Abstract
A possible approach to enhance the performance of microbial electrochemical system such as microbial fuel cells is to increase the conductivity of catalytic biofilms and thereby the direct extracellular electron transfer within the biofilms and from the electrode. In the present study, we evaluated the impact of static low-intensity magnetic field on the anodic biofilms in microbial fuel cells (MFCs). Results demonstrated that the application of a low-intensity magnetic field (105 and 150 mT) can significantly shorten the startup time and enhance the overall performance of single-chamber MFCs in terms of current density (300%) and power density (150%). In situ conductance evaluation indicated that short-term application of magnetic field can increase biofilm conductivity, although the long-term enhancements were likely results of increased conductivity of the anodic biofilms associated with enriched population of Geobacteraceae. The peak-manner response of conductivity over gate potentials and the positive response of mature biofilm conductance to low intensity of magnetic field support the redox conduction model of the conductive exoelectrogenic biofilms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial electrochemical technologies use exoelectrogens on anode to convert the chemical energy stored in reduced substances to electricity, hydrogen, and other products. They have the potential to be used for wastewater treatment, bioenergy production, bioremediation, and biosensoring (Logan 2009). However, low current output has prevented this technology from practical applications (Logan 2009; Malvankar et al. 2012c). Many previous studies have attempted to enhance the power/current production of MFCs through developing novel electrode materials and improving reactor designs (Fan et al. 2011). An alternative is to enhance the unique ability of anodic exoelectrogenic microbial communities in directly transferring extracellular electrons to electrode (Leang et al. 2013).
Critical to direct extracellular electron transfer (DEET) to electrode is the establishment of electrical connections facilitated through conductivity of anodic exoelectrogenic biofilms (Lovley 2011a, b). The detailed conduction mechanisms of anodic exoelectrogenic biofilms are still under investigation and diverged into two distinct models: the redox conductivity model (Phan et al. 2016; Yates et al. 2016b) and the metallic-like conductivity model (Malvankar et al. 2012b, 2015, 2011). Despite the controversy of conduction mechanisms, conductivity of anodic exoelectrogenic biofilms supports the long distance electron transfer beyond the molecular scale and conserves energy during electron transfer, and therefore is critical to the ability of anodic exoelectrogenic microbial communities in directly transferring extracellular electrons to electrode (Li et al. 2017; Lovley 2011a, b).
Previous studies have demonstrated that the conductivity of anodic exoelectrogenic biofilms directly correlates their ability to produce current in microbial fuel cells (MFCs) and Geobacteraceae remain to be the major exoelectrogens within the communities of these biofilms (Lee et al. 2016; Malvankar et al. 2012c). Increasing the conductivity of anodic exoelectrogenic biofilms through manipulating of the pilus expression of Geobacter sulfurreducens (Leang et al. 2013) has been suggested as effective approaches to increase the power/current production of MFCs using pure cultures. However, low-cost strategies with less environmental impact are preferred for practical applications of MFCs, especially those using mixed cultures.
Recently, static low-intensity magnetic field (SLIMF) had been applied to the anode side of MFCs and demonstrated enhancement on power/current output of MFCs (Li et al. 2011; Tao and Zhou 2014; Yin et al. 2013; Zhao et al. 2016; Zhou et al. 2017). It has been hypothesized that the observed enhancement of current production was a result of biological effects of SLIMF had on the anodic exoelectrogenic community including stimulating enzyme activity (Zhao et al. 2016) and enhancing oxidation stress (Yin et al. 2013). While it is a well-known phenomenon that the resistivity of conductive materials can vary in the presence of magnetic field (Nalwa 2001), the impact of SLIMF on the conductivity of mixed-species anodic biofilms has not been investigated.
In the present study, we first investigated the magnitude and persistency of the impact of SLIMF on startup time and overall performance of single-chamber MFCs with carbon cloth anode. Changes of conductance were then examined over time with SLIMF applied from the inoculation and with SLIMF applied to mature biofilms at different intensities of magnetic field by using a split anode design adapted from previous studies (Li et al. 2016b; Malvankar et al. 2011, 2012a).
