with identified homologues serratamolide B to G (Wasserman et al. 1961; Matsuyama et al. 1985; Dwivedi et al. 2008; Zhu et al. 2018). In addition, studies have identified serrawettin W2 (and its analogues) (Matsuyama et al. 1992; Su et al. 2016) and serrawettin W3 has been partially characterised (Matsuyama et al. 1986). In addition to serrawettins, Ganley et al. (2018) reported the discovery of antimicrobial lipodepsipeptides known as stephensiolides A to K.
Serrawettin family
Serrawettins are solely produced by members of the Serratia genus; serrawettin W1 is produced by strains of S. marcescens, serrawettin W2 is produced by S. marcescens and S. surfactantfaciens strains, and the partially characterised serrawettin W3 is also produced by a S. marcescens strain (Matsuyama et al. 1985, 1992; Matsuyama and Nakagawa 1996; Su et al. 2016). The first serrawettin was isolated in 1985 from a S. marcescens strain and was found to be identical to serratamolide A that was previously identified by Wasserman et al. (1961) (Matsuyama et al. 1985). The general structure of serrawettin W1 (also known as serratamolide A) includes a symmetric dilactone structure composed of two L-serine amino acids linked to two β-hydroxy fatty acids (comprised of 3-hydroxydecanoic acids) (molecular weight is 514.66 Da) (Fig. 1a) (Eckelmann et al. 2018). The diversity in the structure of serrawettin W1 (serratamolide A) exists due to the variation in the length of the fatty acid chain (C8 to C14) and the presence or absence of double bonds, resulting in homologues of serrawettin W1 (serratamolide A), namely serratamolide B to G (Dwivedi et al. 2008; Zhu et al. 2018). The molecular weight of the homologues range within 486.61 to 665.40 Da (Dwivedi et al. 2008; Zhu et al. 2018). In contrast to serrawettin W1, the general structure of serrawettin W2 includes five amino acids (D-leucine/isoleucine-L-serine-L-threonine-D-phenylalanine-L-isoleucine/leucine), bonded to a β-hydroxy fatty acid moiety (molecular weight of 731.93 Da) (Fig. 1b) (Matsuyama et al. 1992; Motley et al. 2016).
The diversity in the structure of serrawettin W2 results from variation at the first, second or fifth amino acid positions or the length of the fatty acid chain (C8 or C10), resulting in the detection of analogues with a molecular weight ranging from 703.3 to 759.3 Da (Matsuyama et al. 1986; Motley et al. 2016; Su et al. 2016). While the exact chemical composition of serrawettin W3 has yet to be elucidated, the cyclodepsipeptide was found to be composed of a fatty acid (one dodecanoic acid) and five amino acids, including threonine, serine, valine, leucine and isoleucine (Matsuyama et al. 1986). Serrawettins are described as non-ionic biosurfactants as they have no amino acid residues with ionic hydrophilicity (Matsuyama et al. 2011).
Stephensiolide family
Stephensiolide lipodepsipeptides (A to K) are produced by a Serratia strain that was isolated from the midgut and salivary glands of an Anopheles stephensi mosquito (Ganley et al. 2018). Although stephensiolide mimics the overall structure of serrawettin W2 (as both structures are cyclic pentapeptides), the peptide sequence of the stephensiolides differs. The general chemical structure of stephensiolide includes five amino acids (threonine-serine-serine-valine/isoleucine-isoleucine/valine) attached to a long alkyl chain. The diversity in the structure of stephensiolides exists due to variation in the length of the acyl chain, variation in amino acid residues (presences of either isoleucine or valine at the fourth and fifth amino acid residues) or the presence or absence of a double bond in the fatty acyl chain, resulting in congeners of stephensiolide A to K (Ganley et al. 2018). This results in the molecular masses of the stephensiolides ranging from 599 to 695 Da depending on the congener (A to K). Although these lipopeptides have only recently been discovered, preliminary antimicrobial testing of the stephensiolide mixture (A to K) revealed activity against Bacillus subtilis and the malaria parasite, Plasmodium falciparum (Ganley et al. 2018).
Glycolipids produced by Serratia species
Glycolipids are a class of low molecular weight biosurfactants, which are comprised of a hydrophilic carbohydrate attached to a hydrophobic aliphatic or hydroxyl-fatty acid (Shekhar et al. 2015). A number of structurally diverse glycolipid structures have been identified and are produced by a wide range of bacterial and fungal genera. A microorganism can produce homologues of the same glycolipid due to variation in the length and conformation of the fatty acid moiety, while the carbohydrate moiety is comprised of mono-, di-, tri- or tetra-saccharides (Banat et al. 2014). Previous studies have isolated glycolipids produced by Serratia species, including rubiwettin R1, rubiwettin RG1 and rhamnolipids (Matsuyama et al. 1990; Nalini and Parthasarathi 2014). In addition, a number of studies have detected glycolipids such as a sucrose lipid (Pruthi and Cameotra 2000), a glycolipid composed of glucose and palmitic acid (Dusane et al. 2011) and an arabinolipid (Bidlan et al. 2007).
Rubiwettin family
Serratia rubidaea ATCC 27593 is currently the only strain reported to produce rubiwettins and was found to produce both rubiwettin R1 and rubiwettin RG1 (Matsuyama et al. 1990). The general structure (undetermined carbohydrate moiety) and molecular weight of rubiwettin R1 have yet to be fully elucidated. However, a mixture of linked 3-hydroxy fatty acids comprised of major components, including 3-(3′-hydroxytetradecanoyloxy) decanoate and 3-(3′-hydroxyhexadecanoyloxy) decanoate, and minor molecular isomers have been identified and a proposed structure of the fatty acid moiety (Fig. 2a) was provided by Matsuyama et al. (1990). The general structure of rubiwettin RG1 (Fig. 2b) was also proposed and consists of β-D-glucopyranosyl 3-(3′-hydroxytetradecanoyloxy) decanoate minor fatty acid isomers (molecular weight of 576.77 Da) (Matsuyama et al. 1990). Therefore, RG1 was found to have a rhamnolipid-like glycolipid structure; however, rhamnose is substituted with a glucose moiety (Matsuyama et al. 1990).
