Abstract
D-Lactic acid is a chiral, three-carbon organic acid, that bolsters the thermostability of polylactic acid. In this study, we developed a microbial production platform for the high-titer production of D-lactic acid. We screened 600 isolates of lactic acid bacteria (LAB) and identified twelve strains that exclusively produced D-lactic acid in high titers. Of these strains, Lactobacillus saerimneri TBRC 5746 was selected for further development because of its homofermentative metabolism. We investigated the effects of high temperature and the use of cheap, renewable carbon sources on lactic acid production and observed a titer of 99.4 g/L and a yield of 0.90 g/g glucose (90% of the theoretical yield). However, we also observed L-lactic acid production, which reduced the product’s optical purity. We then used CRISPR/dCas9-assisted transcriptional repression to repress the two Lldh genes in the genome of L. saerimneri TBRC 5746, resulting in a 38% increase in D-lactic acid production and an improvement in optical purity. This is the first demonstration of CRISPR/dCas9-assisted transcriptional repression in this microbial host and represents progress toward efficient microbial production of D-lactic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Producing chemicals and fuels from biological sources, rather than petroleum, offers a promising alternative that could help reduce global greenhouse gas emissions (Liao et al., 2016). Examples of bio-based production include creating alternatives to gasoline and organic acids, and developing monomers for bioplastics (Chen et al., 2018; Choi & Lee, 2013; Kruyer & Peralta-Yahya, 2017; Moon et al., 2009). Lactic acid, an organic compound with three carbon atoms, is utilized across various sectors like food and plastic production (Castro-Aguirre et al., 2016; Lahtinen et al., 2012). In the plastic industry, lactic acid is especially valuable for making biodegradable and eco-friendly polylactic acid (PLA) plastics, which can replace non-biodegradable petroleum-based plastics. The chirality of lactic acid used, whether it is pure L-lactic or D-lactic acid, or a mixture of both, significantly impacts the properties of the resulting PLA (Bai et al., 2017). Stereocomplexation of poly-D-lactic acid with poly-L-lactic acid leads to plastics that are notably heat resistant. L-Lactic acid production through fermentation by microbes is well-known and widely practiced by several large biotech companies, while D-lactic acid production is less common, with few published reports (Abdel-Rahman & Sonomoto, 2016; Abdel-Rahman et al., 2013). This gap signals the need to develop a more effective system for producing pure D-lactic acid.
Several strains of bacteria, naturally occurring in the wild, are known for their prolific production of D-lactic acid (Lahtinen et al., 2012). These include members of the Sporolactobacillus, Leuconostoc, and Lactobacillus genera. Many research teams have used these microorganisms effectively to achieve high levels of D-lactic acid through fermentation. For instance, fermenting sugarcane juice with Leuconostoc mesenteroides B512 in a shake-flask led to a yield of 60.2 g/L of D-lactic acid, equivalent to 0.51 g/g of glucose (Coelho et al., 2011). Similarly, fermenting corncob residue hydrolysates with Sporolactobacillus inulinus YBS1-5 in a fed-batch process produced 107.2 g/L of D-lactic acid, equivalent to 0.85 g/g of glucose (Bai et al., 2016). Apart from these natural D-lactic acid producers, certain industrial hosts that originally could not produce D-lactic acid have been genetically modified to do so (Juturu & Wu, 2016; Sornlek et al., 2022; Watcharawipas et al., 2021).
In this study, we systematically screened 600 lactic acid bacterial isolates archived at the Thailand Bioresource Research Center (TBRC) and BIOTEC’s culture collection (BCC) to identify strains that produce high levels of D-lactic acid. Our screening led us to Lactobacillus saerimneri (recently reclassified as Ligilactobacillus saerimneri) TBRC 5746, which stood out due to its conversion yield of 0.96–0.99 g/g of glucose. Fed-batch fermentation of L. saerimneri TBRC 5746 resulted in a lactic acid titer of 99.4 g/L. However, at this high production level, the strain began to also produce a noticeable amount of L-lactic acid. Based on genome and real-time PCR analysis of L. saerimneri TBRC 5746, we found that two putative Lldh genes were likely responsible for this L-lactic acid production. By using CRISPR/dCas9-assisted transcriptional repression to down-regulate these genes, we achieved a 38% improvement in D-lactic acid production. This research represents a step forward towards the efficient microbial production of D-lactic acid.
Materials and Methods
Strain, Media, and Transformation
The bacterial strains used in this study were obtained from Thailand Bioresource Research Center (TBRC) and BIOTEC Culture Collection (BCC). The primers used in this study are listed in Table S1. The bacterial strains were stored in 25% glycerol at a temperature of − 80 °C. Lactic acid bacteria were grown in de Man, Rogosa and Sharpe (MRS) medium, with the addition of 5 μg/ml chloramphenicol as needed. Escherichia coli was grown in Luria–Bertani (LB) medium, with the addition of 100 μg/ml ampicillin and 5 μg/ml chloramphenicol as necessary. Lactobacillus saerimneri TBRC 5746 cells were transformed via electroporation using a modified protocol (Huang et al., 2019). The transformed bacteria were selected on MRS medium supplemented with 5 μg/ml chloramphenicol. The CRISPR-Cas9 plasmids used for gene repression were generated from pHSP02 and pLH01 (Huang et al., 2019).