Materials and methods
MFC design and operation
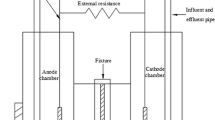
Single-chamber MFCs (total volume of 12 mL) were constructed according to the design of previous studies (Fan et al. 2007). Carbon cloth (type B, fuelcellearth.com) with a projected surface area of 3 cm2 was used as the anode of the MFCs (labeled as CCA-MFC) to evaluate the effect of magnetic field on the performance of anodic biofilms. Gold foil sheet (projected surface area of 7 cm2, Alfa Aesar, Haverhill, MA, USA) was used as the anode of the MFCs (labeled as GA-MFC) to evaluate the effect of SLIMF on the conductivity of anodic biofilms. A nonconductive gap in the middle of the gold electrode was created according to our previous studies (Li et al. 2016a, b). Carbon cloth/activated carbon air cathode (projected surface area of 7 cm2) was fabricated following a previously developed protocol (Janicek et al. 2015) and used in both the CCA-MFCs and GA-MFCs. Magnets (2.54 cm diameter, K&J Magnetics, Inc. Pipersville, PA, USA) were applied to the anode side of the MFCs (0.2 cm away from anode (Fig. 1a, b)) to create SLIMF with intensities of 105 and 150 mT at the surface of anodes. The intensity of magnetic field was determined using a Gauss meter (7010, Sypris Solution, Inc. Louisville, KY, USA). Control CCA-MFCs and GA-MFCs were constructed but were not subjected to the impact of SLIMF. Magnets were applied to the magnetic CCA-MFCs right after the inoculation of a mixed exoelectrogenic culture. After 100 days of operation, the magnets were removed from the magnetic CCA-MFCs to investigate the persistency of the SLIMF effects when the current production of CCA-MFCs reached plateau. The GA-MFCs were operated in two ways to evaluate the effects of SLIMF on the conductance of anodic biofilms at different growth stages: (1) applying magnets right after the inoculation and (2) applying magnets after power production became stable and biofilms were mature.
A lab-maintained active MFC culture was used as inoculum for all the MFCs. The culture was originally enriched from active sludge collected from the Corvallis Wastewater Treatment Plant (Corvallis, OR). This anodic exoelectrogenic community has been demonstrated to produce one of the highest reported power densities (Fan et al. 2007). Acetate (30 mM) was used as the electron donor during the startup of MFCs and the concentration increased to 60 mM after power outputs became stable. Modified Geobacter medium (MGM) (pH 7) was used in all experiments (Fan et al. 2012). The medium consists of the following ingredients (per liter): KCl, 0.13 g; NH4Cl, 0.31 g; NaH2PO4·H2O, 5.84 g; Na2HPO4·7H2O, 15.5 g; vitamin, 12.5 mL; and mineral 12.5-mL solution as previously reported. MFCs were operated in fed-batch mode with external resistance decreased from 10,000 to 75 Ω for CCA-MFCs and from 10,000 to 500 Ω for GA-MFCs between batches as the biofilms grew in order to maintain maximum cell voltages around 0.3 V. When voltages were under 10% of batch maximum, the media was removed and replaced with new media. When power/current production of all CCA-MFCs became stable (after day 14), polarization curves were made by changing external resistance from 1000 to 33 Ω with interval time of 20 min. Power/current density was calculated by normalizing to the surface area of anode. To evaluate the impact of SLIMF on charge transfer resistance (Rct) in anodic biofilms, electrochemical impedance spectroscopy (EIS) analysis was performed as described previously (Malvankar et al. 2012a).
In situ measurement of biofilm conductance
Two-probe method adapted from previous studies (Li et al. 2016a, b; Malvankar et al. 2011) was used to evaluate the in situ anodic biofilm conductance of MFCs during growth period at open circuit potential (OCP). GA-MFC anodes were temporarily disconnected from the cathode and allowed OCP (− 470 mV vs. Ag/AgCl) to be reached. Then, a voltage bias (Vapp) was straddled between two halves of a split anode (0, 25, and 50 mV) in steps of 25 mV by using a source meter (Model 2405, Keithley, USA). For each voltage step, transient ionic current was allowed to decline until a steady state was reached. Conducting current flowing between the two halves of split anode was then recorded every 30 s over a period of 3 min by using the same source meter. Biofilm resistance was calculated by plotting Vapp against average measured current. Conductance was then calculated from the inverse of resistance. Measurements were taken approximately twice a week during MFC operation. Measurements of conductance conducted at OCP were performed in duplicate reactors.
