Abstract
Bacterial wilt, caused by Ralstonia solanacearum, occurs occasionally during tobacco planting and potentially brings huge economic losses in affected areas. Soil microbes in different management stages play important roles in influencing bacterial wilt incidence. Studies have focused on the impacts of species diversity and composition during cropping periods on disease morbidity; however, the effects of the soil bacterial biomass, species diversity, species succession, and population interactions on morbidity remain unclear during non-cropping periods. In this study, we explored the soil bacterial communities in the non-cropping winter fallow (WF) and cropping late growing (LG) periods under consecutive monoculture systems using 16S ribosomal RNA gene sequencing and qPCR and further analyzed their effects on tobacco bacterial wilt incidence. We found that the bacterial communities in the WF period were significantly different from those in the LG period based on detrended correspondence analysis and dissimilarity tests. Crop morbidity was significantly related to bacterial community structure and to the presence of some genera during WF and LG periods. These genera, such as Arthrobacter, Pseudomonas, Acidobacteria GP6, and Pasteuria, may be potential biological control agents for bacterial wilt. Further analysis indicated that low soil bacterial diversity during the WF period, decrease of bacterial interactions from the WF to LG periods, and low soil biomass during the LG period all have the potential to increase morbidity. In conclusion, an increase of soil bacterial diversity and control of some bacterial abundances in the WF period might be an effective approach in controlling bacterial wilt incidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial wilt can infect many crops, such as tobacco and tomato, and therefore, causes huge economic losses. It is a general response of morbidity to soil environments, including soil type, nutrients, bacteria, and fungi (Kyselková et al. 2009). The traditional approaches to control bacterial wilt are as follows: (i) application of pesticides such as streptomycin sulfate, (ii) soil removal and replacement, and (iii) crop rotation (Niu et al. 2017). However, the use of pesticides is damaging to the soil ecosystem and unhealthy for human, soil removal and replacement is expensive, and it is difficult to select crops for rotation with tobacco. In addition, disease incidence is determined by many factors, e.g., disease resistance ability of the plant, infection ability of the pathogen, soil conditions, climate, and soil microbial communities (Schmidt et al. 2014; Berkovitch 1996; Bramley et al. 1996; Franke-Whittle et al. 2015). Factors such as infection ability of pathogen and climate are uncontrollable, whereas the soil microbial community can be artificially controlled. Therefore, management of the soil microbial community is a good and optional approach for biocontrol of plant diseases.
The cycle of annual crop agriculture includes both non-cropping and cropping periods. The cropping periods include planting, seedling, growing, and late growing (LG)/mature periods. Soil microbial components and structure differ between these periods, because of the variation in type and quantity of root exudates from plants during different developmental periods (Zhan et al. 2005). The quantity of soil microbes is more abundant during the LG period than the planting period (Hu et al. 2004). However, during the non-cropping winter fallow (WF) period, soil fertility recovers and the soil microbial community changes accordingly. Many bacteria, including pathogenic bacteria such as Ralstonia, are able to survive and propagate in soils during the WF period (Huo et al. 2007). Therefore, the impacts of soil microbial community in the WF period on crops should not be neglected.
Soil microbial diversity plays an important role in plant disease suppression. van Elsas and Salles (2012) reported a decrease in the competitive ability of the invader (Escherichia coli O157:H7) in species-rich bacterial communities, compared with species-poor communities. This is reflected in the amount of resources used and the rate of their consumption, which may explain the negative correlation between the diversity of the soil microbiota and the survival of the invading species, suggesting that microbial diversity can inhibit the invasion of a bacterial pathogen on crops (van Elsas and Salles 2012). Similar phenomenon was also discovered in many other reports (Granér et al. 2003; Mengesha et al. 2017; van Elsas et al. 2002; Shiomi et al. 1999). For instance, Mengesha et al. (2017) demonstrated that bacterial wilt of potatoes grown under controlled conditions could be suppressed by a non-aerated compost tea with a high microbial diversity. However, these studies largely focused on the microbial community during the cropping period, while ignoring the non-cropping fallow period.