Screening of Lactic Acid Bacteria from TBRC
Lactic acid bacteria strains from TBRC and BCC were cultured in 5-ml aliquots in MRS medium in 15-ml Falcon tubes. The cultures were grown at either 30 °C or 37 °C in an incubator without shaking. Samples were taken at 24 h to determine optical density at 600 nm (OD600) and D-lactic acid production. The amounts of D-lactic acid and L-lactic acid were determined using high-performance liquid chromatography (HPLC) as previously described with some modifications (Watcharawipas et al., 2021). Briefly, 1 ml of the bacterial culture was filtered through a 0.2-micron nylon syringe filter (Filtrex), and the purified sample was then analyzed on an Agilent 1100 series HPLC with a Shodex ORpak CRX-853 chiral column at 50 °C. The eluent used was 0.5 mM CuSO4 at a flow rate of 1.0 ml/min. D-lactic acid and L-lactic acid were detected using a UV detector set at 230 nm.
Plasmid and Strain Construction
To construct the CRISPR/Cas9 plasmids for specific gene repression, the specific crRNA for each gene (Lldh_Scaffold9 and Lldh_Scaffold15) was designed using the CRISPR RGEN tools (Bae et al., 2014; Park et al., 2015).
Plasmid pHSP02-dCas9: The gene encoding SpCas9 was mutated to encode the nuclease-deficient mutant (SpCas9D10A,H840A or SpdCas9) using overlap-extension PCR (OE-PCR). Three DNA fragments were amplified from pHSP02 using primers pHSP02dCas9-Fr1-F and pHSP02dCas9-Fr1-R, primers pHSP02dCas9-F2-F and pHSP02dCas9-Fr2-R, and primers pHSP02dCas9-Fr3-F and pHSP02dCas9-Fr3-R. The three fragments were then assembled by OE-PCR and ligated to the PstI/BglII sites of pHSP02 to create pHSP02-dCas9.
Plasmid pHSP02-dCas9-Lldh-Scaffold9-Cm: The crRNA for Lldh_Scaffold9 was amplified from pHSP02 using primers Sc9pHSP-F3 and Sc9pHSP-XbaI-R3. The 1.0-kb upstream and downstream fragments of Lldh_Scaffold9 were amplified from the genomic DNA of L. saerimneri TBRC 5746 using primers Sc9pHSP-SOE-F and Sc9pHSP-R1, and primers Sc9pHSP-F2 and Sc9pHSP-R2, respectively. The three DNA fragments were assembled by OE-PCR and ligated to the ApaI/XbaI sites of pHSP02-dCas9 to create pHSP02-dCas9-Lldh-Scaffold9. The chloramphenicol selectable marker (Cm) was amplified from pLH01 using primers Cm-ApaI-F and Cm-ApaI-R and ligated to the ApaI site of pHSP02-dCas9-Lldh-Scaffold9 to create pHSP02-dCas9-Lldh-Scaffold9-Cm.
Plasmid pHSP02-dCas9-Lldh-Scaffold15-Cm: The crRNA for Lldh_Scaffold15 was amplified from pHSP02 using primers Sc15pHSP-F3 and Sc15pHSP-XbaI-R3. The 1.0-kb upstream and downstream fragments of Lldh_Scaffold15 were amplified from the genomic DNA of L. saerimneri TBRC 5746 using primers Sc15pHSP-SOE-F and Sc15pHSP-R1, and primers Sc15pHSP-F2 and Sc15pHSP-R2, respectively. The three DNA fragments were assembled by OE-PCR and ligated to the ApaI/XbaI sites of pHSP02-dCas9 to create pHSP02-dCas9-Lldh-Scaffold15. The chloramphenicol selectable marker (Cm) was amplified from pLH01 using primers Cm-ApaI-F and Cm-ApaI-R and ligated to the ApaI site of pHSP02-dCas9-Lldh-Scaffold15 to create pHSP02-dCas9-Lldh-Scaffold15-Cm.
Isolation and Sequencing of Genomic DNA from L. saerimneri TBRC 5746
Lactobacillus saerimneri TBRC 5746's genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega). The purified DNA sample was sent to Novogene for whole genome sequencing. The 150 nt pair-end reads were checked for quality using FASTP, and the clean sequences were compared to the genome sequence of L. saerimneri 30a (Romano et al., 2013). The genome sequence was uploaded to GenBank with the Accession Number JAPXFN000000000.