Electrochemical gating analysis
To further examine the conductive changes of the anodic mixed-species biofilms under the impact of SLIMF, electrochemical gating analysis, which measures biofilm conductance as a function of redox potential, was performed based on the three-electrode configuration described previously (Li et al. 2016b; Malvankar et al. 2012b). A potentiostat (References 100, Gamry Instruments Inc., Warminster, PA) was used to apply a series of gate potentials (Vg) from − 500 to − 300 mV with increments of 50 mV (vs. Ag/AgCl) (Li et al. 2016b). Concurrent with the setting of Vg, a source meter (Model 2405, Keithley, USA) was used to apply voltages (Vapp) between the source and drain anode. The conducting currents at various Vapp (0, 25, and 50 mV) were measured and used to calculate resistance from the slope of the voltage-current curve.
Electrochemical gating analysis was also conducted to investigate the conductive changes of the mixed-species exoelectrogenic biofilms in the absence of substrate as described previously (Li et al. 2016b). To remove substrate, acetate-containing growth media was replaced with MGM containing no acetate. Cell voltages of the MFCs dropped below 0.001 V within 24 h following acetate removal. Liquid samples were also collected and analyzed using high-performance liquid chromatography (HPLC) to confirm that acetate was completely removed following medium replacement. To prevent potential of damage to biofilms, all electrochemical gating analysis was limited with 48 h and deoxygenated media were used. Experiments of electrochemical gating analysis were conducted using duplicate reactors with six replicates.
DNA extraction and microbial community analysis of anodic biofilms
Following electrochemical gating analysis (day 80), duplicate biofilms from both magnetic and control GA-MFCs were collected for DNA extraction. DNA extraction was performed using the MoBio PowerBiofilm DNA Isolation Kit (Carlsbad, CA) following the protocol suggested by the manufacture. The quality of the DNA extraction was checked on an agarose gel and further verified through use of spectrophotometer (NanoDrop, Wilmington, DE, USA).
DNA from both magnetic and control GA-MFCs was amplified with primers containing a linker sequence, an 8-bp index sequence, and universal primers designed to amplify the 16S rRNA gene V3-V4 region (Fadrosh et al. 2014). Amplicon pools were purified/cleaned using AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA). An Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, US) was used to check the size and quality of the amplicon library. The amplicon library was sequenced together using standard Illumina sequencing primers for a 250-bp paired-end run (v3) on the MiSeq platform (Illumina, San Diego, CA, US). Image analysis, base calling, and data quality assessment were performed on the MiSeq instrument.
QIIME (version 2.7.0) was used to process raw sequencing result. Samples were demultiplexed and the 8-bp barcode sequences were removed. Sequence reads that did not have an average phred quality of 20 were filtered out for initial quality pre-processing. Taxonomic assignment was conducted using Ribsomal Database Project (RDP) Naïve Bayesian Classifier on Greengene database. Sequences of 13 identified representative taxa (> 1.0% abundance) were deposited in the NCBI sequence read archive under the following accession number MF497868-MF497875 and MG438546-MG438547. Rarefaction curve was also generated using QIIME.
Statistical analysis
Single-factor analysis of variance (ANOVA) was also performed by using data analysis package in Microsoft Excel and numbers were considered statistically different when P < 0.05.
Results
Effects of magnetic field on MFC performance
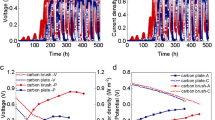
In the present study, we focused our evaluation on the impact of magnetic field on the performance of anodic biofilms, which can be reflected by the startup time of MFCs, the anodic current density, and the persistency of the impact. To reduce the cathode limitation, an MFC configuration with a small anode to cathode projected surface area ratio (0.4) was used. Increases in power density over time were observed for all CCA-MFC reactors during startup period (Fig. 2a). While CCA-MFC reactors amended with 105 and 150 mT SLIMF generated more than 3 W/m2 power density (normalized to anodic surface area) in less than 8 days, the power density generated by the control CCA-MFC reactors was near 1 W/m2 after 12 days even similar approach (maintaining output voltage of 0.3 V) was used to operate MFCs. Polarization experiment was conducted at day 14 when power production became relatively stable. The average maximum current densities of 105 and 150 mT CCA-MFCs were 18.4 ± 1.0 and 20.1 ± 0.4 A/m2, respectively, which were significantly greater than the control CCA-MFCs (4.8 ± 0.2 A/m2) (P < 0.05) (Fig. 2b). The average maximum power densities of 105 and 150 mT CCA-MFCs were also greater than that of control CCA-MFCs (4.31 ± 0.16 and 4.56 ± 0.07 W/m2 compared to 1.90 ± 0.45 W/m2) (P < 0.05) (Fig. 2b).
