Abstract
Mercury-resistant (HgR) bacteria occur in various bacterial species from a wide variety of environmental sources. Resistance is conferred by a set of operon genes termed the mer operon. Many HgR bacteria have been isolated from diverse environments and clinical samples, and it is recognized that mer operons are often localized on transposons. Previous research reports have suggested that HgR transposons participate in the horizontal gene transfer of mer operons among bacteria. This was confirmed by a study that found that mer operons were distributed worldwide in Bacilli with dissemination of TnMERI1-like transposons. In this mini review, possible strategies for transposon-mediated in situ molecular breeding (ISMoB) of HgR bacteria in their natural habitat are discussed. In ISMoB, the target microorganisms for breeding are indigenous bacteria that are not HgR but that are dominant and robust in their respective environments. Additionally, we propose a new concept of bioremediation technology for environmental mercury pollution by applying transposon-mediated ISMoB for environmental mercury pollution control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Elemental mercury and its compounds are distributed widely in the environment as a result of geological and anthropogenic activities (Selin 2009). Microbes convert mercurial compounds and play an important role in the global mercury cycle (Barkay et al. 2003). Although mercury and its compounds are toxic to all living organisms, certain bacteria possess resistance genes to mercurials. Mercury-resistant (HgR) bacteria occur in various bacterial species from a wide variety of clinical and environmental sources (Barkay et al. 2003; Osborn et al. 1997). Different resistance mechanisms against mercury compounds have been found in a wide range of bacterial genera that have also been isolated from clinical, intestinal, and environmental samples (Silver and Phung 1996). The most studied mechanism of bacterial mercury resistance is enzymatic reduction of Hg2+ to its metallic form, Hg0 (Barkay et al. 2003; Misra 1992; Osborn et al. 1997; Silver and Phung 1996). A high vapor pressure and a very low aqueous solubility of Hg0 result in its volatilization from the bacterial cytoplasm into the outer atmospheric environment (Barkay et al. 2003).
This underlying resistance mechanism is conferred by a set of operon genes, the mer operon. The mer operon consists of a cluster of linked genes that encode proteins with functions related to regulation, transport, decomposition, and reduction of mercurial compounds. Although mer operons regulating the same mechanisms have been identified from various Gram-negative and Gram-positive bacteria, the component genes of the mer operons are not uniform, and genetic variations within each gene exist (Barkay et al. 2003; Silver and Phung 1996). In general, merR (regulatory gene), merT and merP (mercury transport genes), and merA (mercury reductase gene) are commonly conserved as core mer operon genes, while additional genes such as merB (organomercury lyase) (Begley et al. 1986), merC (mercury transport) (Liebert et al. 2000), merD (regulatory gene) (Nucifora et al. 1989), merE and merF (mercury transport genes) (Liebert et al. 1999; Wilson et al. 2000), and merG (phenylmercury resistance gene) (Kiyono and Pan-Hou 1999) are optional.
These mer genes are often localized on mobile elements, such as transposons (Hobman and Brown 1997; Mindlin and Petrova 2013; Osborn et al. 1997). Transposons carrying mer operons have been identified from both clinical and environmental species. The majority of characterized HgR transposons belong to the Tn3-family of replicative transposons (previously designated class II transposons; DNA without an RNA intermediate) that are generally typified by encoding 35–48 bp terminal inverted repeat (IR) sequences, the tnpA gene (transposase), the tnpR gene (resolvase), and res sites (co-integrated resolution sites) (Grinsted et al. 1990; Liebert et al. 1999; Nicolas et al. 2015). The first investigated HgR transposons were Tn21 and Tn501 (Liebert et al. 2000; Nucifora et al. 1989). The distribution and diversity of transposition genes relating to Tn21 and Tn501 have been studied using environmental bacteria or bacterial community DNA isolated from different environments (Holt et al. 1999; Huang et al. 1999a; Liebert et al. 1999; Mindlin and Petrova 2013).
Environmental mercury contamination, particularly by organomercurial compounds, has caused very serious disasters such as methylmercury poisoning cases in Minamata Bay, Japan, during the 1950s, after the region was polluted with methylmercury compounds from industrial wastewater (Harada 1995; Tsubaki and Irukayayama 1977). Based on reported mercury concentrations in 1959 and the late 1980s, natural processes (largely microbial) had removed between 75 and 90% of the mercury in Minamata Bay sediment (Nakamura and Silver 1994; Silver et al. 1994). The frequency of multiple organomercurial resistant bacteria in isolates from Minamata Bay sediment were at least 20-fold higher than that found in isolates from a nearby unpolluted control site (Nakamura et al. 1990). These records suggest the contribution of bacteria in in situ remediation activity in mercury-polluted environments. In the Minamata Bay sediments, HgR bacteria had been identified from ten different genera (Bacillus, Enterobacter, Flavobacterium, Moraxella, Pseudomonas, Vibrio, Corynebacterium, Micrococcus, Staphylococcus, and Clostridium) (Nakamura et al. 1988, 1990; Narita et al. 1999). Among these bacteria, Bacillus was the most abundant and was a major contributor of mercury cycling in Minamata Bay sediment (Nakamura et al. 1988, 1990). Similar mer operons among isolated Minamata Bacillus strains suggest horizontal spread of mercury resistance determinants (Silver et al. 1994).