The chemical structure of rubiwettin a R1 [3-(3′-hydroxytetradecanoyloxy) decanoate and 3-(3′-hydroxyhexadecanoyloxy) decanoate] and b RG1 [β-D-glucopyranosyl 3-(3′-hydroxytetradecanoyloxy) decanoate minor fatty acid isomers] (adapted from Matsuyama et al. 1990)
Rhamnolipid family
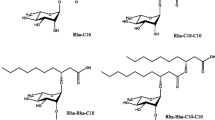
Rhamnolipids consist of one (mono-rhamnolipid) or two (di-rhamnolipid) rhamnose sugars bonded to lipid moieties by an O-glycosidic linkage. Although rhamnolipids are primarily produced by Pseudomonas species, studies by Nalini and Parthasarathi (2013, 2014) isolated a S. rubidaea SNAU02 strain from hydrocarbon-contaminated soil that was found to produce this class of glycolipid. Chemical characterisation of the compounds produced indicated that S. rubidaea SNAU02 was able to produce eight rhamnolipid congeners with varying β-hydroxy fatty acid chains ranging from C8 to C16 (Nalini and Parthasarathi 2013, 2014). Amongst the detected mono- and di-rhamnolipids, the di-rhamnolipid rha-rha-C10-C8 (β–α rhamnosyl (1 → 2) rhamnosyl-β-hydroxydecanoyl-β-hydroxyoctadecanoic acid) (Fig. 3a) was found to be the most abundant (Nalini and Parthasarathi 2014). The chemical structure of another major component, a mono-rhamnolipid produced by S. rubidaea SNAU02, was also determined, which was rha-C10-C10 (rhamnosyl-β-hydroxydecanoyl-β-hydroxydecanoic acid) (Fig. 3b) (Nalini and Parthasarathi 2014).
The proposed chemical structures for the a mono-rhamnolipid (Rha-C10-C10) and b di-rhamnolipid (Rha-Rha -C8-C10) produced by S. rubidaea SNAU02 (adapted from Nalini and Parthasarathi 2014)
Biosynthesis of biosurfactants produced by Serratia species
The biosynthesis of lipopeptides involves multistep processes mediated by various non-ribosomal peptide synthetase (NRPS) enzymes which catalyse the condensation and selection of amino acid residues to yield various metabolites (including lipopeptides). The mechanisms involved in the biosynthesis of serrawettin W1, serrawettin W2 and the stephensiolides have been identified, while the mechanisms involved in the biosynthesis of serrawettin W3 and the glycolipids produced by Serratia species are not fully elucidated. Investigation into the biosynthesis of serrawettin W1, serrawettin W2 and stephensiolides revealed open reading frames (ORF), namely swrW, swrA and sphA (Fig. 4), respectively, that displayed high homology with the NRPS family (Marahiel et al. 1997; Li et al. 2005; Ganley et al. 2018). The ORF is comprised of a unimodular or multimodular region, where each module consists of specific condensation (C), adenylation (A), thiolation (T) and thioesterase (TE) domains in functional order (Fig. 4) (Marahiel et al. 1997; Li et al. 2005).
Comparison of the proposed biosynthetic pathways for the biosynthesis of a serrawettin W1 encoded by swrW, b serrawettin W2 encoded by swrA and c stephensiolide encoded by sphA with their respective domain regions [condensation (C), adenylation (A), thiolation (T) and thioesterase (TE)] and general structures produced by each gene cluster (adapted from Ganley et al. 2018)
The systematic functioning of NRPSs involved in the biosynthesis of secondary metabolites has been determined and indicates that the precursor molecules (e.g. amino acids) are linked to the phosphopantetheinyl moiety of each thiolation domain in the multimodular enzyme (Matsuyama et al. 2011). A previous study by Sunaga et al. (2004) revealed a novel gene, pswP, in S. marcescens that encodes for 40-phosphopantetheinyl transferase (PPTase). PPTase is the activator of peptidyl carrier protein (PCP) (also known as the thiolation domain in the NRPS family) and was shown to be essential for the biosynthesis of serrawettin W1 (Sunaga et al. 2004). The PPTase is presumed to be involved in the incorporation reaction of L-serine as a molecular component of serrawettin W1 (Sunaga et al. 2004). Therefore, the thiolation domain must be activated by acquiring the phosphopantetheinyl moiety by the action of PPTase for the accurate functioning of serrawettin W1 synthetase (Li et al. 2005). After the activation by PPTase, the adenylation domain is able to adenylate L-serine to an activated form. This activated L-serine will then bind as a thioester to the thiolation domain (that has already been phosphopantetheinylated by PPTase) (Li et al. 2005). The amino group of the L-serine bound to the thiolation domain will react and create an amide linkage with the 3-D-hydroxydecanoyl moiety by detaching from the acyl carrier protein (ACP). This reaction results in the formation of a serratamic acid linked to the thiolation domain (Li et al. 2005). The serratamic acid is transferred to the thioesterase domain and the biosynthesis of a second serratamic acid bound to the free thiolation domain will follow. The two neighbouring serratamic acids will form an intramolecular linkage which results in the release of a symmetric and circular product, serrawettin W1, from the swrW gene region (Li et al. 2005). The serrawettin W2 biosynthetic pathway has a similar mechanism; however, it is encoded by the swrA gene (Su et al. 2016).
The biosynthesis of serrawettin W2 was investigated in S. surfactantfaciens sp. YD25T and a hybrid polyketide synthases (PKS)-NRPS gene cluster putatively involved in the biosynthesis of serrawettin W2 was identified (Su et al. 2016). The biosynthetic pathway was determined based on the presence of PKS and NRPS encoding genes. The precursor molecule, a C10 fatty acid (FA), is synthesised by the PKS SwrEFG gene cluster and additional undetermined proteins and is released as a fatty acyl-CoA (Su et al. 2016). The NRPS gene cluster encodes the core W2-peptide chain (5-amino acid peptide moiety) and contains five modules (Fig. 4b) (Su et al. 2016).
Similar to serrawettin W1 and serrawettin W2, the biosynthesis of stephensiolides was investigated in a Serratia strain using bioinformatic analysis (sequencing of the Serratia sp. genome) and a NRPS gene cluster encoded by the sphA gene putatively involved in the biosynthesis of stephensiolides was identified (Fig. 4c) (Ganley et al. 2018). Further bioinformatics analysis indicated that seven sequenced S. marcescens strains contained a homologue with identical predicted domain regions as the sphA gene (Ganley et al. 2018). Stephensiolides contain D- and L-amino acids and these lipopeptides are cyclised through a macrolactone ring. Both of these characteristic properties provide an indication that the peptides are synthesised by NRPSs. Although both serrawettin W2 and stephensiolide are cyclic pentapeptides and are similarly biosynthesised, they are cyclised in a different manner. Serrawettin W2 is cyclised through a 3-hydroxyl group of the fatty acid, while stephensiolides are cyclised through a hydroxyl group of the threonine (Ganley et al. 2018). Therefore, serrawettin W1, serrawettin W2 and stephensiolides are synthesised by various NRPS enzymes. The biosynthesis of these biosurfactants by Serratia species is influenced by the producer strain, growth conditions and nutrient composition.