Although the importance of soil microorganisms in crop production is well recognized, the relationship between crop morbidity and soil microbial community is complex and remains unclear. On the one hand, plants provide nutrients, such as root exudates or exfoliative root cells for soil microorganism growth and development (Shi et al. 2012). On the other hand, soil microorganisms can either promote or are harmful to plant growth (Lugtenberg and Kamilova 2009). Plant probiotic bacteria benefit plants in many ways (Defoirdt et al. 2007; Kailasapathy 2002; Kesarcodi-Watson et al. 2008; Hai 2015). They can (i) promote nutrient uptake of plants, through increasing the availability of refractory mineral nutrients or enlarging plant root surface area; (ii) remove the toxicity of H2S to roots by sulfur oxidation; and (iii) synthesize and secrete vitamin, amino acid, auxin, and growth stimulant substances (e.g., indole acetic acid and gibberellin), which are helpful for plant growth. Furthermore, some microorganisms can produce antibiotics to inhibit pathogenic bacterial growth and development. Although both fungi and bacteria can be potential biological control agents (BCAs), the majority of BCAs are bacteria (Pal and Gardener 2006). It has been reported that Pseudomonas fluorescens is a common antagonistic bacterium for many plant diseases, because of its ability to produce antibiotics (Mazurier et al. 2009), hydrogen cyanide (Voisard et al. 1989), 2,4-diacetylphloroglucinol (Sharifi-Tehrani et al. 1998), and siderophores (Kloepper et al. 1980). Some Bacillus strains can secrete certain antibiotics and antimicrobial proteins (Leifert et al. 1995), which can efficiently inhibit various plant diseases, such as bacterial wilt (Ahimou and Deleu 2000; Moyne et al. 2004). Bacillus cereus UW85 has shown a wide range of biological effects on plants, including alfalfa (Handelsman et al. 1990), tobacco (Handelsman et al. 1991), and cucumber (Smith et al. 1993). Kloepper et al. (1999) suggested that plant growth-promoting rhizobacteria, endophytic bacteria, and the indigenous soil bacterial community could also effectively decrease crop morbidity. However, BCAs are not effective for all pathogens or plants; under certain conditions, they are harmful to plants, as they can become pathogens and compete with crop for water and nutrients or produce toxic substances (Mazurier et al. 2009).
Bacterial wilt, caused by Ralstonia solanacearum, is distributed globally with a wide host range of 200 plant species, such as tobacco, tomato, potato, peanut, pepper, and eggplant (Mansfield et al. 2012). However, little is known about relationships between the soil bacterial community during the WF period and bacterial wilt morbidity. Understanding the interaction between soil microorganisms during the WF period and morbidity will provide better suggestions for agriculture management. According to the above reports, two hypotheses are put forward in tobacco planting as follows: (i) soil bacterial communities in the WF period, similar to those in the LG period, might be closely related to morbidity of tobacco bacterial wilt, and (ii) some key genera of soil microbial community might be potential biological control agents for bacterial wilt. In the present study, to verify the hypotheses, we investigated the soil bacterial communities of consecutive monoculture in WF and LG periods with high-throughput sequencing technology and explored the effects of soil bacterial community during the WF and LG periods on bacterial wilt disease.
Materials and methods
Field design and sampling
Soil samples were collected from four tobacco consecutive monoculture experiment areas in a long-term ecological research site located in Hunan, China (Table 1). The four areas were continuously cultivated with tobacco for 1 year (N1), 4 years (N4), 5 years (N5), and 12 years (N12), respectively. These four areas were randomly arranged in a complete block design, and the plot size was about 80 m2 (8 m × 10 m). The agricultural management practices and fertilization regimes were similar in all four areas. The applied fertilizer consisted of 50 kg/ha special basal fertilizer, 20 kg/ha special top dressing, 15 kg/ha bio-organic fertilizer, and 5 kg/ha hole-applied fertilizer. The ratio of N/P/K (N, P2O5, and K2O) applied was 1:1.2:2.43. The transplanted crop was irrigated with 300 kg/ha water and 5 kg/ha hole-applied fertilizer. No pest or disease controls were applied during the experiment.
Eight samples were collected from each area using a checkerboard sampling method in the non-cropping WF period (at the end of March) and cropping LG/mature period (at the end of July), respectively. In the checkerboard sampling method, each field was divided into eight areas (each 2 m × 5 m) and the central point of each area was the sampling site. Only topsoil samples (0–20 cm, 3 cm diameter) were obtained. Soil samples were stored at − 80 °C for molecular analyses. The morbidity of each area was determined according to the disease symptoms described previously (Huo et al. 2007), and the formula was as follows: Morbidity = N i /N t × 100%, where N i represents the number of tobacco plants with bacterial wilt symptoms and N t represents the total number of tobacco plants.