The first HgR Bacillus was isolated from a sediment from a site polluted with heavy metals in Boston Harbor, USA (Mahler et al. 1986), and the genetic properties of the mercury resistance determinant of the isolated strain, Bacillus cereus RC607, were characterized (Wang et al. 1989). Subsequently, Huang et al. (1999b) identified a broad-spectrum mercury resistance Tn3-family replicative transposon, TnMERI1, from a Minamata Bay isolate, Bacillus megaterium MB1 (collection number: NBRC 110925), which showed resistance to both organomercurials and inorganic mercury salt, and demonstrated the mobile nature of mercury resistance determinants among Bacillus organisms. Moreover, the findings of the worldwide distribution of TnMERI1-like mercury resistance transposons indicated the ubiquitousness of the catabolic genes (Bogdanova et al. 1998; Matsui et al. 2016; Narita et al. 2004). These studies prompted us to propose a new mercury bioremediation strategy targeting the mercury resistance transposon of Bacilli.
In this mini review, we discuss a possible strategy of transposon-mediated in situ molecular breeding (ISMoB) of HgR Bacilli in natural habitats. In ISMoB, breeding target microorganisms are indigenous Bacilli that are not HgR but are dominant in the environment and have excellent survival. We thus propose a new concept of environmental mercury pollution bioremediation using transposon-mediated ISMoB and ways to apply this concept.
Diversity of HgR determinants in Bacilli isolated from Minamata Bay sediment
Toxic mercurials enhanced the abundance of mercury resistance genes and bacteria in Minamata Bay sediment in Japan (Nakamura et al. 1986, 1990). Previous studies have also suggested the contribution of bacterial in the in situ remediation activity of mercury-polluted environments (Huang et al. 1999b; Narita et al. 2003). B. megaterium MB1, a broad-spectrum HgR Bacillus strain, was isolated from Minamata Bay sediment. The sediment was collected in June 1984, before the sediment was dredged by authorities to remove mercury contamination, and was stored under air-dry conditions at 4 °C. The detailed mercury resistance genetic module was characterized from B. megaterium MB1 chromosomal DNA (Huang et al. 1999a, b). This genetic module is almost identical to the mer operon of Gram-positive bacteria previously found from B. cereus RC607 (Gupta et al. 1999; Wang et al. 1989), which was isolated in the USA, and an Exiguobacterium sp. strain TC38-2b, which was isolated in Ukraine (Bogdanova et al. 1998; Bogdanova and Mindlin 1991). The mer genes from B. megaterium MB1 are encoded on a Tn3-family replicative transposon designated as TnMERI1 (Huang et al. 1999b). This indicates that the transposons are involved in the horizontal dissemination of mercury resistance among Gram-positive bacteria. The previous observation of low genetic diversity in Minamata Bay sediment may reflect a peculiarity of this site (Huang et al. 1999b; Nakamura and Silver 1994), enabling us to further determine the molecular diversity of the Minamata Bay mercury-resistant Bacillus population.
In addition to the B. megaterium MB1, 30 HgR Bacilli (designated MB2 to MB31) were also isolated from the same Minamata Bay sediment samples, and the mercury resistance genes among these regional Bacilli were characterized in detail (Table 1) (Narita et al. 2003). All isolates were Gram-positive and grew as rod-shaped spore-forming cells. Eight isolates (MB11, MB12, MB13, MB22, MB24, MB25, MB26, and MB27) were facultative anaerobes while the other 22 were strict aerobes. Their mercury resistance was divided into a narrow spectrum, showing resistance to inorganic mercury salt only and broad-spectrum mercury resistance by their phenotype. Polymerase chain reaction (PCR) amplification analysis targeting different mer genes against the 11 broad-spectrum HgR Bacillus isolates resulted in the amplification of the PCR fragments of the same size as those from the B. megaterium MB1 strain. Southern hybridization analysis showed that these 11 isolates carried the merA, B1, B2, and B3 genes similar to B. megaterium MB1. Eleven of the 30 broad-spectrum HgR Bacillus isolate HgR strains possessed merB genes; three of 30 unidentified-genotype phenyl-mercury acetate (PMA) resistance Bacillus isolates (MB19, MB20, and MB21) did not contain MB1-type merB genes. A different PMA resistance mechanism may exist in these bacteria. Furthermore, three isolates (MB11, MB12, and MB13) did not hybridize with the merA probes from B. megaterium MB1 and did not produce mer-related PCR products. As these three isolates showed high minimum inhibition concentrations (MIC) toward mercury chloride, other resistance mechanisms may be employed by these strains.
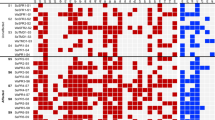
PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the entire 6.8 kb mer operon encoding merB3, R1, E, T, P, A, R2, B2, and B1 in the 11 isolates showed identical results with that of the B. megaterium MB1 mer operon. Thus, 11 of 30 isolates had the same mer operon as that of TnMERI1 in B. megaterium MB1. Targeting the 1.3 kbp merA core region, the diversity of merA gene structure configurations was determined using RFLP profiles. The 11 broad-spectrum HgR Bacillus isolates showed identical RFLP patterns, whereas the 16 narrow-spectrum isolates and the three unidentified genotype HgR Bacilli were classified into six classes including five new RFLP classes not previously observed (Fig. 1) (Hart et al. 1998). The most abundant merA sequences were class 2 type and were shared by eight narrow-spectrum Minamata Bay Bacillus isolates and 13 previously identified English Bacillus isolates (Hart et al. 1998). Thus, the class 2 merA sequences are distributed globally and are shared by different Bacillus species in different geographic regions.