Physiochemical characterisation of biosurfactants produced by Serratia species
In recent years, there has been an increased scientific interest in the isolation of microorganisms that produce biosurfactants with unique physicochemical properties due to their potential application in diverse industries and in bioremediation processes (Fracchia et al. 2012). The amphiphilic nature of biosurfactants allows for their accumulation at the interface between immiscible fluids or between a fluid and a solid, thereby reducing surface (liquid-air) tension and interfacial (liquid-liquid) tension (Varjani and Upasani 2017). The accumulation of these compounds at surfaces or interfaces also decreases the repulsive forces (cohesive forces that hold water molecules together) between two immiscible phases, such as water and oil (Peele et al. 2016). This results in the dispersion of one liquid into another leading to the emulsification of the two immiscible liquids (Soberón-Chávez and Maier 2011). The physicochemical properties of biosurfactants thus include their ability to reduce surface tension, form hydrocarbon emulsions (emulsification) and thus enhance the water solubility of hydrophobic compounds (Desai and Banat 1997).
Surface tension is considered to be the measure of free energy per unit area associated with a surface or interface and is measured using a tensiometer (DuNouy ring method). This is a common screening method to detect the presence of biosurfactant compounds produced by a microorganism (Satpute et al. 2010). Typically, a biosurfactant is considered effective if it can reduce the surface tension between water and air from 72 to 35 mN/m and the interfacial tension between water and n-hexadecane from 40 to 1 mN/m (Soberón-Chávez and Maier 2011). Furthermore, a bacterial strain is considered to be a good biosurfactant producer if it is able to reduce the surface tension of a growth medium by ≥ 20 mN/m compared to distilled water (Walter et al. 2010). Previous studies have identified lipopeptides and glycolipids capable of reducing the surface tension of a growth medium. For example, Dusane et al. (2011) isolated a S. marcescens strain that produced a glycolipid that was able to reduce the surface tension of the growth medium from 52.0 to 27 mN/m. Similarly, previously characterised serrawettin W1, serrawettin W2 and serrawettin W3 produced by S. marcescens ATCC 13380, NS 25 and NS 45 strains, respectively, were capable of reducing the surface tension of water to 32.2, 33.9 and 28.8 mN/m, respectively (Matsuyama et al. 2011). In addition, Matsuyama et al. (2011) indicated that rubiwettin R1 and rubiwettin RG1 produced by S. rubidaea ATCC 27593 reduced the surface tension of water to 25.5 and 25.8 mN/m, respectively.
Biosurfactants are also known to increase the solubility and bioavailability of hydrophobic organic compounds (Mnif and Ghribi 2015). Emulsification activity is thus an indirect method often used to screen for biosurfactant-producing microorganisms and the emulsification index can be calculated by measuring the emulsion height divided by the total height of the solution (equal volume of hydrocarbon to cell-free broth culture). Although kerosene is the most commonly used to test for emulsification, previous studies have tested the ability of biosurfactants produced by Serratia species to emulsify various hydrocarbons, such as diesel and crude oil (Pruthi and Cameotra 2000; Wei et al. 2004; Ibrahim et al. 2013). For example, Wei et al. (2004) identified a pigmented S. marcescens SS-1 strain that was able to emulsify both kerosene (72%) and diesel (40%).
The amphiphilic nature and structure of these compounds thus confer a diverse range of useful properties, such as emulsification activity, wetting, foaming, dispersion traits, surface activity and the reduction in viscosity of heavy liquids, allowing for their application in many industrial and commercial processes (Satpute et al. 2010; Aparna et al. 2012).
Production of biosurfactants by Serratia species
Biosurfactants are promising alternatives to synthetic surfactants and have been incorporated into commercialised products, such as Bio Surfactants ACS-Sophor® (sophorolipids) produced by Allied Carbon Solutions Co., Ltd., NatSurFact (rhamnolipids) produced by Logos Technologies, LLC and Yashinomi Vegetable Wash (sophorolipids) produced by Saraya Co. Ltd., amongst others (Geetha et al. 2018). However, the increased global biosurfactant market has resulted in the need for cost-effective, industrial-scale production and purification processes that result in maximum biosurfactant yield (Nitschke and Silva 2018). Biosurfactant production is dependent on the producer strain, physicochemical conditions (temperature, pH, agitation and aeration) and medium composition (carbon source, nitrogen source and salinity). Table 1 indicates various biosurfactant-producing Serratia species, the type of biosurfactant produced by each strain and the media and culture conditions used for small-scale biosurfactant production (excluding production in bioreactors).
The production of biosurfactants by Serratia species occurs during the late log and early stationary phase of growth indicating that the biosurfactants are secondary metabolites (Pruthi and Cameotra 2000; Bidlan et al. 2007; Dusane et al. 2011). It is noteworthy that the production of secondary metabolites, including biosurfactants and prodigiosin, by Serratia species is temperature-dependent (Matsuyama et al. 2011; Eckelmann et al. 2018). Matsuyama et al. (1986, 1990) investigated the production of secondary metabolites by Serratia species at varying temperatures and found that while the bacterial strains grew well at both 30 °C and 37 °C, serrawettin (W1, W2 and W3), rubiwettin (R1 and RG1) and the pigment (prodigiosin) were produced at 30 °C and were significantly reduced or absent when the bacterial suspension was grown at 37 °C. Numerous studies have subsequently utilised 30 °C as the optimum growth temperature for the production of biosurfactants by Serratia species (Pruthi and Cameotra 2000; Anyanwu et al. 2010; Dusane et al. 2011; Kadouri and Shanks 2013; Su et al. 2016; Almansoory et al. 2017).
Another physicochemical condition that influences production is oxygen transfer during cell growth. Several factors may contribute to the transfer of oxygen from the gas phase to the aqueous phase within the growth medium, such as agitation speed and aeration, amongst others, thereby affecting cell growth and, ultimately, biosurfactant production (Fakruddin 2012). Various agitation speeds have been used during the growth and production of biosurfactants by Serratia species (Table 1). In addition, the media composition, pH values (5 to 9) and cultivation times (24 to 168 h) similarly influence the quality and quantity of the biosurfactants produced. Almansoory et al. (2017) investigated the effects of varying cultivation times (24 to 168 h), agitation speeds (100, 125, 150, 180 or 200 rpm) and pH (5 to 9) for the production of biosurfactants by a Serratia strain. Results indicated that the optimum cultivation time, agitation speed and pH for maximum biosurfactant production and surface tension reduction were after 120 h at 200 rpm with the pH of the growth medium at 8.0. However, for the small-scale production of biosurfactants by Serratia species, an agitation speed of 200 rpm, pH of 7.2 and a cultivation time of 72 h are extensively used (Table 1). The salt concentration within the growth medium was also hypothesised to influence microbial growth and biosurfactant production by Serratia species (Almansoory et al. 2017). Almansoory et al. (2017) further indicated that the growth and production of a biosurfactant by an S. marcescens strain was reduced in the absence of salt. The authors additionally tested varying salt concentrations (ranging from 1 to 5%), with 1% recorded as optimum for biosurfactant production. The production of biosurfactants can also be improved by the presence of different carbon and nitrogen sources, as these factors strongly influence cell growth and the accumulation of metabolic products (Santos et al. 2016). Moreover, research has indicated that the concentration and type of biosurfactant compounds synthesised by the producer strain are influenced by the type of carbon substrate (Rahman and Gakpe 2008; Ndlovu et al. 2017).