After the current/power production of CCA-MFCs became stable (after day 14), the anodic charge transfer resistance was analyzed using electrochemical impedance spectroscopy and integrated by using a model that has been established previously (Malvankar et al. 2012a). Anodic Rct of the control CCA-MFCs was 322.1 ± 74.6 Ω, which was much higher than the anodic Rct of the magnetic CCA-MFCs (110.8 ± 18.9 Ω) (Fig. S1), indicating an improvement of extracellular electron transfer ability of the anodic biofilms in the magnetic CCA-MFCs (Malvankar et al. 2012a). No significant difference was observed between the Rct of CCA-MFC reactors amended with 105 and 150 mT SLIMF (P > 0.05).
To evaluate the persistency of magnetic effects on MFC performance after current/power production reached plateau, magnets were removed from the magnetic CCA-MFCs after 100 days of operation. Removal of SLIMF did not significantly affect the power production (P < 0.05, Fig. 3). The difference in peak power density around day 100 compared with the startup period was likely due to the aging effect of cathode (Zhang et al. 2014).
Effects of magnetic field on the conductivity of anodic biofilms
Conductivity of anodic biofilms that facilitates the DEET from exoelectrogenic biofilms to anode is one of the most critical parameters of anodic biofilms in high-performance MFCs (Lovley 2011a, b). In the present study, we examined both long- and short-term effects of SLIMF on the anodic biofilm conductivities. In the experiment evaluating the long-term effects of SLIMF, magnets of 105 and 150 mT were applied to GA-MFCs starting from the inoculation. The changes of biofilm conductivity were monitored overtime. The conductance increased as biofilms grew across non-conductive gaps in both control and magnetic GA-MFCs (Fig. 4a). The conductance of anodic biofilms in the magnetic GA-MFCs became higher than the control GA-MFCs starting from day 12. After the conductance plateaued, conductance was then further analyzed over a range of potentials. Results demonstrated that the conductivity of anodic biofilms in both magnetic and control GA-MFCs changed in a peak-manner based on gate potential (Vg) (Fig. 4b). The peak conductance of the anodic biofilms was 1516.1 ± 23.0 μS at Vg of − 400 mV (vs. Ag/AgCl) in the magnetic GA-MFCs, which was approximately 150% higher than that in control GA-MFCs (613.3 ± 1.0 μS). No significant difference of conductance can be observed between the GA-MFCs with 105 and 150 mT SLIMF (P > 0.05).
a Conductances of biofilms in magnetic and control GA-MFC reactors over times. Error bars represent the standard deviation (n = 2). b Electrochemical gating analysis of anodic biofilms in magnetic and control GA-MFC reactors. Error bars represent the standard deviation of 24 replicates in 4 biofilms exposed to magnetic fields with intensity of both 105 and 150 mT
In the experiment evaluating the short-term effects of SLIMF, magnets of 105 and 150 mT were applied to the GA-MFCs containing mature biofilms that had not been affected by magnetic field prior. Electrochemical gating analysis demonstrated that the peak conductivity of mature anodic biofilms changed positively upon the application of SLIMF. The highest conductance (680.0 ± 1.3 μS) was observed at the field intensity of 150 mT followed by 655.3 ± 4.6 μS at the intensity of 105 mT (Fig. 5a). Although these conductances were 10 and 6% higher than that of the control (613.3 μS), they were much lower than the 1516.1 μS observed in the biofilms with SLIMF applied from the startup of MFCs. In the absence of electron donor (acetate), the conductance still increased with the increase of SLIMF intensity, but the conductance (less than 130 μS) of all biofilms was much lower than that in the presence of electron donors (Fig. 5b). The differences in biofilm conductance may reflect changes of electron accumulation within the biofilm, as has been suggested previously (Li et al. 2016b; Liu and Bond 2012).