Quantification of D-Lactic Acid Production and Other Fermentation Metabolites in Lactic Acid Bacteria Strains
For small-scale fermentation, the engineered strains were first grown in 5 ml of MRS medium overnight, then used to inoculate 5 ml of fresh MRS medium in 50-ml Falcon tubes to reach an initial OD600 of 0.05. The cultures were incubated at 30 °C without shaking. At various time points, samples were taken to measure OD600, biomass, extracellular metabolites, and the production of D-lactic acid. D-Lactic acid and other extracellular metabolites were analyzed using HPLC. Specifically, 1 ml of the culture was filtered through a 0.2-micron nylon syringe filter (Filtrex), and the purified sample was then analyzed on an Agilent 1100 series HPLC with an Aminex HPX-87H ion exchange column (Bio-Rad). The LC program was performed using 5 mM H2SO4 as the solvent at a flow rate of 0.68 ml/min for 30 min. The column was maintained at 60 °C. All metabolites were detected with Agilent 1200 series DAD and RID detectors.
Batch and Fed-Batch Bioreactor Fermentations
Fed-batch fermentation of L. saerimneri TBRC 5746 was performed in a 5-L stirred-tank bioreactor (Biostat B; Sartorius) with an initial volume of 2 L semi-defined medium with the following composition: 10 g/L peptone No.3, 10 g/L meat extract, 5 g/L yeast extract, 2 g/L K2HPO4, 2 g/L C6H8O7.3NH3, 5 g/L CH3COO-Na, 1 g/L Tween 80, 0.2 g/L MgSO4.7H2O, 0.05 g/L MnSO4.H2O, and 0.1 ml/L antifoam. The pH was maintained at pH 6 by adding NH4OH solution (25% solution in water). Glucose was prepared separately and added at a concentration of 40 g/L during batch fermentation. Fed-batch fermentation using intermittent feeding started after 10 h by adding 60 ml of 800 g/L glucose for 20 min at 0, 4, 8, 12, and 18 h time points to maintain approximately 15 g/L glucose per feeding cycle.
RNA Isolation and Transcript Quantification
Fed-batch fermentation of Lactobacillus saerimneri TBRC 5746 was performed in a 5-L stirred-tank bioreactor as described above. At 2, 4, 6, and 8 h time points, 1 ml aliquots of each culture were collected and centrifuged for 2 min at 9000 × g. Total RNA was extracted from the samples using Qiagen’s RNeasy Kit and the protocol provided by the manufacturer. The RNA was treated with DNaseI enzyme to remove any genomic DNA contaminants. The quantity of the RNA was measured using a NanoDrop ND-1000 spectrophotometer, and the samples were stored at − 80 °C until they were used for RT-PCR analysis. cDNA was produced from the RNA using the RevertAid Reverse Transcriptase kit and protocol provided by Thermo Fisher Scientific. The relative expression levels of the genes Dldh, Lldh_Scaffold 9, and Lldh_Scaffold 15 were then quantified using the iQ SYBR Green Kit (Bio-Rad) on a CFX96 Touch Real-time PCR Detection System (Bio-Rad). Real-time PCR was performed in triplicates, and the 16S ribosomal RNA (rRNA) gene was used to normalize the amount of the total mRNA in all samples. Primers for real-time PCR are listed in Table S1.
Results and Discussion
Screening of Lactic Acid Bacteria to Identify High D-Lactic Acid-producing Strains
In this study, 600 lactic acid bacteria (LAB) strains previously isolated from various sources, such as fermented meat, fermented fish, and vegetables, were tested for their ability to produce D-lactic acid. These strains had been deposited at the Thailand Bioresource Research Center (TBRC) and the BIOTEC Culture Collection (BCC). Each LAB isolate was cultured in de Man, Rogosa and Sharpe (MRS) broth at 37 °C for 24 h. The cell-free supernatant was then used to analyze D-lactic acid production using an HPLC equipped with a chiral column. The screening results showed that among 600 isolates from 8 genera of LAB including Lactobacillus, Weissella, Tetragenococcus, Pediococcus, Leuconostoc, Lactococcus, Trichococcus, and Fructobacillus, only 15 isolates from the genera Weissella and Leuconostoc showed potential as D-lactic producers (Supplementary file 1). Of these 15 isolates, 12 produced exclusively D-lactic acid (Table 1).
Interestingly, during the screening, we found that L. saerimneri TBRC 5746 had the highest production of D-lactic acid without any other metabolic by-products such as ethanol or acetic acid. This is likely due to L. saerimneri TBRC 5746 belonging to the group of homofermentative LAB, which produce only lactic acid as the primary by-product in glucose fermentation. Homofermentative LAB produce two lactic acid molecules as a major end-product per molecule of consumed glucose, with a theoretical yield of 1.0 g/g. In contrast, the isolates from the genera Weissella and Leuconostoc belong to the group of heterofermentative LAB, which produce lactic acid simultaneously with several organic acids such as succinic acid and acetic acid, resulting in lower lactic acid production. Because of this potential, we further investigated L. saerimneri TBRC 5746.