Restriction fragment length polymorphism (RFLP) patterns with seven restriction endonucleases of amplified 1.3-kbp merA PCR products from Minamata Bay Bacillus isolates. Modified from Narita et al. (2003). For HinfI digests, doublets are represented as bands of double thickness. The RFLP classes I and VI were previously described in Hart et al. (1998), and classes XXIII to XXVII are new classes identified in our study. The RFLP classes XXVIII to XXXI are also new classes obtained from 1.3-kbp merA sequences of Russian and Ukrainian Bacillus isolates
A previous study demonstrated that variations of mer genes among bacteria from less polluted soils are greater than those in bacteria from mercury-polluted soils (Olson et al. 1991). The employed Minamata Bay sediment sample was highly polluted by Hg2+ and methylmercury, and a low genetic diversity was observed from the studied broad-spectrum HgR Bacillus isolates. These results are consistent with the strong selective pressure of organomercurials, mainly methylmercury, on the broad-spectrum HgR Bacilli in the studied sediment. However, these results also indicate a weaker selective pressure of inorganic mercury on narrow-spectrum mercury detoxification genes among Bacilli in the same sediment. These differences demonstrate that broad-spectrum HgR Bacillus may be responsible for resistance to toxic pollutants in Minamata Bay sediment and that bacteria carrying broad-spectrum HgR were selected. At the same time, horizontal dissemination of mer genes may occur under weaker selective pressure of inorganic mercury and may play a key role in the adaptation of bacterial populations to environmental contaminants.
Worldwide dissemination of mercury resistance transposons in Bacilli
Bacteria are ubiquitous in the atmosphere and can be transported by wind over long distances (Burrows et al. 2009). Generally, the survival of disseminated non-indigenous microorganisms is expected to be low. However, spores of Bacilli show high tolerance against harsh environmental conditions such as heat and desiccation (Nicholson et al. 2000). Previous studies showed that spore-forming Clostridium spp. and Bacillus spp. were viable after 30 years on the Antarctic Peninsula, while fecal coliforms lost their viability rapidly under the same conditions (Hughes and Nobbs 2004). Thus, spore-forming Bacilli have advantages for long-term survival and transportation over long distances.
Bacilli showing mercury resistance have been described repeatedly in various geographically distinguished regions worldwide (Table 2). Indeed, mercury-resistant Bacilli of different genera and species have been described. Their mercury resistance determinants were identified on mobile genetic elements such as plasmids and transposons. In Gram-negative bacteria, transposons carrying mercury resistance determinants have been well documented including the Tn21-, Tn501-, and -Tn5053 families (Mindlin and Petrova 2013). However, few studies have investigated Bacilli. The linkage between mer operons of Gram-positive bacteria and transposition genes was first reported by Bogdanova et al. in 1998. Further studies subsequently confirmed that some mercury resistance determinants of Gram-positive bacteria are located on transposons, similar to Tn3 (Bogdanova et al. 2001; Huang et al. 1999b). TnMERI1 was reported in the chromosome of Bacillus megaterium MB1, an isolate from mercury polluted sediment in Minamata Bay, Japan (Huang et al. 1999b). TnMERI1 carries genes that participate in the resistance to organomercurials and inorganic mercury salts. Three closely related TnMERI1-like transposons are Tn5083 from B. megaterium MK64-1 in the Kuril Islands, Russia; Tn5084 from B. cereus RC607 in Boston Harbor, USA; and Tn5085 from Exiguobacterium sp. TC38-2b in Carpathian, Ukraine (Bogdanova et al. 2001). Among these, the DNA sequence of Tn5083 has yet to be completely determined. Our laboratory also identified TnMERI1, Tn5084, and Tn5085 transposons in 21 of 56 Bacilli isolates from worldwide environmental samples (Narita et al. 2004).
We further investigated 12 of 65 spore-forming HgR bacteria that were isolated from natural environments worldwide to understand the acquisition of additional genes by and dissemination of HgR Tn3-family of replicative transposons across related Bacilli genera via horizontal gene transfer (HGT) (Matsui et al. 2016). The finding of different TnMERI1-like transposons, including Tn6294 (a newly identified mercury resistance transposon) and TndMER3 (a newly identified deleted transposon-like fragment carrying mercury resistance determinants), suggests the diversity of mercury resistance transposons among Bacilli, similar to mercury resistance transposons in Gram-negative bacteria. Moreover, the identification of Tn6294 in Bacillus sp. from Taiwan and in Paenibacillus sp. from Antarctica is noteworthy. The horizontal dissemination of TnMERI1-like transposons across bacterial species and geographical barriers indicates the worldwide distribution of Bacilli carrying mercury resistance transposons in the environment.
Transposition of Bacilli Tn3-family of replicative transposon
TnMERI1-like transposons are generally unitary non-composite structures belonging to the Tn3-family (Nicolas et al. 2015). Tn3-family transposons replicate during integration into the target sequence, and this replicative mode allows the success of proliferation of catabolic genes in bacteria (Nojiri et al. 2004). Although the molecular mechanisms underlying Tn3-family transposons have been well characterized, the triggers that stimulate and attenuate the transposition process are not well understood.
Transposition activity of TnMERI1-like transposon (Tn5085) was first revealed experimentally in Escherichia coli cells (Bogdanova et al. 2001). The transposition of Tn5085 into the recipient plasmid was shown to occur with cointegration of plasmid formation in a recombinase A (recA)-deficient E. coli HB101 strain. These cointegrates were resolved in E. coli IF238, which has a complete recombination system. To evaluate the effect of RecA on translocation of the TnMERI1-like transposon, we constructed a mini-TnMERI1 and confirmed the participation of RecA in the resolution of the cointegrated transposon structure (Matsui et al. 2005). Other studies have also shown that the efficiency of cointegrated resolution is low in the recA-deficient E. coli strain with different Tn3-type transposons from Gram-negative bacteria (e.g., Tn4652 and TnHad2) (Sota et al. 2002; Tsuda and Iino 1987). RecA may contribute to resolution of the cointegrates and facilitate the translocation of transposons to other replicons. However, in the case of TnMERI1, cell treatment with stress agents, including UV irradiation doses of up to 3000 J m−2, did not alter transposition frequencies, indicating that RecA functions independently from SOS stress responses (Matsui et al. 2005).