Electrochemical gating analysis of anodic biofilms in control GA-MFC reactors under different intensities of SLIMF: a with substrate and b without substrate. The conductance measurements were proceeded in two independent biofilms and with 6 replicates for each biofilms. All biofilms behaved in a similar pattern upon applications of magnetic field. Error bar represents the standard deviation of 6 replicates in one biofilm
Effect of magnetic field on microbial community of anodic biofilms
To evaluate the effect of SLIMF on the community structures of the anodic biofilms, microbial communities on the anodes of both magnetic and control GA-MFCs were characterized after 80 days of operation. Number of observed OTUs and Chao1 estimator were used to examine and compare the abundance and diversity of both communities (Fig. 6). MiSeq yielded over 250,000 and 200,000 high-quality sequences for microbial communities from magnetic GA-MFC and control MFC, respectively. Chao1 alpha diversity analysis indicated that anodic community of magnetic GA-MFC affected by the SLIMF from the inoculation had a higher richness of species and diversity. Though the numbers of observed OTUs were not plateaued, 2563 observed OTUs could be assigned from the sequences from anodic community of magnetic GA-MFC, which is 14.7% more than the OTUs from anodic community of control MFC (2234 OTUs). Phylogenetic comparison was also performed between two anodic communities by assigning qualified OTUs to known taxonomic level (Fig. 7a, b). At the order level, 12 bacterial orders were identified with over 1.0% abundance in each community. Desulfuromonadales accounted for the most dominant order in both communities with abundances of 32.1 and 21.5% for magnetic and control GA-MFCs, respectively. Though orders like Clostridales and Synergistales also showed differences of 5–7% between the two communities, the dominant orders were similar. At the family level, 20 families with over 1.0% abundance were identified in control MFCs and 10 families with over 1.0% abundance were identified in magnetic MFCs. Geobacteraceae, well known as the core exoelectrogenic species (Lesnik and Liu 2014), was more abundant in magnetic MFCs (32.1%) than control MFCs (21.5%). A recent MFC study that applied pulse electromagnetic fields on magnetite polyaniline modified anodes also have a similar observation of enriched Geobacteraceae (Zhou et al. 2017). Porphyromonadaceae and Aminiphilaceae, as the potential critical players in the syntrophic interaction within the anodic community, accounted for 10.1 and 8.9%, respectively, in magnetic MFCs and 8.0 and 3.8%, respectively, in control MFCs. All other families detected in control MFC were also identified in anodic communities of magnetic MFCs with less than 1.0% difference in terms of relative abundance.
Discussion
Application of magnetic field enhanced the performance of anodic biofilms
SLIMF has been suggested as a beneficial factor to overall performance of MFCs (Li et al. 2011; Tao and Zhou 2014; Yin et al. 2013; Zhao et al. 2016). The reported enhanced current densities in these studies ranged from 0.005 to 8.5 A/m2, corresponding to a current increase of 10–50% (compared to control MFCs). In the present study, we observed a much greater increase in current (over 300%) to even higher current densities (18 to 20 A/m2). Compared to results from previous studies, the higher power production in this study was likely a result of using single-chamber MFCs with smaller anodes (to reduce anode limitation) and the use of mixed culture as the inoculum. Many of the previous studies involving SLIMF utilized reactor designs containing two chambers systems and pure culture of Shewanella oneidensis as inoculum, which have been suggested to be less pragmatic in MFCs aiming for harvest of power (Fan et al. 2007; Logan 2009). Overall, the significant enhancement in the present study suggests that the SLIMF impact can be more significant than previous observation and this enhancement can be extended to a higher current density range, indicating the potential of this approach for practical application.
In addition, in the present study, the enhanced performance of MFCs appeared to be irreversible when current and power production reached plateau (100-day operation), in contrast with previous observations (Li et al. 2011; Tao and Zhou 2014; Zhou et al. 2017). While the impact of SLIMF on MFC may not be limited to the anode of the MFCs, previous studies have demonstrated that the effects of SLIMF on cathodic and solution resistance were insignificant compared to its impact on anodic resistance (Yin et al. 2013). In addition, the magnetic field at the cathode surface was less than 20% of the intensity on the anode surface in this study. Therefore, it is reasonable to believe that the enhanced performance of MFCs was mainly due to the impact of SLIMF on anodic biofilms.
Application of magnetic field increased the conductivity of anodic biofilm
Results of both long-term and short-term effects suggest that the SLIMF can increase the conductivity of anodic biofilms. The peak-manner response of conductivity over gate potentials confirmed our previous observation that the mixed-species anodic biofilms utilize redox conduction as the major conductive mechanism (Li et al. 2016b). The model of redox conductivity describes the conduction network within anodic exoelectrogenic biofilms as a matrix composed by localized reduced and oxidized redox cofactors, in which electrons transfer through via multi-step hopping process (Li et al. 2016b; Phan et al. 2016; Snider et al. 2012; Yates et al. 2016a, b). Compared to the long-term effect, the short-term effect of SLIMF was less significant. The slightly increased conductivities might be due to the change of intrinsic property of the anodic biofilms as a conductive material.