Bioprocess Development for High-titer Production of D-Lactic Acid Using L. saerimneri TBRC 5746
Effects of temperature on lactic acid production: To investigate the effect of cultivation temperature on L. saerimneri TBRC 5746 growth and lactic acid production, a shake flask fermentation was performed at 37 °C, 42 °C, and 45 °C, using glucose, sucrose, and fructose as carbon sources. The results showed that the maximum growth and lactic acid production was obtained at 42 °C for all three carbon sources (Fig. S1). However, achieving higher titers of lactic acid was difficult in shake flask experiments due to pH control issues. As the pH dropped to around 4.5, the growth of the microorganism was inhibited. Therefore, the effects of temperature were re-examined in a 5-L fermenter using batch fermentation with pH control at 6. Ammonium hydroxide was used to control the pH as it can also serve as an additional nitrogen source. The results showed that the maximum growth and lactic acid production of L. saerimneri TBRC 5746 was obtained after 11 h with a titer of 42.1 g/L, without ethanol production (Figs. S2–S4). This demonstrated the strain's ability to convert glucose to lactic acid with a conversion yield of 0.96–0.99 g/g glucose, close to the theoretical yield of homofermentative lactic acid production (1.0 g/g glucose).
To further improve lactic acid production, a fed-batch fermentation was performed using glucose as the carbon source (Fig. 1A and B). The initial glucose concentration was 40 g/L, with an additional 70 g/L added during the fed-batch fermentation. The fermentation was performed at 40 °C (Fig. 1A) and 42 °C (Fig. 1B) as these temperatures were deemed suitable for industrial application because of the potential for reduced operational costs for temperature control. The results showed that lactic acid production at 40 °C and 42 °C were similar, with titer values of 98.7 g/L and 99.4 g/L, respectively. These values correspond to lactic acid productivity of 2.08 g/L/h and 2.09 g/L/h at 40 °C and 42 °C, respectively. No ethanol was detected during fermentation, confirming the homofermentative characteristic of the strain. This is an important characteristic to have, as ethanol production would decrease the yield and purity of the final lactic acid product. The ability to produce lactic acid at high temperatures allows for flexibility and potential scalability in industrial applications.
Fed-batch fermentation profiles of L. saerimneri TBRC 5746 in a 5-L fermenter at 40 °C (A) and 42 °C (B). Fed-batch fermentation was performed with an initial volume of 2 L semi-defined medium. Fed-batch fermentation using intermittent feeding of 280 ml glucose stock solution (500 g/L) started after 10 h at 0, 4, 8, 12, and 18 h time points to maintain approximately 15 g/L glucose per feeding cycle. Total lactic acid and other metabolites were determined using HPLC equipped with an Aminex HPX-87H ion exchange column
Although accelerated growth and sugar consumption rates were obtained when the strain was cultivated at elevated temperatures, a reduced enantiopurity of D-lactic acid was observed as shown in Fig. 2. At 30 °C, a trace amount of L-lactic acid was detected after 24 h of fermentation. In contrast, at higher fermentation temperatures (37 °C, 40 °C, and 42 °C), L-lactic acid was detected from the early stages and persisted throughout the fermentation, culminating at 24 h. This resulted in decreased optical purity of D-lactic acid at higher temperatures. This phenomenon aligns with numerous reports indicating lower thermostability of D-lactate dehydrogenase compared to L-lactate dehydrogenase, which directly impacts the efficiency of commercial microbial production of D-lactic acid (Gu et al., 2014; Jun et al., 2013; Kim et al., 2014).
Effects of carbon source (glucose and sucrose) on lactic acid production: Reducing operational costs is crucial for developing a bioprocess that is economically feasible and can be scaled up for industrial production (Alves de Oliveira et al., 2018). In bacteria, a disaccharide such as sucrose is transported into the bacterial cell via a phosphotransferase system (PTS)-dependent sucrose system or a non-PTS permeases transport system. For sucrose catabolism in lactobacilli, the major mechanism of sucrose uptake is mediated by phosphotransferase system (PTS) coupled to a phosphoenolpyruvate (PEP) dependent phosphorylation leading to sucrose-6-phosphate (S-6-P). S-6-P is cleaved by sucrose-6-phosphate-hydrolase into glucose-6-P (G-6-P) and fructose. If fructose is phosphorylated by fructokinase, the yielding fructose-6-phosphate (F-6-P) will be metabolized via the glycolytic pathway as same as G-6-P. Alternatively in a non-PTS permease system, sucrose transportation by a solute-cation symport system by sucrose permease followed by sucrose phosphorolysis by sucrose phosphorylase yielding glucose-1-phosphate (G-1-P) and fructose. Then, the generated G-1-P and fructose can be readily converted to G-6-P and F-6-P by the bacterial cell and incorporated into the glycolytic pathway (Gänzle & Follador, 2012; Reid & Abratt, 2005). Although, sucrose phosphorylase exhibit a higher specificity for sucrose as a substrate than sucrose hydrolase, a less frequently identified in genome of lactobacilli was reported (Reid & Abratt, 2005). In homofermentative lactobacilli, the catabolism of G-6-P primarily proceeds via the Embden-Meyerhof pathway, leading to pyruvate. As a result, the end-products of sucrose fermentation in most homofermentative lactobacilli are two moles of lactate per mole of glucose derived from sucrose, and theoretically, per mole of fructose derived from sucrose as well. In addition to environmental factors such as temperature and pH that affect lactic acid production, the choice of media composition is also relevant. In this study, we investigated the use of sucrose as a cheaper carbon source for lactic acid production and attempted to increase the sugar content in the culture media during fed-batch fermentation. However, the results showed that increasing the sugar content in the media above 100 g/L did not improve lactic acid production. Lactic acid titers of 94.0 g/L and 97.9 g/L were obtained when 200 g/L of glucose and sucrose were used as carbon sources, respectively (Fig. 3A and B, Tables S2 and S3). These correspond to lactic acid productivity of 1.85 g/L/h and 1.91 g/L/h, respectively. This may be due to end-product inhibition (Aguirre-Ezkauriatza et al., 2010; Luedeking & Piret, 1959) as a lower rate of lactic acid production was observed when the accumulation of produced lactic acid exceeded 80 g/L in the culture. Additionally, excess glucose and sucrose were detected (50–58 g/L) after the fermentation was finished at 72 h, resulting in a decrease in production yield and overall process productivity compared to using lower sugar concentrations.