Although environmental stress seems to be an important parameter facilitating transposition activity, recent studies have indicated the facilitation of HGT under non-selected conditions. It has been shown that the conjugative mercury resistance plasmid pQBR57 is expanded among Pseudomonas fluorescens populations via HGT without mercury selection. Selection with mercury stimulated the clonal expansion of mercury-resistant bacteria but did not stimulate HGT (Stevenson et al. 2017). Similar results were obtained in our previous study on the Minamata Bay Bacillus populations, as described in the previous section (Narita et al. 2003). Environmental and cellular parameters that influence transposition activity of TnMERI1-like transposons are scarcely characterized; however, the dissemination of transposons may occur without mercury selection. Frequent findings of mercury-resistant Bacilli from non-contaminated regions (Table 2) support these findings. The findings from 74 of 78 Bacillus isolates from mercury-polluted Minamata Bay sediment, showing that these carry identical mer determinant to Bacillus sp. RC607 (Nakamura and Silver 1994), agree with the study by Stevenson et al. (2017). Further characterization of these mobile elements will be valuable to disseminate TnMERI1-like transposons for remediation purposes.
Concept of transposon-mediated ISMoB
In conventional bioremediation methods, purification of contaminated environments by microbes is often achieved using specific microbes that degrade or convert environmental pollutants to non-dangerous substances (El Fantroussi and Agathos 2005). Under a mercury contamination scenario, purifying microbes are isolated from the polluted environment and are grown in pure cultures. They are subsequently used for augmentation of capable microbes in the polluted environment, as shown in Fig. 2. In another case, these bacteria are genetically modified by molecular methods and then introduced into the polluted environment. In the latter method, a selected host microorganism is genetically transformed with the functional genes from an isolated microorganism that possesses pollutant degradation or conversion activity, markedly increasing the microbial purification activity (Singh et al. 2011).
However, host microorganisms for use in artificial molecular-breeding are limited in the environment (El Fantroussi and Agathos 2005; Tyagi et al. 2011). Additionally, genetically modified microorganisms must be assessed before their introduction, and their elimination from the site is sometimes required when the purification process is completed (Keese 2008). Usually, microbes introduced into the environment that are not indigenous and that do not acclimatize are less competitive against wild microbes and are targeted by grazing organisms in the environment (Cunningham et al. 2009; Kota et al. 1999). If we are able to transfer special genetic components for environmental purification from introduced microbes to indigenous and predominant microbes in a habitat, and if the transferred microbes can express these genetic features, naturally bred transformant microbes will be preferable for environmental bioremediation (Wiedenbeck and Cohan 2011). Indeed, these microbes are more familiar with the environment and have survival advantage over introduced microbes (Ikuma and Gunsch 2013).
As described in the above sections, the same genetic components involved in microbial response to environmental deterioration are common within various microbial species due to transposon transfer. Therefore, transposon-mediated HGT is considered an effective tool for natural breeding of remediating microbes in polluted environments (Shahi et al. 2017). We propose this as “transposon-mediated in situ molecular breeding (ISMoB)”; ISMoB is a fundamentally natural process. Introduced donor microbes are not indigenous but possess transposons for environmental purification, and recipient microbes are indigenous, dominant, and robust in the given environment. However, the ISMoB process can be enhanced by adjusting conditional factors.
Three major factors are involved in transposon-mediated ISMoB. First, effective transposons and mobilization vectors are required. Next, excision of the transposons from vectors and insertion of transposons to the genomic DNA in the recipients must occur. Lastly, there must be mating probability between the donor and recipient microbes. As previously mentioned, useful transposons for environmental purification exist, and some of them are conjugative transposons and self-transmissible (Burrus et al. 2002; Salyers et al. 1995). However, many others are non-conjugative transposons. In the process of intercellular transfer of non-conjugative transposons, lysogenic phages, conjugative plasmids, genomic islands, or other unclassified elements are needed as transferring vectors (Shahi et al. 2017; Tan 1999). The excision and insertion capabilities of a transposon originate from the genetic elements of the transposon itself, while other host cellular components may enhance the excision and insertion of transposons (Bellanger et al. 2014).
Transposon-harboring microbes that contain genetic elements for pollutant removal and for excision/insertion can be found and isolated from natural or polluted environments (Wright et al. 2008). In addition, vectors can be used for intercellular transfer of these transposons from the environment (Wyndham et al. 1994). By combining effective transposons and transfer vectors, two of the three required factors for ISMoB are provided. The third factor requires the development of methodologies to enhance the possibility of mating between donor and recipient microbes. In laboratories, transposon-mediated transfer of genetic elements beyond the border of microbial species has been established. However, in situ mating methods for ISMoB are not yet established. An important first step of transposon-mediated in situ gene transfer is to establish microbial mating with cell-to-cell contact or cell aggregation of both donor and recipient microbes.
Figure 3 shows a schematic diagram depicting ISMoB use for bioremediation of environments polluted with mercury. A facility with biofilm or immobilization technology to enhance the mating of donor and recipient microbes is required to perform ISMoB. Biofilm technology is considered an effective way to provide an adequate mating environment for these microbes. Biofilm formation media can be used to provide adhesion surfaces to increase mating opportunities, and use of entrapping medium is also effective to enable prolonged co-existence of mating microbes. Nutrients are supplied to increase numbers of the introduced donor and indigenous recipient microbes and mating activities of them in the polluted environment.