Water miscible substrates, such as glucose, sucrose, fructose, mannitol and glycerol, and water immiscible substrates, such as mahua oil and olive oil, have been used for biosurfactant production by Serratia species (Table 1; Almansoory et al. 2017). At low nitrogen levels, bacterial growth is also limited, which favours cell metabolism towards the production of secondary metabolites (Santos et al. 2016). Numerous nitrogen sources have subsequently been used for the production of biosurfactants by Serratia species, including peptone, yeast extract, tryptone, ammonium sulphate, ammonium nitrate and casamino acids. Of the various carbon and nitrogen sources utilised for the production of biosurfactants by Serratia species, the most widely used are glycerol and peptone (Table 1), respectively. Almansoory et al. (2017) also investigated the effects of different carbon (glycerol, olive oil, glucose, sucrose and fructose) and nitrogen sources (ammonium sulphate, yeast extract, peptone and combinations of these three nitrogen sources) on lipopeptide production by S. marcescens by measuring the biosurfactant yield and the reduction of surface tension. Results indicated that of the five carbon sources used, glycerol resulted in the highest yield of 1.05 g/L and the lowest surface tension with a value of 30.4 mN/m recorded. Furthermore, the combination of ammonium sulphate and peptone (shown in Table 1) as nitrogen sources resulted in the highest yield of the biosurfactant compounds (1.33 g/L) and the lowest reduction in surface tension with a value of 29.9 mN/m recorded. Although biosurfactant production by Serratia species is strongly dependent on the producer strain, in summary the most widely used growth conditions and media composition for the small-scale production include an incubation temperature of 30 °C in a medium containing glycerol as a carbon source and peptone as a nitrogen source at a pH of 7.2 with agitation at 200 rpm.
In addition to the small-scale production of biosurfactants, studies have evaluated the use of bioreactors for the large-scale production of biosurfactants by Serratia species. A study by Granada et al. (2018) investigated the effects of dissolved oxygen on the large-scale production of bioactive metabolites, including serratamolide A, prodigiosin and haterumalide NC (a cytotoxic molecule), by Serratia sp. ARP5.1 using a 7-L stirred tank bioreactor. The strain was inoculated into 4 L of mineral medium (with glucose as a carbon source) at 28 °C for 96 h. Additionally, three agitation speeds (150, 300, 450 rpm) and three aeration rates (0.5, 1.0 and 1.5 vvm) were tested for optimal production. It was found that oxygen was a crucial factor for the biosynthesis of these secondary metabolites in a bioreactor, with the best combination of agitation speed and aeration observed at 450 rpm and 1.5 vvm, respectively. It was therefore recommended that dissolved oxygen be included as a parameter for the large-scale production of secondary metabolites by Serratia species. In addition, Roldán-Carrillo et al. (2011) utilised a Box-Behnken experimental design to evaluate the effect of nutrient ratios (C/N, C/Mg and C/Fe) on biosurfactant production by a S. marcescens strain. The results indicated that a nutrient ratio of C/N = 5, C/Mg = 30 and C/Fe = 26,000 was optimal for biosurfactant production. This media composition was then utilised for large-scale production in a 3-L bioreactor. The large-scale production of the biosurfactant by this strain was conducted in a volume of 1.5 L of the growth medium within the 3-L bioreactor. After 48 h, the biosurfactant was extracted using two volumes of ethanol after acid precipitation from the cell-free broth and the crude extract was freeze-dried and weighed. It was thus found that the nutrient ratios optimised by the Box-Behnken experimental design for biosurfactant production by this strain successfully yielded 21.6 g/L of crude extract after 48 h.
Although studies have used various methods to recover biosurfactants, further optimisation of the large-scale production process and downstream recovery of biosurfactants produced by Serratia species is still required. Furthermore, biosurfactants can be used to replace chemically synthesised surfactants in various industries as they exhibit a low toxicity, high biodegradability, can be produced from cost-effective materials and they are stable at extreme temperatures, pH and salinity (Satpute et al. 2010; Santos et al. 2016).
Applications of biosurfactant compounds produced by Serratia species
Biosurfactants have several advantages over chemical surfactants as they exhibit a low toxicity, high biodegradability, can be produced from cost-effective materials and exhibit stability at extreme temperature, pH and salinity (Satpute et al. 2010; Santos et al. 2016). Thus, due to their diverse chemical properties and biological activity, biosurfactants have the potential to replace their chemical counterparts in a number of industries, such as the petroleum, medical, pharmaceutical, food, agriculture, beverage, cosmetics, textiles and mining industries as well as in bioremediation strategies. Furthermore, biosurfactants produced by Serratia species have the potential to be applied as antimicrobial compounds, antifouling agents and antitumour compounds and as emulsifying agents of hydrocarbons.
Medical and pharmaceutical industries
Biosurfactant compounds as antimicrobial agents
Although the exact mode of action of biosurfactant compounds has yet to be elucidated, both lipopeptides (including serrawettin W1, serrawettin W2 and stephensiolides) and glycolipids (rhamnolipid and glucose-palmitic acid glycolipid) produced by Serratia species have been reported to display antimicrobial activity (Dwivedi et al. 2008; Dusane et al. 2011; Kadouri and Shanks 2013; Nalini and Parthasarathi 2014; Su et al. 2016; Eckelmann et al. 2018; Ganley et al. 2018). Dwivedi et al. (2008) purified serratamolide A (serrawettin W1) and homologues (serratamolide B to F) and tested these compounds for antimicrobial activity against Mycobacterium species. It was found that serratamolide A and all the homologues exhibited antibacterial activity against M. diernhoferi at a minimum inhibitory concentration of 0.18 mM. A recent study by Su et al. (2016) also investigated the antimicrobial activity of secondary metabolites produced by S. surfactantfaciens sp. YD25T. The secondary metabolite was identified as serrawettin W2 and was found to exhibit inhibitory activity against Staphylococcus aureus, Pseudomonas aeruginosa and Shigella dysenteriae, amongst other bacterial pathogens, at a concentration of 300 μg/mL (Su et al. 2016). Hage-Hülsmann et al. (2018) further investigated the synergistic antibiotic effects of prodigiosin and biosurfactants produced by S. marcescens DSM12481 strain against a soil bacterium, Corynebacterium glutamicum. As results indicated that the combination of prodigiosin and serrawettin W1 generated a larger zone of inhibition compared to the individual compounds, employing a combination of biomolecules may be a useful strategy for future antimicrobial formulations.