Fermentation profiles of L. saerimneri TBRC 5746 in a 5-L fermenter at 42 °C using different carbon sources (glucose A and sucrose B). Fed-batch fermentation was performed with an initial volume of 2 L semi-defined medium. Fed-batch fermentation using intermittent feeding of glucose stock solution (500 g/L, A) or sucrose stock solution (40 g/L, B) started after 10 h at 0, 4, 8, 12, 16, 20, 26, 32, 38 and 50 h time points. Total lactic acid and other metabolites were determined using HPLC equipped with an Aminex HPX-87H ion exchange column
It has been reported that the concentration of lactic acid that causes a 50% inhibition of growth is around 420 mM, while the critical concentration is 700 mM (Loubiere et al., 1997). This is thought to be due to the solubility of undissociated lactic acid within the cytoplasmic membrane and the insolubility of dissociated lactate. The latter causes acidification of the cytoplasm, leading to a failure of the proton motive forces (Othman et al., 2017). Despite being effective in countering end-product inhibition, pH control during fed-batch fermentation can lead to issues with osmotic pressure and result in bacteriostatic phenomena. To overcome these challenges, a fermentation process using an extractive fermentation system has been developed to overcome end-product inhibition and improve product recovery (Bai et al., 2003; Othman et al., 2017).
Bioprocess Development for High Titer Production of D-Lactic Acid Using Inexpensive, Renewable Carbon Sources
To enhance the feasibility of the fermentation process for industrial application, inexpensive, renewable carbon sources such as raw sugar and enzyme-hydrolyzed cassava chips were tested as alternatives to glucose during fed-batch fermentation. Before testing, sugar analysis was performed on both substrates using HPLC. The results showed that both substrates contained glucose, fructose, and sucrose at different concentrations and ratios (Table 2).
To investigate the suitability of raw sugar and enzyme-hydrolyzed cassava chips as alternative carbon sources for L. saerimneri TBRC 5746, fed-batch fermentation was conducted at 42 °C, pH 6, and without aeration (Fig. 4A and B). The results showed that the strain was able to grow and produce lactic acid, achieving maximum titers of 96.3 g/L and 79.5 g/L using raw sugar and hydrolyzed cassava chips, respectively. These alternative carbon sources have potential as cost-effective and sustainable options for lactic acid production, however, residual sugar was detected. It is possible that inhibitors or toxic substances present in the hydrolysates may impede efficient substrate utilization and result in glucose accumulation. The possibility of contamination and the cost of substrate pretreatment and downstream processing should be considered when scaling up the fermentation process for industrial use with alternative carbon sources.
Fed-batch fermentation profiles of L. saerimneri TBRC 5746 in a 5-L fermenter at 42 °C using cheap renewable carbon sources (raw sugar (A) and enzyme-hydrolyzed cassava chip [B]). Fed-batch fermentation was performed with an initial volume of 2 L semi-defined medium. Fed-batch fermentation using intermittent feeding of raw sugar solution (223 ml of 159.424 g/L total sugar equivalent, A) or enzyme-hydrolyzed cassava chip solution (223 ml of 169.81 g/L total sugar equivalent, [B]) started after 8 h at 0, 4, 8, 12, 16, 20 and 24 h time points. Total lactic acid and other metabolites were determined using HPLC equipped with an Aminex HPX-87H ion exchange column
Genome Sequencing and Identification of Putative L-ldh Genes in L. saerimneri TBRC 5746
Although preliminary fermentation studies of L. saerimneri TBRC 5746 discussed above confirmed that the strain exhibits homofermentative metabolism. A 5-L fed-batch fermentation of L. saerimneri TBRC 5746 resulted in lactic acid production at a titer of 99.4 g/L and yield of 0.90 g/g glucose or 90% of the theoretical yield. However, employing this fermentation condition at elevated temperature, we also observed L-lactic acid production, which reduced the product's optical purity. In order to improve the production titer and optical purity of D-lactic acid from L. saerimneri TBRC 5746 as well as to understand the basis of the strain's L-lactic acid production, we performed genome sequencing using the Illumina platform. The genome of L. saerimneri TBRC 5746 was found to be 1.6 Mb in size with a GC content of 42%. We identified putative Dldh and Lldh genes in the genome of L. saerimneri TBRC 5746, including D-lactate dehydrogenase Dldh on Scaffold1:13,437:14,438:- and L-lactate dehydrogenase Lldh on Scaffold9:17,046:18,014: + and Scaffold15:547:1455:-. Our fermentation results were consistent with the presence of two Lldh genes in the genome of L. saerimneri TBRC 5746 (Fig. 5). To improve D-lactic acid production and optical purity, we used CRISPR/dCas9-assisted transcriptional regulation to repress these genes.