As mentioned above, we found that mer genes were distributed worldwide in Bacilli with the dissemination of TnMERI1-like transposons (Matsui et al. 2016). To confirm the mobile nature of mer genes and the ways in which HgR Bacilli take root in the environment, we developed a reliable quantitative procedure with a real-time PCR-based method for identifying mer genes in environmental soil samples. Using the developed method, we could only quantify mer genes in from 7 out of the 70 soil samples analyzed. The other 63 sites, including the sites augmented with Bacilli harboring known HgR transposons, contained quantities of genetic material that were below the detection limit of the real-time PCR assay. However, further cultivation of the unsterilized soils under nutrient-rich conditions allowed the detection of the mer genes from HgR Bacillus via PCR (data not published). These results suggest that recently appeared HgR Bacilli could propagate under suitable soil environmental conditions. This finding prompted us to propose transposon-mediated ISMoB of HgR bacteria in the environments. We are now seeking direct evidence of HGT with the HgR transposons in soil samples, and the results of this research will be presented in our next original paper.
Bioreactors are also available for ISMoB. Using bioreactors for removal of contaminants is an ex situ procedure. However, bioreactors can be more effective in enhancing transposon transfers than in situ methods. In bioreactors, microbes that are gel-entrapped or surface-immobilized onto fixed beds or fluidized bed systems can be used to provide adequate room and time for mating between donor and recipient microbes. Propagating conditions for microbes including nutrients, temperature, and pH after mating, as well as genetic transfer, can also be easily delivered in bioreactors.
New environmental biotechnology for mercury bioremediation using transposon-mediated ISMoB
Recently, Garbisu et al. (2017) proposed plasmid-mediated bioaugmentation and its utilization in bioremediation of contaminated soils. This technology is effective if plasmids with environmental purifying genes are stable in the environment of the recipient microbes. However, possession of genes with plasmids usually renders bacteria less stable than genes integrated in recipient microbe chromosomal DNA. Therefore, in ISMoB, transposon-mediated gene integration into microbial chromosomes is newly proposed.
To remove mercury from contaminated environments by applying transposon-mediated ISMoB, microbes that are highly HgR and can actively reduce and volatilize mercury from the environments are isolated. It is then determined whether the isolates possess HgR operons and their specific location. Possession of vectors for interspecies transfer of HgR transposons is also investigated. If an isolate with a HgR transposon does not possess a vector for intercellular gene transfer, the microbe must be transformed with appropriate conjugative plasmids or phages (Garbisu et al. 2017; Shahi et al. 2017).
Non-indigenous microbes with HgR transposons and conjugative plasmids are introduced into mercury-contaminated sites as donors of HgR transposons. In these sites, procedures to enhance HGT from the donor to the indigenous recipient microbes are applied, which include supplementation with nutrients and mating location to activate ISMoB. After HGT, indigenous recipient microbes survive and propagate, executing more effective remediation activity.
As described above, B. megaterium MB1 isolated from Minamata Bay, Japan possesses a HgR transposon, TnMERI1; B. cereus RC607 isolated from Boston Harbor, USA possesses a HgR transposon, Tn5084; and Exiguobacterium sp. Tc38-2b isolated from Carpathian Mountains, Ukraine possesses a HgR transposon, Tn5085 (Bogdanova et al. 2001; Narita et al. 2004). These HgR bacteria can be used as donors for transposon-mediated ISMoB. However, no vector from these HgR bacteria has been identified for interspecies HGT. Effective HgR transposon vehicles must be found for interspecies transfer and use in ISMoB.
A gene transfer module of the HgR transposon TnMERI1 from B. megaterium MB1 contains tnpT and tnpR for genetic transposition and a bacterial group II intron named B.me.I1 (Huang et al. 1999b; Chien et al. 2008). However, TnMERI1 does not contain the same IR sequences at each end of the transposon. In this case, the IR sequences should be repaired before using this bacterium for ISMoB to increase the possibility of transfer. The use of B.me.I1 as a gene transfer carrier may also be considered since group II bacterial introns can splice themselves from the transcribed intron RNA and home to other genetic regions. Further research on intron-mediated gene transfer between microbes is warranted.
In conclusion, interspecies gene transfer in natural environments actively occurs via transposon mediation and is applicable to ISMoB. To use transposon-mediated ISMoB as a new biotechnology for environmental mercury pollution control, finding and using effective vectors such as conjugative and broad-range transferable plasmids, providing appropriate mating conditions for both the donor and recipient microbes, and developing augmentation methods for naturally bred microbes are required.