DNA extraction
After mixing separately, four copies of 0.25 g soil from each sample was used for DNA extraction using a PowerSoil DNA Isolation Kit (Mo Bio, San Diego, USA) with freeze grinding with a sodium dodecyl sulfate (SDS) lysis buffer, and then four copies of extracted DNA were mixed together. Crude DNA extracts were purified by electrophoresis on a 0.7% agarose gel, followed by phenol extraction, according to the manufacturer’s protocol. The DNA quality was assessed based on the absorbance ratios of OD260/OD280 nm and OD260/OD230 nm using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA), and the DNA concentration was quantified using a PicoGreen (Life Technologies, Grand Island, NY, USA) assay with a FLUOstar Optima (BMG Labtech, Jena, Germany). The purified DNA was stored at − 80 °C for further analysis.
Amplification, 16S ribosomal RNA gene sequencing, and data processing
Tagged PCR products were generated using primer pairs with unique barcodes through PCR. The V4 region of the 16S ribosomal RNA (rRNA) genes was amplified with a primer pair. The primer pair was 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) combined with Illumina adapter sequences, a pad and a linker of two bases, and barcodes on the reverse primers. The 50 μL 16S rRNA PCR amplification reaction mixtures included 0.5 μL of Taq DNA polymerase (TaKaRa, Tokyo, Japan), 5 μL of 10× PCR buffer, 1.5 μL of dNTP mix, 1.5 μL of forward primers (10 μM), 1.5 μL of reverse primers (10 μM), 1 μL of DNA extracts (20–30 ng/μL), and 39 μL of double-distilled water (ddH2O). Samples were amplified using the following program: denaturation at 94 °C for 1 min and 30 cycles of 94 °C for 20 s, 53 °C for 25 s, and 68 °C for 45 s, with a final extension at 68 °C for 10 min. Positive PCR products were confirmed by agarose gel electrophoresis and recovered according to the instructions of the DNA gel extraction kit (Omega, Norcross, GA, USA). The concentration of PCR products was quantified with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA).
Exactly 200 ng of the PCR products of each sample was used to establish sample libraries. Illumina 2× 250-bp paired-end sequencing was performed on a MiSeq machine (Illumina, San Diego, CA) using a MiSeq 500-cycle kit (Wu et al. 2015). The raw data of samples for MiSeq paired-end sequencing were in FASTQ data format, and the adapters and other Illumina-specific sequences from the read were cut and removed. Sequences perfectly matching to barcodes were split into sample libraries and then were trimmed using Btrim. The quantity of reads joined together was counted based on forward and reverse reads with at least 10-bp overlap and lower than 5% mismatches through Flash (Edgar 2010). The joined pairs were then handled with many steps, such as removal of sequences less than 220 bp with ambiguous base “N” and an average base quality score lower than 20. The trimmed sequences were chimera detected and removed using the UCHIME algorithm (Edgar et al. 2011). These processes were conducted using the Quantitative Insights Into Microbial Ecology (QIIME) pipeline (version 1.17). The remaining and unique sequences were clustered at 97% similarity level to generate operational taxonomic units (OTUs) by UPARSE (version 7.1, http://drive5.com/uparse/), and taxonomic assignment was conducted with a RDP classifier at a minimum of 50% confidence (http://rdp.cme.msu.edu/). The abovementioned steps were conducted through the in-lab Galaxy pipeline platform. All the 16S rRNA gene sequences were submitted to the Sequence Read Archive (SRA) of NCBI, and the accession number is SUB3011624.
PCR primers and real-time quantitative PCR
According to the results of Illumina sequencing, five specific primers (Supplemental Table S1) were used for real-time qPCR, including 16S rRNA universal primer for bacteria, primer for phylum Acidobacteria, and primers for genus Acidobacteria GP2, Ralstonia, and Pseudomonas. The reasons for the choice of these five specific primers and typical taxa were as follows: 16S rRNA universal primer for bacteria was used to the bacterial biomass per gram; generally, phylum Acidobacteria and genus Acidobacteria GP2 were dominant in soil, Ralstonia was the pathogen causing bacterial wilt incidence, and Pseudomonas was one potential BCA for the control of bacterial wilt. The primers were synthesized at Shanghai Sangon Company (China). The ordinary PCR program was as follows: step 1, 95 °C for 5 min; step 2, 30 cycles of 30 s at 95 °C, 30 s at 55/59/62 °C, and 30 s at 72 °C; and step 3, 72 °C for 10 min.