Biosurfactant compounds as antifouling agents
Biosurfactants have been reported to effectively inhibit biofilm formation on various surfaces (Epstein et al. 2011). When a surface is conditioned with a layer of a biosurfactant, it becomes more hydrophilic and is thus expected to have reduced microbial attachment (Gudiña et al. 2010; Zeraik and Nitschke 2010). In addition, many biosurfactant compounds disrupt preformed biofilms. McLandsborough et al. (2006) hypothesised that a biosurfactant has to penetrate the biofilms’ extracellular matrix (possibly through the water channels) and adhere to the interface, thereby reducing the interfacial tension between the substratum surface and the biofilm. The interactions involved in bacterial adhesion are also reduced, ultimately leading to biofilm removal. Dusane et al. (2011) investigated the antifouling activity of a glycolipid, composed of palmitic acid esterified to glucose, produced by a S. marcescens strain to disrupt a preformed biofilm or prevent biofilm formation on polystyrene microtitre plates. Results indicated that 100 μg/mL of the glycolipid prevented the attachment of 94%, 88% and 82% of Bacillus pumilus TiO1, P. aeruginosa PAO1 and Candida albicans BH cells, respectively, to polystyrene microtitre plate surfaces. A glycolipid concentration of 50 μg/mL also resulted in a reduction of up to 55% C. albicans, 62% P. aeruginosa and 55% B. pumilus reduction of preformed biofilms when compared to untreated control biofilms grown on polystyrene microtitre plates (Dusane et al. 2011). A similar study conducted by Motley et al. (2016) investigated the ability of serrawettin W2 produced by a Serratia strain to inhibit the microbial adhesion of C. albicans to a 96-microwell plate. This was conducted by incubating the test compound with the inoculum in the wells of the 96-microwell plate and determining half the inhibitory concentration (IC50) value using a tetrazolium salt (XTT) reduction assay. An IC50 value of 7.7 ± 0.7 μM for serrawettin W2 inhibited the biofilm formation of C. albicans and it was concluded that the cyclic lipodepsipeptides produced by the Serratia strain may be used to control C. albicans infections associated with biofilm modulation.
Other medical and pharmaceutical applications
Kadouri and Shanks (2013) suggested that Serratia species could serve as a potential source of antibiotics to combat multidrug-resistant opportunistic pathogens such as methicillin-resistant S. aureus (MRSA) and Staphylococcus epidermis. Despite their antimicrobial properties, some compounds such as serrawettin W1 have cytotoxic activity and are therefore unlikely to be used directly as a systemic antibiotic. In addition to antimicrobial activity, Tomas et al. (2005) filed a patent for the use of a serratamolide (serrawettin W1) produced by a S. marcescens 2170 strain as a chemotherapeutic agent. The serratamolide was found to reduce the cell viability (induce apoptosis) of various cancer cell lines (Jurkat, Molt-4, NSO, HGT-1, HT-29 and GLC-4S), while displaying no effect on non-malignant cell lines (NIH-3T3, NRK-49F and IEC-18). In addition to serratamolides (serrawettin W1), serrawettin W2 produced by S. surfactantfaciens sp. YD25T was also shown to exhibit anticancer activity by suppressing the growth of cancer cell lines (HeLa and Caco-2 cell lines), while not significantly affecting the viability of non-malignant cells (Vero and HEK293 cell lines) (Su et al. 2016). Based on this research, serrawettins have the potential to be used as anticancer chemotherapeutic agents against leukaemia, lymphoma, myeloma, carcinoma, melanoma and sarcoma (Tomas et al. 2005).
Agricultural industry
Although biosurfactants produced by Serratia species have been shown to display emulsification activity against hydrocarbons, such as diesel and kerosene (Wei et al. 2004), limited research on the use of lipopeptides and glycolipids produced by Serratia species in the food industry has been conducted. Research has however indicated that biosurfactants produced by Serratia species can be used in the agricultural industry, due to their antifungal and plant-protecting properties. A patent was filed by Strobel et al. (2005) for the use of serratamolides produced by a S. marcescens strain in the agricultural industry, due to its anti-mycotic activity against oomycete pathogens, which has the potential to be applied for crop protection. Similarly, a study by Nalini and Parthasarathi (2014) investigated the antifungal activity of a rhamnolipid produced by S. rubidaea SNAU02. The study found that the rhamnolipid exhibited antifungal activity against Fusarium oxysporum and Colletotrichum gloeosporioides (plant pathogens) at a concentration of 100 μg/mL. In addition, the rhamnolipid (at a concentration of 100 μg/mL) did not exhibit toxicity towards the seeds of Brassica oleracea and Artemia salina employed as bio-indicators. The biosurfactant produced by S. rubidaea SNAU02 thus exhibits potential as a biocontrol agent against plant pathogens (Nalini and Parthasarathi 2014). Similarly, Granada et al. (2018) investigated the use of crude extracts obtained from a Serratia strain as a biological control agent of avocado pathogens. The study identified serratamolide A, prodigiosin and haterumalide NC in the crude extract and found that the crude extract displayed high activity against the pathogens Phytophthora cinnamomi and C. gloeosporioides. It was therefore concluded that the secondary metabolites produced by this strain may be used for crop protection or to maintain plant health.
Bioremediation and petroleum industries
Chemically synthesised surfactants have been used for the bioremediation of oil-contaminated sites as well as to enhance the recovery of oil from oil reservoirs in the petroleum industry (Banat 1995). However, as synthetic surfactants are not biodegradable and can be toxic to the environment, biosurfactants provide an environmentally friendly alternative and have been shown to exhibit equivalent emulsification properties. In addition to emulsifying hydrocarbons, studies have indicated that biosurfactants produced by Serratia species have the potential for application as microbial enhanced oil recovery (MEOR) agents (Pruthi and Cameotra 2000; Ibrahim et al. 2013; Nalini and Parthasarathi 2013). MEOR is considered a tertiary recovery method that could recover the residual oil using microorganisms or their products (biosurfactants) and can be evaluated using a sandpack technique (sand previously saturated with oil in a column) (Pruthi and Cameotra 2000; Amani et al. 2010). A study by Ibrahim et al. (2013) isolated a S. marcescens strain that was found to produce a lipopeptide and investigated the effects of the lipopeptide to enhance the recovery of crude oil from a sandpack column. The lipopeptide was able to recover 76% of the crude oil from the column and was earmarked as a potential enhanced oil recovery agent. In addition, a sucrose lipid (glycolipid) produced by a S. marcescens strain was found to exhibit excellent emulsification activity against a wide range of hydrocarbons and effectively recovered 90% of residual oil from an oil-saturated sandpack column (Pruthi and Cameotra 2000). Pruthi and Cameotra (2000) further indicated that the glycolipid displayed a strong ability to recover oil from the walls of containers and therefore could possibly be applied in cleaning operations. Similarly, Nalini and Parthasarathi (2013) demonstrated that a concentration of 0.05% rhamnolipid solution produced by a S. rubidaea SNAU02 strain was able to recover 92% of used engine oil previously absorbed to a sand sample, while the commercially available surfactant (sodium dodecyl sulphate) only removed 60% of the oil from the contaminated sand sample.