References
Amin A, Latif Z (2017) Screening of mercury-resistant and indole-3-acetic acid producing bacterial-consortium for growth promotion of Cicer arietinum L. J Basic Microbiol 57:204–217. https://doi.org/10.1002/jobm.201600352
Baldi F, Marchetto D, Gallo M, Fani R, Maida I, Covelli S, Fajon V, Zizek S, Hines M, Horvat M (2012) Chlor-alkali plant contamination of Aussa River sediments induced a large Hg-resistant bacterial community. Estuar Coast Shelf Sci 113:96–104. https://doi.org/10.1016/j.ecss.2012.04.017
Barkay T, Miller SM, Summers AO (2003) Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev 27:355–384. https://doi.org/10.1016/s0168-6445(03)00046-9
Begley TP, Walts AE, Walsh CT (1986) Mechanistic studies of a protonolytic organomercurial cleaving enzyme-bacterial organomercurial lyase. Biochemistry-US 25:7192–7200. https://doi.org/10.1021/bi00370a064
Bellanger X, Payot S, Leblond-Bourget N, Guedon G (2014) Conjugative and mobilizable genomic islands in bacteria: evolution and diversity. FEMS Microbiol Rev 38:720–760. https://doi.org/10.1111/1574-6976.12058
Bogdanova ES, Mindlin SZ (1991) Occurrence of 2 structural types of mercury reductases among gram-positive bacteria. FEMS Microbiol Lett 78:277–280
Bogdanova ES, Bass IA, Minakhin LS, Petrova MA, Mindlin SZ, Volodin AA, Kalyaeva ES, Tiedje JM, Hobman JL, Brown NL, Nikiforov VG (1998) Horizontal spread of mer operons among gram-positive bacteria in natural environments. Microbiology-UK 144:609–620
Bogdanova E, Minakhin L, Bass I, Volodin A, Hobman JL, Nikiforov V (2001) Class II broad-spectrum mercury resistance transposons in gram-positive bacteria from natural environments. Res Microbiol 152:503–514
Burrows SM, Elbert W, Lawrence MG, Pöschl U (2009) Bacteria in the global atmosphere—part 1: review and synthesis of literature data for different ecosystems. Atmos Chem Phys 9:9263–9280. https://doi.org/10.5194/acp-9-9263-2009
Burrus V, Pavlovic G, Decaris B, Guedon G (2002) Conjugative transposons: the tip of the iceberg. Mol Microbiol 46:601–610. https://doi.org/10.1046/j.1365-2958.2002.03191.x
Chien MF, Huang CC, Kusano T, Endo G (2008) Facilities for transcription and mobilization of an exon-less bacterial group II intron nested in transposon TnMERI1. Gene 408:164–171. https://doi.org/10.1016/j.gene.2007.10.032
Cunningham JJ, Kinner NE, Lewis M (2009) Protistan predation affects trichloroethene biodegradation in a bedrock aquifer. Appl Environ Microbiol 75:7588–7593. https://doi.org/10.1128/aem.01820-09
Dash HR, Mangwani N, Das S (2014) Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05. Environ Sci Pollut Res 21:2642–2653. https://doi.org/10.1007/s11356-013-2206-8
El Fantroussi S, Agathos SN (2005) Is bioaugmentation a feasible strategy for pollutant removal and site remediation? Curr Opin Microbiol 8:268–275. https://doi.org/10.1016/j.mib.2005.04.011
Figueiredo NL, Canario J, O'Driscoll NJ, Duarte A, Carvalho C (2016) Aerobic mercury-resistant bacteria alter mercury speciation and retention in the Tagus Estuary (Portugal). Ecotoxicol Environ Saf 124:60–67. https://doi.org/10.1016/j.ecoenv.2015.10.001
Garbisu C, Garaiyurrebaso O, Epelde L, Grohmann E, Alkorta I (2017) Plasmid-mediated bioaugmentation for the bioremediation of contaminated soils. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.01966
Grinsted J, Delacruz F, Schmitt R (1990) The Tn21 subgroup of bacterial transposable elements. Plasmid 24:163–189. https://doi.org/10.1016/0147-619x(90)90001-s
Gupta A, Phung LT, Chakravarty L, Silver S (1999) Mercury resistance in Bacillus cereus RC607: transcriptional organization and two new open reading frames. J Bacteriol 181:7080–7086
Harada M (1995) Minamata disease—methylmercury poisoning in Japan caused by environmental-pollution. Crit Rev Toxicol 25:1–24. https://doi.org/10.3109/10408449509089885
Hart MC, Elliott GN, Osborn AM, Ritchie DA, Strike P (1998) Diversity amongst Bacillus merA genes amplified from mercury resistant isolates and directly from mercury polluted soil. FEMS Microbiol Ecol 27:73–84
Hobman JL, Brown NL (1997) Bacterial mercury-resistance genes. In: Sigel A, Sigel H (eds) Metal ions in biological systems, vol 34: Mercury and its effects on environment and biology. Marcel Dekker, New York, pp 527–568
Holt RJ, Bruce KD, Strike P (1999) Conservation of transposon structures in soil bacteria. FEMS Microbiol Ecol 30:25–37. https://doi.org/10.1016/s0168-6496(99)00036-7
Huang CC, Narita M, Yamagata T, Endo G (1999a) Identification of three merB genes and characterization of a broad-spectrum mercury resistance module encoded by a class II transposon of Bacillus megaterium strain MB1. Gene 239:361–366
Huang CC, Narita M, Yamagata T, Itoh Y, Endo G (1999b) Structure analysis of a class II transposon encoding the mercury resistance of the gram-positive bacterium Bacillus megaterium MB1, a strain isolated from Minamata Bay, Japan. Gene 234:361–369
Hughes KA, Nobbs SJ (2004) Long-term survival of human faecal microorganisms on the Antarctic Peninsula. Antarct Sci 16:293–297. https://doi.org/10.1017/s095410200400210x