CRISPR-Cas9-based Repression of Putative Lldh Genes to Improve D-Lactic Acid Optical Purity
The presence of two Lldh genes in the genome of L. saerimneri TBRC 5746 provides clues to the production of both D- and L-lactic acid at the bioreactor scale. In order to improve the production titer and optical purity of D-lactic acid, we employed the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease (Cas9) system to repress the expression of the putative Lldh genes in L. saerimneri TBRC 5746 (Dominguez et al., 2016). Despite efforts to delete the L-LDH genes from L. saerimneri, no viable colonies were observed post-transformation, possibly due to essential roles of these genes or cellular stress from double-strand breaks in a strain with limited homology-directed repair capacity. The CRISPR-Cas9 system, which has been widely used in recent years, allows for efficient and precise editing of the bacterial genome, including those from LAB (Doudna & Charpentier, 2014; Jinek et al., 2012; Wu et al., 2021). For example, Song and coworkers developed a RecE/T-assisted CRISPR/Cas9 genome editing system in L. plantarum and L. brevis that utilizes the phage-derived recombination system to overcome the challenges posed by the sensitivity of some Lactobacillus species to double-strand breaks (Huang et al., 2019). Leenay and coworkers demonstrated CRISPR-Cas9 editing in three strains of L. plantarum and observed variable editing outcomes that appeared to be strain-specific (Leenay et al., 2019).
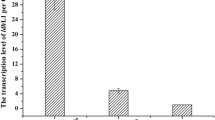
Based on these studies, we developed a CRISPR/dCas9-assisted transcriptional repression system in L. saerimneri TBRC 5746 that targets the two Lldh genes (named Lldh_Scaffold9 and Lldh_Scaffold15). The CRISPR/dCas9 plasmids contain a gene encoding the nuclease-deficient Cas9 from Streptococcus pyogenes (SpCas9D10A,H840A or SpdCas9) as well as the crRNA that targets the individual Lldh genes. Three L. saerimneri TBRC 5746 transformants harboring each CRISPR/dCas9 plasmid were selected for real-time PCR (RT-PCR) analysis of Dldh and Lldh gene expression in test tube cultures (Fig. 6). In the L. saerimneri TBRC 5746 strains harboring the CRISPR/dCas9 plasmid targeting Lldh_Scaffold9, we observed decreased expression of Lldh_Scaffold9 in two (clone #1 and clone #3) out of the three selected clones. Similarly, in strains harboring the CRISPR/dCas9 plasmid targeting Lldh_Scaffold15, we observed decreased expression of Lldh_Scaffold15 in two (clone #2 and clone #14) out of the three selected clones. These RT-PCR experiments using small-scaler cultures provide a preliminary and qualitative indication of the CRISPR/dCas9 system’s ability to downregulation gene expression in L. saerimneri TBRC 5746.
We then evaluated the effect of Lldh repression on D-lactic acid production in a 5-L bioreactor (Fig. 7). We hypothesized that the expression of Ldh genes would be up-regulated in fed-batch fermentation in the presence of high concentrations of glucose, making the effects of the CRISPR/dCas9-assisted transcriptional repression of Ldh genes more pronounced. Our observations supported this hypothesis: we saw a significant improvement in D-lactic acid production in the strain harboring the CRISPR/dCas9 plasmid targeting Lldh_Scaffold9, reaching a D-lactic acid titer of 78.5 g/L, a 38% increase over the titer observed in the control strain (L. saerimneri TBRC 5746; 57.0 g/L) (Fig. 7B vs. 7A). This increase in D-lactic acid production was also accompanied by a decrease in L-lactic acid production, with the engineered strain producing L-lactic acid at a titer of 21.9 g/L, a 24% decrease from the level observed in the control strain (28.8 g/L). In contrast, the strain harboring the CRISPR/dCas9 plasmid targeting Lldh_Scaffold15 saw a more modest increase in D-lactic acid production (62.6 g/L) and negligible change in L-lactic acid production (31.8 g/L) (Fig. 7C). These results suggest that repression of Lldh genes using CRISPR/dCas9 system has the potential to improve D-lactic acid production in L. saerimneri TBRC 5746.