Conclusions and future perspectives
The review provided insight into the classification and structure of biosurfactants produced by Serratia species that have been characterised and identified as serrawettins, stephensiolides, rubiwettins and rhamnolipids. Furthermore, the biosynthetic pathways of three lipopeptides synthesised by NRPS enzymes, including serrawettin W1, serrawettin W2 and stephensiolides, encoded by swrW, swrA and sphA, respectively, were discussed. However, further research is still required to elucidate the chemical structure of serrawettin W3 and rubiwettin R1 as well as the biosynthetic pathway of glycolipids and serrawettin W3 produced by Serratia species. While the small-scale production of biosurfactants produced by Serratia species has been extensively applied, the large-scale production and downstream recovery of these compounds still require optimisation. Furthermore, a few biosurfactants produced by Serratia species have been patented for use in industries such as the agricultural sector, due to their antifungal and plant-protecting properties, and numerous studies have demonstrated that these compounds have the potential to be applied as hydrocarbon emulsifiers for enhanced oil recovery. The potential of these compounds as antitumor agents is also being explored and while biosurfactants produced by Serratia species display antibacterial and antifungal activity, the mode of action of these compounds still needs to be extensively investigated. Biosurfactants produced by Serratia species are thus a vital source of antimicrobial, antifouling, surface active (emulsifier) and antitumor compounds and should be considered one of the major candidates for application in the medical, agricultural and petroleum industries.
References
Almansoory AF, Hasan HA, Idris M, Abdullah SRS, Anuar N (2017) Biosurfactant production by the hydrocarbon-degrading bacteria (HDB) Serratia marcescens: optimization using central composite design (CCD). J Ind Eng Chem 47:272–280. https://doi.org/10.1016/j.jiec.2016.11.043
Amani H, Sarrafzadeh MH, Haghighi M, Mehrnia MR (2010) Comparative study of biosurfactant producing bacteria in MEOR applications. J Pet Sci Eng 75(1–2):209–214. https://doi.org/10.1016/j.petrol.2010.11.008
Anyanwu CU, Obi SKC, Okolo BN (2010) Production of surface active glycolipid by Serratia marcescens NSK-1 isolated from petroleum contaminated soil. Our Nature 8(1):1–11
Aparna A, Srinikethan G, Smitha H (2012) Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf B Biointerfaces 95:23–29. https://doi.org/10.1016/j.colsurfb.2012.01.043
Banat IM (1995) Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresour Technol 51(1):1–12. https://doi.org/10.1016/0960-8524(94)00101-6
Banat IM, Franzetti A, Gandolfi I, Bestetti G, Martinotti MG, Fracchia L, Smyth TJ, Marchant R (2010) Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol 87(2):427–444. https://doi.org/10.1007/s00253-010-2589-0
Banat IM, De Rienzo MAD, Quinn GA (2014) Microbial biofilms: biosurfactants as antibiofilm agents. Appl Microbiol Biotechnol 98(24):9915–9929. https://doi.org/10.1007/s00253-014-6169-6
Bar-Ness R, Avrahamy N, Matsuyama T, Rosenberg M (1988) Increased cell surface hydrophobicity of a Serratia marcescens NS 38 mutant lacking wetting activity. J Bacteriol 170(9):4361–4364. https://doi.org/10.1128/jb.170.9.4361-4364.1988
Bidlan R, Deepthi N, Rastogi NK, Manonmani HK (2007) Optimized production of biosurfactant by Serratia marcescens DT-1P. Res J Microbiol 2:705–716. https://doi.org/10.3923/jm.2007.705.716
Bodour AA, Drees KP, Maier RM (2003) Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid southwestern soils. Appl Environ Microbiol 69(6):3280–3287. https://doi.org/10.1128/AEM.69.6.3280-3287.2003
Cameotra SS, Makkar RS (2004) Recent applications of biosurfactants as biological and immunological molecules. Curr Opin Microbiol 7(3):262–266. https://doi.org/10.1016/j.mib.2004.04.006
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61(1):47–64
Dusane DH, Pawar VS, Nancharaiah YV, Venugopalan VP, Kumar AR, Zinjarde SS (2011) Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 27(6):645–654. https://doi.org/10.1080/08927014.2011.594883
Dwivedi D, Jansen R, Molinari G, Nimtz M, Johri BN, Wray V (2008) Antimycobacterial serratamolides and diacyl peptoglucosamine derivatives from Serratia sp. J Nat Prod 71(4):637–641. https://doi.org/10.1021/np7007126
Eckelmann D, Spiteller M, Kusari S (2018) Spatial-temporal profiling of prodiginines and serratamolides produced by endophytic Serratia marcescens harbored in Maytenus serrata. Sci Rep 8(1):5283. https://doi.org/10.1038/s41598-018-23538-5
Epstein AK, Pokroy B, Seminara A, Aizenberg J (2011) Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc Natl Acad Sci U S A 108(3):995–1000. https://doi.org/10.1073/pnas.1011033108
Escobar-Díaz E, Lopez-Martin EM, Del Cerro MH, Puig-Kroger A, Soto-Cerrato V, Montaner B, Giralt E, García-Marco JA, Perez-Tomas R, García-Pardo A (2005) AT514, a cyclic depsipeptide from Serratia marcescens, induces apoptosis of B-chronic lymphocytic leukemia cells: interference with the Akt/NF-κB survival pathway. Leukemia 19(4):572–579. https://doi.org/10.1038/sj.leu.2403679
Fakruddin (2012) Biosurfactant: production and application. J Pet Environ Biotechnol 3:4. https://doi.org/10.4172/2157-7463.1000124
Fiechter A (1992) Biosurfactants: moving towards industrial application. Trends Biotechnol 10:208–217. https://doi.org/10.1016/0167-7799(92)90215-H
Foulds J (1972) Purification and partial characterization of a bacteriocin from Serratia marcescens. J Bacteriol 110(3):1001–1009
Fracchia L, Cavallo M, Martinotti MG, Banat IM (2012) Biosurfactants and bioemulsifiers biomedical and related applications–present status and future potentials. Biomed Sci, Eng Technol InTech. https://doi.org/10.1002/cbic.201800124