The impact of magnetic field intensity on biofilm conductivity in this study may offer a new angle to further investigate the conduction mechanism in conductive anodic biofilms, which differs from previous methods based on temperature dependency of conductance (Phan et al. 2016; Yates et al. 2016a) and double potential step chronoamperometry (Zhang et al. 2017). The increase of conductance in anodic biofilms when being exposed to SLIMF in short period of time resembles a conductive behavior of thin polymer films called “negative magnetoresistance (NMR)”, which describes the resistivity of a conductive material decrease as the magnetic field strength increases (Hu and Wu 2007). This phenomenon has not been observed in conductive biofilms before. The short-term effects of SLIMF to conductive biofilms would more possibly be associated with changes that have faster response, such as changes in the intrinsic properties of the conduction network rather than community change. The causes of NMR in conductive materials could be various, from reducing the shrinkage of wave function to decreasing the activation energy of hopping (Raikh et al. 1992). The model of metallic-like conductivity suggests that conductivity of anodic exoelectrogenic biofilms is conferred by the special type of pilus filaments produced by Geobacteraceae which possess π-π delocalized electronic state similar to organic metal polymer such as polyaniline polymers (Holmes et al. 2016; Malvankar et al. 2012b, 2015, 2011; Tan et al. 2016; Vargas et al. 2013). This NMR-like behavior of anodic biofilms in the present study is distinct from the conductive behavior of polyaniline polymers, which has been used as an analog to the metallic-like conductivity model of Geobacter pili (Malvankar et al. 2011). Polyaniline polymers display positive magnetoresistance (the resistivity of a conductive material increases as the field strength increases) in the presence of magnetic fields with similar intensity to SLIMF in the present study (Gu et al. 2013, 2014).
Application of magnetic field enriched Geobacteraceae
The positive correlation between the population of Geobacteraceae and conductivity of microbial aggregates has been observed in both biofilms of phylogenetically distinct communities originated from the same inoculum (Li et al. 2018) and methanogenic granules treating with brewery wastewater (Shrestha et al. 2014). When the SLIMF was applied, the observed increase in conductivities of anodic biofilms may positively relate to the enriched population of Geobacteraceae in a feedback loop in which the short-term increase of conductivity permits the Geobacteraceae to conserve more energy during electron transfer to the anode and the prosperous population of Geobacteraceae further encourages the construction of a more conductive biofilm. It has been suggested that certain exoelectrogens in the family of Geobacteraceae and Shewanellaceae may possess the ability to produce and utilize extracellular magnetite particles as a pathway for anaerobic respiration (Lovley et al. 1987; Vali et al. 2004). It is also possible that these species may response to the application of SLIMF more sensitively, as increased production of soluble electron shuttles in MFCs has been suspected to be the major response to magnetic field (Yin et al. 2013). Although the ability of Geobacteraceae to perform DEET was thought to be independent from the utilization of redox cofactors (Bond and Lovley 2003; Malvankar et al. 2012b), a recent study indicates that G. sulfurreducens can secrete and utilize flavins as a bound redox cofactor along with outer membrane c-type cytochromes to facilitate DEET (Okamoto et al. 2014).
Besides Geobacteraceae, higher abundance of Proteiniphilum and Aminiphilus spp. was also observed in the anodic biofilms of magnetic GA-MFCs. These species may serve as fermenters in syntrophic interactions to provide substrates and nutrients from cell debris for the exoelectrogens in anodic biofilms of MFCs (Lesnik and Liu 2014; Parameswaran et al. 2010). In addition, application of SLIMF enhanced the overall richness and diversity of anodic community, indicating that species other than the core microbiome could also be stimulated by the presence of SLIMF. Therefore, it is likely that the increased conductivity of anodic biofilms, the enriched Geobacteraceae, and the enhanced syntrophic interactions worked in concert to cause the observed irreversible enhancements in overall performance of magnetic MFCs. These results also suggest that SLIMF should be applied to the anode of a MFC starting from the inoculation in order to gain a more significant enhancement of the overall performance.