Our study showed that the CRISPR/dCas9 system can effectively improve the production of D-lactic acid, which is of great significance for the industrial production of lactic acid. D-lactic acid is widely used in the food and pharmaceutical industries, and high optical purity is often a requirement. However, there are certain applications where a mixture of L-lactic acid and D-lactic acid may be more desirable than a single isomer. One example is in the food industry, where a mixture of L-lactic acid and D-lactic acid can be used as a flavoring agent or a preservative. In this case, the mixture can provide a more complex and nuanced flavor profile than a single isomer. In future work, we will focus on optimizing the CRISPR/dCas9 system for improved efficiency and broader applicability to other lactic acid bacterial strains. Additionally, more research is needed to investigate the potential of this system for improving other fermentation processes and product yields.
Conclusion
In this study, we screened 600 lactic acid bacterial isolates for D-lactic acid production and identified twelve strains that produce high titers of exclusively D-lactic acid. Initial fermentation experiments showed that only L. saerimneri TBRC 5746 exhibited homofermentative metabolism. A 5-L fed-batch fermentation of L. saerimneri TBRC 5746 resulted in a lactic acid titer of 99.4 g/L, with a yield of 0.90 g/g glucose, or 90% of the theoretical yield. However, at this titer, we also observed the production of L-lactic acid, which lowered the product's optical purity. Genome sequencing of L. saerimneri TBRC 5746 revealed the presence of two putative Lldh genes, providing support for the production of L-lactic acid as a minor product. Repressing these genes using CRISPR/dCas9-assisted transcriptional repression resulted in a 38% increase in D-lactic acid production and improved optical purity. To our knowledge, this is the first demonstration of CRISPR/dCas9-assisted transcriptional repression in L. saerimneri. Our work represents progress towards efficient microbial production of D-lactic acid.
References
Abdel-Rahman, M. A., & Sonomoto, K. (2016). Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. Journal of Biotechnology, 236, 176–192.
Abdel-Rahman, M. A., Tashiro, Y., & Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31, 877–902.
Aguirre-Ezkauriatza, E. J., Aguilar-Yáñez, J. M., Ramírez-Medrano, A., & Alvarez, M. M. (2010). Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresource Technology, 101, 2837–2844.
Alves de Oliveira, R., Komesu, A., Rossell, C. E. V., & Maciel Filho, R. (2018). Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochemical Engineering Journal, 133, 219–239.
Bae, S., Park, J., & Kim, J. S. (2014). Cas-OFFinder: A fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics, 30, 1473–1475.
Bai, D. M., Wei, Q., Yan, Z. H., Zhao, X. M., Li, X. G., & Xu, S. M. (2003). Fed-batch fermentation of Lactobacillus lactis for hyper-production of L-lactic acid. Biotechnology Letters, 25, 1833–1835.
Bai, Z., Gao, Z., Sun, J., Wu, B., & He, B. (2016). D-Lactic acid production by Sporolactobacillus inulinus YBS1-5 with simultaneous utilization of cottonseed meal and corncob residue. Bioresource Technology, 207, 346–352.
Bai, H., Deng, S., Bai, D., Zhang, Q., & Fu, Q. (2017). Recent Advances in Processing of Stereocomplex-Type Polylactide. Macromolecular Rapid Communications, 38, 1700454.
Castro-Aguirre, E., Iniguez-Franco, F., Samsudin, H., Fang, X., & Auras, R. (2016). Poly(lactic acid)—Mass production, processing, industrial applications, and end of life. Advanced Drug Delivery Reviews, 107, 333–366.
Chen, N., Wang, J., Zhao, Y., & Deng, Y. (2018). Metabolic engineering of Saccharomyces cerevisiae for efficient production of glucaric acid at high titer. Microbial Cell Factories, 17, 67.
Choi, Y. J., & Lee, S. Y. (2013). Microbial production of short-chain alkanes. Nature, 502, 571–574.
Coelho, L. F., de Lima, C. J., Bernardo, M. P., & Contiero, J. (2011). D(-)-lactic acid production by Leuconostoc mesenteroides B512 using different carbon and nitrogen sources. Applied Biochemistry and Biotechnology, 164, 1160–1171.
Dominguez, A. A., Lim, W. A., & Qi, L. S. (2016). Beyond editing: repurposing CRISPR–Cas9 for precision genome regulation and interrogation. Nature Reviews Molecular Cell Biology, 17, 5–15.
Doudna, J. A., & Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science, 346, 1258096.
Gänzle, M. G., & Follador, R. (2012). Metabolism of oligosaccharides and starch in lactobacilli: a review. Frontiers in Microbiology, 3, 340.
Gu, S. A., Jun, C., Joo, J. C., Kim, S., Lee, S. H., & Kim, Y. H. (2014). Higher thermostability of L-lactate dehydrogenases is a key factor in decreasing the optical purity of D-lactic acid produced from Lactobacillus coryniformis. Enzyme and Microbial Technology, 58–59, 29–35.
Huang, H., Song, X., & Yang, S. (2019). Development of a RecE/T-Assisted CRISPR–Cas9 Toolbox for Lactobacillus. Biotechnology Journal, 14, e1800690.
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J. A., & Charpentier, E. (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 337, 816–821.
Jun, C., Sa, Y. S., Gu, S. A., Joo, J. C., Kim, S., Kim, K. J., & Kim, Y. H. (2013). Discovery and characterization of a thermostable D-lactate dehydrogenase from Lactobacillus jensenii through genome mining. Process Biochemistry, 48, 109–117.