Ikuma K, Gunsch CK (2013) Successful genetic bioaugmentation with Pseudomonas putida for toluene degradation in soil columns. Environ Chem Lett 11:365–370. https://doi.org/10.1007/s10311-013-0416-4
Kannan SK, Mahadevan S, Krishnamoorthy R (2006) Characterization of a mercury-reducing Bacillus cereus strain isolated from the Pulicat Lake sediments, south east coast of India. Arch Microbiol 185:202–211. https://doi.org/10.1007/s00203-006-0088-6
Keese P (2008) Risks from GMOs due to horizontal gene transfer. Environ Biosaf Res 7:123–149. https://doi.org/10.1051/ebr:2008014
Kiyono M, Pan-Hou H (1999) The merG gene product is involved in phenylmercury resistance in Pseudomonas strain K-62. J Bacteriol 181:726–730
Kota S, Borden RC, Barlaz MA (1999) Influence of protozoan grazing on contaminant biodegradation. FEMS Microbiol Ecol 29:179–189
Liebert CA, Hall RM, Summers AO (1999) Transposon Tn21, flagship of the floating genome. Microbiol Mol Biol R 63:507−522
Liebert CA, Watson AL, Summers AO (2000) The quality of merC, a module of the mer mosaic. J Mol Evol 51:607–622
Mahler I, Levinson HS, Wang Y, Halvorson HO (1986) Cadmium- and mercury-resistant Bacillus strains from a salt marsh and from Boston Harbor. Appl Environ Microbiol 52:1293–1298
Matsui K, Narita M, Ishii H, Endo G (2005) Participation of the recA determinant in the transposition of class II transposon mini-TnMERI1. FEMS Microbiol Lett 253:309–314. https://doi.org/10.1016/j.femsle.2005.09.053
Matsui K, Yoshinami S, Narita M, Chien MF, Phung LT, Silver S, Endo G (2016) Mercury resistance transposons in Bacilli strains from different geographical regions. FEMS Microbiol Lett 363:fnw013. https://doi.org/10.1093/femsle/fnw013
Medina JAC, Farias JE, Cruz Hernandez AC, Martinez RG, Valdes SS, Silva GH, Jones GH, Campos-Guillen J (2013) Isolation and characterization of mercury resistant Bacillus sp from soils with an extensive history as substrates for mercury extraction in Mexico. Geomicrobiol J 30:454–461. https://doi.org/10.1080/01490451.2012.705229
Mindlin S, Petrova M (2013) Mercury resistance transposons. In: Roberts AP, Mullany P (eds) Bacterial integrative mobile genetic elements. Landes Bioscience, Austin, pp 33–52
Misra TK (1992) Bacterial resistances to inorganic mercury salts and organomercurials. Plasmid 27:4–16. https://doi.org/10.1016/0147-619x(92)90002-r
Nakamura K, Silver S (1994) Molecular analysis of mercury-resistant Bacillus isolates from sediment of Minamata Bay, Japan. Appl Environ Microbiol 60:4596–4599
Nakamura K, Fujisaki T, Tamashiro H (1986) Characteristics of Hg-resistant bacteria isolated from Minamata Bay sediment. Environ Res 40:58–67. https://doi.org/10.1016/s0013-9351(86)80081-0
Nakamura K, Fujisaki T, Shibata Y (1988) Mercury-resistant bacteria in the sediment of Minamata Bay. Nippon Suisan Gakk 54:1359–1363
Nakamura K, Sakamoto M, Uchiyama H, Yagi O (1990) Organomercurial-volatilizing bacteria in the mercury-polluted sediment of Minamata Bay, Japan. Appl Environ Microbiol 56:304–305
Narita M, Huang CC, Koizumi T, Endo G (1999) Molecular analysis of merA gene possessed by anaerobic mercury-resistant bacteria isolated from sediment of Minamata Bay. Microbes Environ 14:77–84. https://doi.org/10.1264/jsme2.14.77
Narita M, Chiba K, Nishizawa H, Ishii H, Huang C-C, Kawabata Z, Silver S, Endo G (2003) Diversity of mercury resistance determinants among Bacillus strains isolated from sediment of Minamata Bay. FEMS Microbiol Lett 223:73–82. https://doi.org/10.1016/s0378-1097(03)00325-2
Narita M, Matsui K, Huang CC, Kawabata Z, Endo G (2004) Dissemination of TnMERI1-like mercury resistance transposons among Bacillus isolated from worldwide environmental samples. FEMS Microbiol Ecol 48:47–55. https://doi.org/10.1016/j.femsec.2003.12.011
Nazaret S, Brothier E, Ranjard L (2003) Shifts in diversity and microscale distribution of the adapted bacterial phenotypes due to Hg(II) spiking in soil. Microbial Ecol 45:259–269. https://doi.org/10.1007/s00248-002-2035-7
Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P (2000) Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol Mol Biol R 64:548−572. https://doi.org/10.1128/mmbr.64.3.548-572.2000
Nicolas E, Lambin M, Dandoy D, Galloy C, Nguyen N, Oger CA, Hallet B (2015) The Tn3-family of replicative transposons. Microbiol Spectr 3. https://doi.org/10.1128/microbiolspec.MDNA3-0060-2014
Nithya C, Gnanalakshmi B, Pandian SK (2011) Assessment and characterization of heavy metal resistance in Palk Bay sediment bacteria. Mar Environ Res 71:283–294. https://doi.org/10.1016/j.marenvres.2011.02.003
Nojiri H, Shintani M, Omori T (2004) Divergence of mobile genetic elements involved in the distribution of xenobiotic-catabolic capacity. Appl Microbiol Biotechnol 64:154–174. https://doi.org/10.1007/s00253-003-1509-y
Nucifora G, Silver S, Misra TK (1989) Down regulation of the mercury resistance operon by the most promoter-distal gene merD. Mol Gen Genet 220:69–72
Olson BH, Cayless SM, Ford S, Lester JN (1991) Toxic element contamination and the occurrence of mercury-resistant bacteria in Hg-contaminated soil, sediments, and sludges. Arch Environ Contam Toxicol 20:226–233