References
Bond DR, Lovley DR (2003) Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69(3):1548–1555
Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J (2014) An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2(1):6
Fan Y, Hu H, Liu H (2007) Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J Power Sources 171(2):348–354
Fan Y, Xu S, Schaller R, Jiao J, Chaplen F, Liu H (2011) Nanoparticle decorated anodes for enhanced current generation in microbial electrochemical cells. Biosens Bioelectron 26(5):1908–1912
Fan Y, Han SK, Liu H (2012) Improved performance of CEA microbial fuel cells with increased reactor size. Energy Environ Sci 5(8):8273–8280
Gu H, Guo J, Sadu R, Huang Y, Haldolaarachchige N, Chen D, Young DP, Wei S, Guo Z (2013) Separating positive and negative magnetoresistance for polyaniline-silicon nanocomposites in variable range hopping regime. Appl Phys Lett 102(21):212403
Gu H, Guo J, Yan X, Wei H, Zhang X, Liu J, Huang Y, Wei S, Guo Z (2014) Electrical transport and magnetoresistance in advanced polyaniline nanostructures and nanocomposites. Polymer 55(17):4405–4419
Holmes DE, Dang Y, Walker DJF, Lovely DR (2016) The electrically conductive pili of Geobacter species are a recently evolved feature for extracellular electron transfer. Microb Genom 2(8):e000072
Hu B, Wu Y (2007) Tuning magnetoresistance between positive and negative values in organic semiconductors. Nat Mater 6:985–991. https://doi.org/10.1038/nmat2034
Janicek A, Fan Y, Liu H (2015) Performance and stability of different cathode base materials for use in microbial fuel cells. J Power Sources 280:159–165
Leang C, Malvankar N, Franks A, Nevin K, Lovley DR (2013) Engineering Geobacter sulfurreducens to produce a highly cohesive conductive matrix with enhanced capacity for current production. Energy Environ Sci 6(6):1901–1908
Lee HS, Dhar B, An J, Rittmann BE, Ryu H, Domingo JW, Ren H, Chae J (2016) The roles of biofilm conductivity and donor substrate kinetics in a mixed-culture biofilm anode. Environ Sci Technol 50(23):12799–12807
Lesnik KL, Liu H (2014) Establishing a core microbiome in acetate-fed microbial fuel cells. Appl Microbiol Biotechnol 98(9):4187–4196
Li WW, Sheng GP, Liu XW, Cai PJ, Sun M (2011) Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens Bioelectron 26(10):3987–3992
Li C, Lesnik KL, Fan Y, Liu H (2016a) Millimeter scale electron conduction through exoelectrogenic mixed species biofilms. FEMS Microbiol Lett 363(15):fnw153
Li C, Lesnik KL, Fan Y, Liu H (2016b) Redox conductivity of current-producing mixed species biofilms. PLoS One 11(5):e0155247
Li C, Lesnik KL, Liu H (2017) Stay connected: electrical conductivity of microbial aggregates. Biotechnol Adv 35(6):669–680
Li C, Lesnik KL, Liu H (2018) Conductive properties of methanogenic biofilms. Bioelectrochem 119:220–226
Liu Y, Bond DR (2012) Long-distance electron transfer by G. sulfurreducens biofilms results in accumulation of reduced c-type cytochromes. ChemSusChem 5(6):1047–1053
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7(5):375–381
Lovley DR (2011a) Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energy Environ Sci 4(12):4896–4906
Lovley DR (2011b) Reach out and touch someone: potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev Environ Sci Biotechnol 10(2):101–105
Lovley DR, Stolz JF, Nord GL, Phillips EJP (1987) Anaerobic production of magnetite by a dissimilatory iron-reducing microorganism. Nature 330(6145):252–254
Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovley DR (2011) Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol 6(9):573–579
Malvankar NS, Lau J, Nevin KP, Franks AE, Tuominen MT, Lovley DR (2012a) Electrical conductivity in a mixed-species biofilm. Appl Environ Microbiol 78(16):5967–5971
Malvankar NS, Tuominen MT, Lovley DR (2012b) Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energy Environ Sci 5(9):8651–8659
Malvankar NS, Tuominen MT, Lovely DR (2012c) Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energy Environ Sci 5(2):5790–5797
Malvankar NS, Vargas M, Nevin K, Tremblay PL, Evans-Lutterodt K, Nykypanchuk D, Martz E, Tuominen MT, Lovley DR (2015) Structural basis for metallic-like conductivity in microbial nanowires. mBio 6(2):e00084–e00015
Nalwa HS (ed) (2001) Handbook of Thin Films, Five-Volume Set (Vol. 5). Elsevier