Juturu, V., & Wu, J. C. (2016). Microbial production of lactic acid: The latest development. Critical Reviews in Biotechnology, 36, 967–977.
Kim, S., Gu, S. A., Kim, Y. H., & Kim, K. J. (2014). Crystal structure and thermodynamic properties of D-lactate dehydrogenase from Lactobacillus jensenii. International Journal of Biological Macromolecules, 68, 151–157.
Kruyer, N. S., & Peralta-Yahya, P. (2017). Metabolic engineering strategies to bio-adipic acid production. Current Opinion in Biotechnology, 45, 136–143.
Lahtinen, S., Ouwehand, A. C., Salminen, S., & von Wright, A. (2012). Lactic Acid Bacteria: Microbiological and Functional Aspects (4th ed.). CRC Press.
Leenay, R. T., Vento, J. M., Shah, M., Martino, M. E., Leulier, F., & Beisel, C. L. (2019). Genome editing with CRISPR-Cas9 in Lactobacillus plantarum revealed that editing outcomes can vary across strains and between methods. Biotechnology Journal, 14, e1700583.
Liao, J. C., Mi, L., Pontrelli, S., & Luo, S. (2016). Fuelling the future: microbial engineering for the production of sustainable biofuels. Nature Reviews Microbiology, 14, 288–304.
Loubiere, P., Cocaign-Bousquet, M., Matos, J., Goma, G., & Lindley, N. D. (1997). Influence of end-products inhibition and nutrient limitations on the growth of Lactococcus lactis subsp. lactis. Journal of Applied Microbiology, 82, 95–100.
Luedeking, R., & Piret, E. L. (1959). A kinetic study of the lactic acid fermentation. Batch process at controlled pH. Biotechnology and Bioengineering, 1, 393–412.
Moon, T. S., Yoon, S. H., Lanza, A. M., Roy-Mayhew, J. D., & Prather, K. L. (2009). Production of glucaric acid from a synthetic pathway in recombinant Escherichia coli. Applied and Environmental Microbiology, 75, 589–595.
Othman, M., Ariff, A. B., Rios-Solis, L., & Halim, M. (2017). Extractive fermentation of lactic acid in lactic acid bacteria cultivation: a review. Frontiers in Microbiology, 8, 2285.
Park, J., Bae, S., & Kim, J. S. (2015). Cas-Designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics, 31, 4014–4016.
Reid, S. J., & Abratt, V. R. (2005). Sucrose utilisation in bacteria: genetic organisation and regulation. Applied Microbiology and Biotechnology, 67, 312–321.
Romano, A., Trip, H., Campbell-Sills, H., Bouchez, O., Sherman, D., Lolkema, J. S., & Lucas, P. M. (2013). Genome sequence of Lactobacillus saerimneri 30a (Formerly Lactobacillus sp. Strain 30a), a reference lactic acid bacterium strain producing biogenic amines. Genome Announcements, 1, e00097-e112.
Sornlek, W., Sae-Tang, K., Watcharawipas, A., Wongwisansri, S., Tanapongpipat, S., Eurwilaichtr, L., Champreda, V., Runguphan, W., Schaap, P. J., & Martins Dos Santos, V. A. P. (2022). D-Lactic acid production from sugarcane bagasse by genetically engineered Saccharomyces cerevisiae. Journal of Fungi, 8, 816.
Watcharawipas, A., Sae-Tang, K., Sansatchanon, K., Sudying, P., Boonchoo, K., Tanapongpipat, S., Kocharin, K., & Runguphan, W. (2021). Systematic engineering of Saccharomyces cerevisiae for D-lactic acid production with near theoretical yield. FEMS Yeast Research, 21, foab024.
Wu, J., Xin, Y., Kong, J., & Guo, T. (2021). Genetic tools for the development of recombinant lactic acid bacteria. Microbial Cell Factories, 20, 118.
Acknowledgements
The project was funded by the Integrated Technology Platform (Biobased Materials) project, “IBMDL1-High-level Microbial Production of Enantiomerically Pure D-lactic Acid,” supported by the National Science and Technology Development Agency [grant number P-17-522777].
Author information
Authors and Affiliations
Contributions
Conceptualization, WR, WV and KK; methodology, WR and KK; validation, KS, PS, YK, KK and WR; formal analysis, KK and WR; investigation, KS, PS and PP; resources, WR; data curation, KS, PS, YK, KK and WR; writing—original draft preparation, WR and KK; writing—review and editing, WR, KK and PP; visualization, WR and KK; supervision, WV, ST, WR and KK; project administration, WR and KK; funding acquisition, WR All authors have read and agreed to the published version of the manuscript.”
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethical approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sansatchanon, K., Sudying, P., Promdonkoy, P. et al. Development of a Novel D-Lactic Acid Production Platform Based on Lactobacillus saerimneri TBRC 5746. J Microbiol. 61, 853–863 (2023). https://doi.org/10.1007/s12275-023-00077-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00077-x