The ordinary PCR products were purified with gel extraction kit (Omega Biotech, Norcross, GA, USA), quantified using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, USA), and used to construct standard curves. The copy number of the ordinary PCR products were calculated as follows: Copy number (copies/μL) = 6.02 × 1014 × C / (M × W), where C represents the concentration of ordinary PCR products (unit, ng/μL), M represents the length of PCR products (unit, bp), and W represents the constant (660 Da/bp). The ordinary PCR products were diluted serially from 101 to 109 copies/μL and amplified with real-time qPCR to construct standard curves. The real-time PCR was run with an iCycler iQ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, USA). The reaction mixture contained 10 μL SYBR qPCR mix (Toyobo Co., Ltd., Osaka, Japan), 0.4 μL forward/reverse primer (10 mM), and 2 μL of template DNA, and ddH2O was added to reach a total volume of 20 μL. The real-time PCR program was as follows: step 1, 95 °C for 5 min; step 2, 40 cycles of 95 °C for 25 s, 55/59/62 °C for 25 s, and 72 °C for 30 s; step 3, 55 °C for 1 min; and step 4, wherein the temperature was increased in the steps of 0.5 °C from 55 to 95 °C through 80 cycles. In step 4, melting curves for the amplicons were measured while monitoring fluorescence.
The amplification efficiencies of all primers were between 90 and 110%, and the melting curves of five primers all showed a single peak (Supplemental Fig. S1), suggesting that the results were credible. The copies of each sample using 16S rRNA universal primer represented the bacterial biomass per gram. The following formula was used to calculate the relative abundance of each genus/phylum: Relative abundance = C i / C0 × 100, where C i represents the copies of the genus or phylum and C0 represents the bacterial biomass.
Network construction and characterization
Random matrix theory (RMT)-based approaches were used for network construction (Yin et al. 2015), hub, and connector OTU identification, and the topological property was determined with an automatic threshold. To ensure correlation reliability, OTUs presenting in at least six out of eight replicates were used for network analysis. Various network properties were characterized such as average degree, average path distance, average clustering coefficient, and modularity index. The network modules were generated using rapid greedy modularity optimization. The experimental data used for constructing phylogenetic molecular ecological networks (pMENs) based on 16S rRNA gene sequencing analysis, and Cytoscape 2.8.3 software was used to visualize the network graphs (Zhou et al. 2010). The pMENs were constructed separately based on sequencing data of the eight treatments to reveal the temporal and spatial difference of network interactions.
Data analyses
The community diversity was assessed using the Shannon diversity index (H′). Differences in diversity and relative abundances of bacterial composition based on Tukey’s test were conducted by a one-way analysis of variance (ANOVA) and response ratio (Deng et al. 2012a). Detrended correspondence analysis (DCA) and dissimilarity tests were conducted to compare different bacterial community structures. Analysis of similarity (ANOSIM) of two groups was based on Bray–Curtis distances. Pearson correlation tests, regression analysis, and partial least squares path modeling (PLS-PM) were carried out to explore relationships among species abundances, microbial community structure, microbial diversity, and morbidity (Barberán et al. 2014; Cui et al. 2016; Wagg et al. 2014). In PLS-PM analysis, bacterial community structures were represented by the values of DCA1 and DCA2 (the first two axes) after DCA. All analyses were performed using R v.2.6.1 (Supplemental methods) and STAMP v 2.1.3 (http://kiwi.cs.dal.ca/Software/index.php/STAMP).
Results
Morbidity and bacterial biomass
Morbidity of bacterial wilt was lowest in N1 (8.78%), highest in N5 (80.45%), and medium degree in N4 (57.78%) and N12 (40.09%) (Fig. 1a). Bacterial biomass, quantified with real-time qPCR, increased from the WF period to the LG period, particularly in the N1 group (Fig. 1a). Morbidity showed a significantly (p < 0.05) negative correlation with bacterial biomass in the LG period (Fig. 1c), whereas no significant correlation was found in the WF period (Fig. 1b).
Overview of soil bacterial community compositions in the WF and LG periods
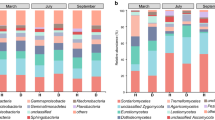
More than 18,000 high-quality 16S rRNA sequences were obtained for each sample. Rarefaction curves showed that the numbers of OTUs showed no clear increase with the increase of sequencing depth (Supplemental Fig. S2). After clustering at 97% sequence identity, 19,422 OTUs were identified, out of which, 171 OTUs were classified as Archaea. In the Archaea domain, 86.40% of reads were unclassified genera of the phylum Crenarchaeota. In the bacteria domain, there were six dominant phyla in the WF period and in the LG period (Supplemental Fig. S3a) and they accounted for 72.40–81.52% of total reads. Those phyla are Proteobacteria (23.97–43.43%), Acidobacteria (10.23–23.24%), Actinobacteria (2.09–9.15%), Chloroflexi (4.85–9.74%), Verrucomicrobia (2.83–6.35%), and Planctomycetes (2.14–7.81%).