Okamoto A, Saito K, Inoue K, Nealson KH, Hashimoto K, Nakamura R (2014) Uptake of self-secreted flavins as bound cofactors for extracellular electron transfer in species. Energy Environ Sci 7(4):1357–1361
Parameswaran P, Zhang H, Torres CI, Rittmann BE, Krajmalnik-Brown R (2010) Microbial community structure in a biofilm anode fed with a fermentable substrate: the significance of hydrogen scavengers. Biotechnol Bioeng 105(1):69–78
Phan H, Yates MD, Kirchhofer ND, Bazan GC, Tender LM, Nguyen TQ (2016) Biofilm as a redox conductor: a systematic study of the moisture and temperature dependence of its electrical properties. Phys Chem Chem Phys 18(27):17815–17821
Raikh ME, Czingon J, Ye QT, Koch F, Schoepe W, Ploog K (1992) Mechanisms of magnetoresistance in variable-range-hopping transport for two-dimensional electron systems. Phys Rev B 45(11):6015–6022
Shrestha PM, Malvankar NS, Werner JJ, Franks AE, Rotaru AE, Shrestha M, Liu F, Nevin K, Angenent LT, Lovely DR (2014) Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour Technol 174:306–310
Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, Tender LM (2012) Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc Natl Acad Sci U S A 109(38):15467–15472
Tan Y, Adhikari RY, Malvankar NS, Ward JE, Nevin KP, Woodard TL, Smith JA, Snoeyenbos-West O, Franks A, Lovley DR (2016) The low conductivity of Geobacter uraniireducens pili suggests a diversity of extracellular electron transfer mechanisms in the genus Geobacter. Front Microbiol 7:980
Tao Q, Zhou S (2014) Effect of static magnetic field on electricity production and wastewater treatment in microbial fuel cells. Appl Microbiol Biotechnol 98(23):9879–9887
Vali H, Weiss B, Li Y-L, Sears SK, Kim SS, Kirschvink JL, Zhang CL (2004) Formation of tabular singledomain magnetite induced by Geobacter metallireducens GS-15. Proc Natl Acad Sci 101(46):16121–16126
Vargas M, Malvankar NS, Tremblay PL, Leang C, Smith JA, Patel P, Synoeyenbos-West O, Nevin KP, Lovley DR (2013) Aromatic amino acids required for pili conductivity and long-range extracellular electron transport in Geobacter sulfurreducens. mBio 4(2):e00105–e00113
Yates MD, Eddie BJ, Kotloski NJ, Lebedev N, Malanoski AP, Lin B, Strycharz-Glaven SM, Tender LM (2016a) Toward understanding long-distance extracellular electron transport in an electroautotrophic microbial community. Energy Environ Sci 9(11):3544–3558
Yates MD, Strycharz-Glaven SM, Golden JP, Roy J, Tsoi S, Erickson JS, El-Naggar MY, Barton S, Tender LM (2016b) Measuring conductivity of living Geobacter sulfurreducens biofilms. Nat Nanotechnol 11(11):910–913
Yin Y, Huang G, Tong Y, Liu Y, Zhang L (2013) Electricity production and electrochemical impedance modeling of microbial fuel cells under static magnetic field. J Power Sources 237:58–63
Zhang X, Philips J, Roume H, Guo K, Rabaey K, Prévoteau A (2017) Rapid and quantitative assessment of redox conduction across electroactive biofilms via double potential step chronoamperometry. ChemElectroChem 4(5):1026–1036
Zhang X, Xia X, Ivanov I, Huang X, Logan BE (2014) Enhanced activated carbon cathode performance for microbial fuel cell by blending carbon black. Environ Sci Technol 48(3):2075–2081. https://doi.org/10.1021/es405029y
Zhao YN, Li XF, Ren YP, Wang XH (2016) Effect of static magnetic field on the performances of and anode biofilms in microbial fuel cells. RSC Adv 6(85):82301–82308
Zhou H, Liu B, Wang Q, Sun J, Xie G, Ren N, Ren ZJ, Xing D (2017) Pulse electromagnetic fields enhance extracellular electron transfer in magnetic bioelectrochemical systems. Biotechnol Biofuels 10(1):238
Acknowledgements
The authors would like to acknowledge Keaton Larson Lesnik and Ningshengjie Gao for their valuable discussion.
Funding
This study was funded by U.S. National Science Foundation (CBET 0955124) and U.S. Department of Energy (DE-EE0007269).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 315 kb)
Rights and permissions
About this article
Cite this article
Li, C., Wang, L. & Liu, H. Enhanced redox conductivity and enriched Geobacteraceae of exoelectrogenic biofilms in response to static magnetic field. Appl Microbiol Biotechnol 102, 7611–7621 (2018). https://doi.org/10.1007/s00253-018-9158-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9158-3