Olukoya DK, Smith SI, Ilori MO (1997) Isolation and characterization of heavy metals resistant bacteria from Lagos Lagoon. Folia Microbiol 42:441–444. https://doi.org/10.1007/bf02826550
Oregaard G, Sorensen SJ (2007) High diversity of bacterial mercuric reductase genes from surface and sub-surface floodplain soil (Oak Ridge, USA). ISME J 1:453–467. https://doi.org/10.1038/ismej.2007.56
Osborn AM, Bruce KD, Strike P, Ritchie DA (1997) Distribution, diversity and evolution of the bacterial mercury resistance (mer) operon. FEMS Microbiol Rev 19:239–262
Rochelle PA, Wetherbee MK, Olson BH (1991) Distribution of DNA-sequences encoding narrow-spectrum and broad-spectrum mercury resistance. Appl Environ Microbiol 57:1581–1589
Sadhukhan PC, Ghosh S, Chaudhuri J, Ghosh DK, Mandal A (1997) Mercury and organomercurial resistance in bacteria isolated from freshwater fish of wetland fisheries around Calcutta. Environ Pollut 97:71–78. https://doi.org/10.1016/s0269-7491(97)00068-7
Salyers AA, Shoemaker NB, Stevens AM, Li LY (1995) Conjugative transposons—an unusual and diverse set of integrated gene-transfer elements. Microbiol Rev 59:579–590
Selin NE (2009) Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resour 34:43–63. https://doi.org/10.1146/annurev.environ.051308.084314
Shahi A, Ince B, Aydin S, Ince O (2017) Assessment of the horizontal transfer of functional genes as a suitable approach for evaluation of the bioremediation potential of petroleum-contaminated sites: a mini-review. Appl Microbiol Biotechnol 101:4341–4348. https://doi.org/10.1007/s00253-017-8306-5
Silva A, de Carvalho MAR, de Souza SAL, Dias PMT, da Silva RG, Saramago CSD, Bento CAD, Hofer E (2012) Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz J Microbiol 43:1620–1631
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789
Silver S, Endo G, Nakamura K (1994) Mercury in the environment and the laboratory. J Jpn Soc Wat Environ 17:234–243
Singh JS, Abhilash PC, Singh HB, Singh RP, Singh DP (2011) Genetically engineered bacteria: an emerging tool for environmental remediation and future research perspectives. Gene 480:1–9. https://doi.org/10.1016/j.gene.2011.03.001
Sota M, Endo M, Nitta K, Kawasaki H, Tsuda M (2002) Characterization of a class II defective transposon carrying two haloacetate dehalogenase genes from Delftia acidovorans plasmid pUO1. Appl Environ Microbiol 68:2307–2315. https://doi.org/10.1128/eam.68.5.2307-2315.2002
Stevenson C, Hall JPJ, Harrison E, Wood AJ, Brockhurst MA (2017) Gene mobility promotes the spread of resistance in bacterial populations. ISME J 11:1930–1932. https://doi.org/10.1038/ismej.2017.42
Tan HM (1999) Bacterial catabolic transposons. Appl Microbiol Biotechnol 51:1–12
Trevors JT (1987) Mercury-resistance and mercuric reductase activity in Chromobacterium, Erwinia, and Bacillus species. Bull Environ Contam Toxicol 38:1070–1075
Tsubaki T, Irukayayama K (1977) Minamata disease: methylmercury poisoning in Minamata and Niigata, Japan. Kodansha, Tokyo, 317p
Tsuda M, Iino T (1987) Genetic analysis of a transposon carrying toluene degrading genes on a TOL plasmid pWWO. Mol Gen Genet 210:270–276. https://doi.org/10.1007/bf00325693
Tyagi M, da Fonseca MM, de Carvalho CC (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22:231–241. https://doi.org/10.1007/s10532-010-9394-4
Wang Y, Moore M, Levinson HS, Silver S, Walsh C, Mahler I (1989) Nucleotide sequence of a chromosomal mercury resistance determinant from a Bacillus sp. with broad-spectrum mercury resistance. J Bacteriol 171:83–92
Wiedenbeck J, Cohan FM (2011) Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol Rev 35:957–976. https://doi.org/10.1111/j.1574-6976.2011.00292.x
Wilson JR, Leang C, Morby AP, Hobman JL, Brown NL (2000) MerF is a mercury transport protein: different structures but a common mechanism for mercuric ion transporters? FEBS Lett 472:78–82. https://doi.org/10.1016/s0014-5793(00)01430-7
Wright MS, Baker-Austin C, Lindell AH, Stepanauskas R, Stokes HW, McArthur JV (2008) Influence of industrial contamination on mobile genetic elements: class 1 integron abundance and gene cassette structure in aquatic bacterial communities. ISME J 2:417–428. https://doi.org/10.1038/ismej.2008.8
Wyndham RC, Nakatsu C, Peel M, Cashore A, Ng J, Szilagyi F (1994) Distribution of the catabolic transposon Tn5271 in a groundwater bioremediation system. Appl Environ Microbiol 60:86–93
Acknowledgements
The authors thank and acknowledge Prof. Simon Silver, Prof. Nigel Brown, Prof. John Hobman, Dr. Elena Bogdanova, Dr. Chieh Chen Huang, and Dr. Masaru Narita for their kind collaboration and cooperation while performing transposon-mediated ISMoB research for mercury bioremediation.
Funding
This work was supported by JSPS KAKENHI (Grant Number: 16K07529).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Matsui, K., Endo, G. Mercury bioremediation by mercury resistance transposon-mediated in situ molecular breeding. Appl Microbiol Biotechnol 102, 3037–3048 (2018). https://doi.org/10.1007/s00253-018-8847-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8847-2