Ganley J, Carr G, Ioerger T, Sacchettini J, Clardy J, Derbyshire E (2018) Discovery of antimicrobial lipodepsipeptides produced by a Serratia sp. within mosquito microbiomes. Chembiochem 19:1590–1594. https://doi.org/10.1002/cbic.201800124
Geetha SJ, Banat IM, Joshi SJ (2018) Biosurfactants: production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal Agric Biotechnol 14:23–32. https://doi.org/10.1016/j.bcab.2018.01.010
Gerc AJ, Song L, Challis GL, Stanley-Wall NR, Coulthurst SJ (2012) The insect pathogen Serratia marcescens Db10 uses a hybrid non-ribosomal peptide synthetase-polyketide synthase to produce the antibiotic althiomycin. PLoS One 7(9):e44673. https://doi.org/10.1371/journal.pone.0044673
Granada SD, Ramírez-Restrepo S, López-Luján L, Peláez-Jaramillo CA, Bedoya-Pérez JC (2018) Screening of a biological control bacterium to fight avocado diseases: from agroecosystem to bioreactor. Biocatal Agric Biotechnol 14:109–115. https://doi.org/10.1016/j.bcab.2018.02.005
Gudiña EJ, Rocha V, Teixeira JA, Rodrigues LR (2010) Antimicrobial and antiadhesive properties of a biosurfactant isolated from Lactobacillus paracasei ssp. paracasei A20. Lett Appl Microbiol 50(4):419–424. https://doi.org/10.1111/j.1472-765X.2010.02818.x
Hage-Hülsmann J, Grünberger A, Thies S, Santiago-Schübel B, Klein AS, Pietruszka J, Binder D, Hilgers F, Domröse A, Drepper T, Kohlheyer D (2018) Natural biocide cocktails: combinatorial antibiotic effects of prodigiosin and biosurfactants. PLoS One 13(7):e0200940. https://doi.org/10.1371/journal.pone.0200940
Ibrahim ML, Ijah UJJ, Manga SB, Bilbis LS, Umar S (2013) Production and partial characterization of biosurfactant produced by crude oil degrading bacteria. Int Biodeterior Biodegradation 81:28–34. https://doi.org/10.1016/j.ibiod.2012.11.012
Kadouri DE, Shanks RM (2013) Identification of a methicillin-resistant Staphylococcus aureus inhibitory compound isolated from Serratia marcescens. Res Microbiol 164(8):821–826. https://doi.org/10.1016/j.resmic.2013.06.002
Khanna A, Khanna M, Aggarwal A (2013) Serratia marcescens—a rare opportunistic nosocomial pathogen and measures to limit its spread in hospitalized patients. J Clin Diagn Res 7(2):243. https://doi.org/10.7860/JCDR/2013/5010.2737
Li H, Tanikawa T, Sato Y, Nakagawa Y, Matsuyama T (2005) Serratia marcescens gene required for surfactant serrawettin W1 production encodes putative aminolipid synthetase belonging to nonribosomal peptide synthetase family. Microbiol Immunol 49(4):303–310. https://doi.org/10.1111/j.1348-0421.2005.tb03734.x
Lindum PW, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M (1998) N-Acyl-L-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J Bacteriol 180(23):6384–6388
Luna M, García S, García O, Trigos Á (2013) Serratin a new metabolite obtained from Serratia marcescens, a bacterium isolated from the microflora associated with banana plantations. Nat Prod Res 27(1):49–53. https://doi.org/10.1080/14786419.2011.650638
Marahiel MA, Stachelhaus T, Mootz HD (1997) Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev 97(7):2651–2674. https://doi.org/10.1021/cr960029e
Matsuyama T, Nakagawa Y (1996) Bacterial wetting agents working in colonization of bacteria on surface environments. Colloids Surf B Biointerfaces 7(5–6):207–214. https://doi.org/10.1016/0927-7765(96)01300-8
Matsuyama T, Fujita M, Yano I (1985) Wetting agent produced by Serratia marcescens. FEMS Microbiol Lett 28(1):125–129. https://doi.org/10.1111/j.1574-6968.1985.tb00777.x
Matsuyama T, Murakami T, Fujita M, Fujita S, Yano I (1986) Extracellular vesicle formation and biosurfactant production by Serratia marcescens. Microbiol 132(4):865–875. https://doi.org/10.1099/00221287-132-4-865
Matsuyama T, Kaneda K, Ishizuka I, Toida T, Yano I (1990) Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J Bacteriol 172(6):3015–3022. https://doi.org/10.1128/jb.172.6.3015-3022.1990
Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I (1992) A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol 174(6):1769–1776. https://doi.org/10.1128/jb.174.6.1769-1776.1992
Matsuyama T, Tanikawa T, Nakagawa Y (2011) Serrawettins and other surfactants produced by Serratia. In: Soberón-Chávez G (ed) Biosurfactants. Microbiology monographs, vol 20. Springer, Berlin, Heidelberg, pp 93–120
McLandsborough L, Rodriguez A, Pérez-Conesa D, Weiss J (2006) Biofilms: at the interface between biophysics and microbiology. Food Biophys 1(2):94–114. https://doi.org/10.1007/s11483-005-9004-x
Mnif I, Ghribi D (2015) Review lipopeptides biosurfactants: mean classes and new insights for industrial, biomedical, and environmental applications. Pept Sci 104(3):129–147. https://doi.org/10.1002/bip.22630
Motley JL, Stamps BW, Mitchell CA, Thompson AT, Cross J, You J, Powell DR, Stevenson BS, Cichewicz RH (2016) Opportunistic sampling of roadkill as an entry point to accessing natural products assembled by bacteria associated with non-anthropoidal mammalian microbiomes. J Nat Prod 80(3):598–608. https://doi.org/10.1021/acs.jnatprod.6b00772
Nalini S, Parthasarathi R (2013) Biosurfactant production by Serratia rubidaea SNAU02 isolated from hydrocarbon contaminated soil and its physico-chemical characterization. Bioresour Technol 147:619–622. https://doi.org/10.1016/j.biortech.2013.08.041
Nalini S, Parthasarathi R (2014) Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application as biocontrol agent. Bioresour Technol 173:231–238. https://doi.org/10.1016/j.biortech.2014.09.051
Ndlovu T, Khan S, Khan W (2016) Distribution and diversity of biosurfactant-producing bacteria in a wastewater treatment plant. Environ Sci Pollut Res Int 23(10):9993–10004. https://doi.org/10.1007/s11356-016-6249-5
Ndlovu T, Rautenbach M, Vosloo JA, Khan S, Khan W (2017) Characterisation and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 7(1):108. https://doi.org/10.1186/s13568-017-0363-8
Nitschke M, Silva SSE (2018) Recent food applications of microbial surfactants. Crit Rev Food Sci Nutr 58(4):631–638. https://doi.org/10.1080/10408398.2016.1208635
Peele KA, Ch VRT, Kodali VP (2016) Emulsifying activity of a biosurfactant produced by a marine bacterium. 3 Biotech 6(2):177. https://doi.org/10.1007/s13205-016-0494-7
Pruthi V, Cameotra SS (2000) Novel sucrose lipid produced by Serratia marcescens and its application in enhanced oil recovery. J Surfactant Deterg 3(4):533–537. https://doi.org/10.1007/s11743-000-0153-9
Rahman PK, Gakpe E (2008) Production, characterisation and applications of biosurfactants—review. Biotechnol 7:360–370. https://doi.org/10.3923/biotech.2008.360.370
Roldán-Carrillo T, Martínez-García X, Zapata-Penasco I, Castorena-Cortés G, Reyes-Avila J, Mayol-Castillo M, Olguín-Lora P (2011) Evaluation of the effect of nutrient ratios on biosurfactant production by Serratia marcescens using a Box-Behnken design. Colloids Surf B Biointerfaces 86(2):384–389. https://doi.org/10.1016/j.colsurfb.2011.04.026
Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants: minireview. Environ Microbiol 3(4):229–236. https://doi.org/10.1046/j.1462-2920.2001.00190.x
Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA (2016) Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci 17(3):401. https://doi.org/10.3390/ijms17030401
Satpute SK, Banpurkar AG, Dhakephalkar PK, Banat IM, Chopade BA (2010) Methods for investigating biosurfactants and bioemulsifiers: a review. Crit Rev Biotechnol 30(2):127–144. https://doi.org/10.3109/07388550903427280
Shekhar S, Sundaramanickam A, Balasubramanian T (2015) Biosurfactant producing microbes and their potential applications: a review. Crit Rev Environ Sci Technol 45(14):1522–1554. https://doi.org/10.1080/10643389.2014.955631
Soberón-Chávez G, Maier RM (2011) Biosurfactants: a general overview. In: Soberón-Chávez G (ed) Biosurfactants. Microbiology monographs, vol 20. Springer, Berlin, Heidelberg, pp 1–11
Srobel G, Li JY, Sugawara F, Koshino H, Harper J, Hess WM (1999) Oocydin A, a chlorinated macrocyclic lactone with potent anti-oomycete activity from Serratia marcescens. Microbiol 145(12):3557–3564. https://doi.org/10.1099/00221287-145-12-3557
Stankovic N, Senerovic L, Ilic-Tomic T, Vasiljevic B, Nikodinovic-Runic J (2014) Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Appl Microbiol Biotechnol 98(9):3841–3858. https://doi.org/10.1007/s00253-014-5590-1
Strobel GA, Morrison SL, Cassella M, HMV Corp (2005) Protecting plants from oomycete pathogens by treatment with compositions containing serratamolide and oocydin A from Serratia marcescens. Patent number: 6926892
Su C, Xiang Z, Liu Y, Zhao X, Sun Y, Li Z, Li L, Chang F, Chen T, Wen X, Zhou Y (2016) Analysis of the genomic sequences and metabolites of Serratia surfactantfaciens sp. nov. YD25T that simultaneously produces prodigiosin and serrawettin W2. BMC Genomics 17(1):865. https://doi.org/10.1186/s12864-016-3171-7
Sunaga S, Li H, Sato Y, Nakagawa Y, Matsuyama T (2004) Identification and characterization of the pswP gene required for the parallel production of prodigiosin and serrawettin W1 in Serratia marcescens. Microbiol Immunol 48(10):723–728. https://doi.org/10.1111/j.1348-0421.2004.tb03597.x
Tomas RP, Ramoneda BM, Lledo EG, Pedemonte MM, Casas MV (2005) Use of cyclic depsipeptide as a chemotherapeutic agent against cancer. Patent Number: EP1553080
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol 232:389–397. https://doi.org/10.1016/j.biortech.2017.02.047
Walter V, Syldatk C, Hausmann R (2010) Screening concepts for the isolation of biosurfactant producing microorganisms. In: Sen R (ed) Biosurfactants. Advances in experimental medicine and biology, vol 672. Springer, New York, NY, pp 1–13
Wasserman HH, Keggi JJ, McKeon JE (1961) Serratamolide, a metabolic product of Serratia. J Am Chem Soc 83(19):4107–4108. https://doi.org/10.1021/ja01480a046
Wei YH, Lai HC, Chen SY, Yeh MS, Chang JS (2004) Biosurfactant production by Serratia marcescens SS-1 and its isogenic strain SMΔR defective in SpnR, a quorum-sensing LuxR family protein. Biotechnol Lett 26(10):799–802. https://doi.org/10.1023/B:BILE.0000025881.95596.23
Wilf NM, Salmond GP (2012) The stationary phase sigma factor, RpoS, regulates the production of a carbapenem antibiotic, a bioactive prodigiosin and virulence in the enterobacterial pathogen Serratia sp. ATCC 39006. Microbiol 158(3):648–658. https://doi.org/10.1099/mic.0.055780-0
Zeraik AE, Nitschke M (2010) Biosurfactants as agents to reduce adhesion of pathogenic bacteria to polystyrene surfaces: effect of temperature and hydrophobicity. Curr Microbiol 61(6):554–559. https://doi.org/10.1007/s00284-010-9652-z
Zhang L, Sun JA, Hao Y, Zhu J, Chu J, Wei D, Shen Y (2010) Microbial production of 2, 3-butanediol by a surfactant (serrawettin)-deficient mutant of Serratia marcescens H30. J Ind Microbiol Biotechnol 37(8):857–862. https://doi.org/10.1007/s10295-010-0733-6
Zhang X, Xu D, Zhu C, Lundaa T, Scherr KE (2012) Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa strains. Chem Eng J 209:138–146. https://doi.org/10.1016/j.cej.2012.07.110
Zhu L, Pang C, Chen L, Zhu X (2018) Antibacterial activity of a novel depsipeptide and prodigiosine of Serratia marcescens S823. Nat Prod Chem Res 6(2):312. https://doi.org/10.4172/2329-6836.1000312
Funding
The authors thank the financial support provided by the Water Research Commission under Grant K5/2728//3 (WRC project) and the National Research Foundation of South Africa (Grant Number: 113849) for funding. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Clements, T., Ndlovu, T., Khan, S. et al. Biosurfactants produced by Serratia species: Classification, biosynthesis, production and application. Appl Microbiol Biotechnol 103, 589–602 (2019). https://doi.org/10.1007/s00253-018-9520-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9520-5