In addition, all OTUs were affiliated to 495 genera, and the dominant genera (more than 1%) included Acidobacteria GP6, Acidobacteria GP4, Dokdonella, Spartobacteria genera incertae sedis, Pseudomonas, Rhodanobacter, and Gemmatimonas (Fig. 2). Among these, the percentage of total unclassified genera was about 40% and the rare genera (less than 1%) occupied about 20% in all treatment. Some genera showed no significant differences among different sampling sites, for example Pseudomonas in the WF period (Fig. 3b) and Arthrobacter in the LG period (Fig. 3c). However, bacterial composition differed substantially among different groups. In the WF period, compared with the N4, N5, or N12 group, the relative abundances of some genera in the N1 group were significantly lower, e.g., Ralstonia (Fig. 3a) and Arthrobacter (Fig. 3c), or significantly higher, e.g., Acidobacteria GP6 (Fig. 3e) and Pasteuria (Fig. 3f). Similar results were found in the LG period, particularly that Spartobacteria genera incertae sedis represented a higher proportion of total genera in N1 than in N4 (Fig. 3d).
Relative abundances of several genera and their correlations with morbidity. a Ralstonia. b Pseudomonas. c Arthrobacter. d Spartobacteria genera incertae sedis. e Acidobacteria GP6. f Pasteuria. *p < 0.05, the significance level of difference between the WF and LG periods; lowercase letters a and b indicate the significance level of difference (p < 0.05) among different sampling sites in the WF period; uppercase letters A and B indicate the significance level of difference (p < 0.05) among different sampling sites in the LG period. Eight replicates are used in this regression analysis
Pearson correlation tests (Supplemental Table S2) showed that in the WF period, abundances of Acidobacteria, Crenarchaeota, and OD1 were significantly negatively correlated to morbidity (p < 0.05). In contrast, abundances of Actinobacteria, Chlamydiae, and Euryarchaeota were significantly positively correlated to morbidity (p < 0.05). In the LG period, abundances of phyla Acidobacteria, BRC1, Euryarchaeota, and WS3 had significantly negative relationships with morbidity (p < 0.05), and abundances of phyla Actinobacteria, Chlorobi, Gemmatimonadetes, OD1, and Proteobacteria showed significantly positive correlation to morbidity (p < 0.05).
Further Pearson correlation and linear regression analyses (Supplemental Table S4, Fig. 3) showed that 37 genera (e.g., Acidobacteria GP6) in the WF period and 59 genera (e.g., Pasteuria) in the LG period were significantly negatively correlated to morbidity (p < 0.05). Moreover, 19 genera (e.g., Arthrobacter) in the WF period and 45 genera (e.g., Gemmatimonas) in the LG period significantly increased morbidity (p < 0.05; Supplemental Table S4). Ralstonia (r = 0.338, p = 0.059, under Pearson correlation test, Supplemental Table S4; R2 = 0.148, p = 0.017, under linear regression analysis, Fig. 3a) in the WF period showed a marginally significantly weak and positive relationship with morbidity.
Real-time qPCR was performed to verify the accuracy of 16S rRNA gene sequencing (Fig. 4). The 16S rRNA sequencing approach provided a higher abundance of phylum Acidobacteria than the qPCR approach. In most samples, there was no significant difference in the relative abundances of the three genera. Due to the deficiency of specific primers of the phylum, the 16S rRNA gene sequencing technology presented more reliable results than qPCR.
Comparison of bacterial communities in the WF and LG periods
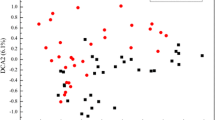
The DCA analysis (Fig. 5a) showed that samples in the WF period were segregated from those samples in the LG period, and similarity analysis (ANOSIM, Fig. 5b) showed that the distances between groups were significantly larger than those within the groups (R = 0.223 and p = 0.001). Similar results were obtained by dissimilarity tests (Supplemental Table S3), suggesting that temporal difference in the bacterial community occurred. The Shannon diversity was significantly different (p < 0.05) between the WF period (6.679 for N1, 6.446 for N4, and 6.636 for N12) and the LG period (6.896 for N1, 5.861 for N4, and 6.099 for N12), whereas no significant difference (p > 0.05) between the two periods was found in N5 (Fig. 5c). Linear regression and polynomial regression analyses were conducted to test the interaction between soil bacterial community and crop morbidity. The results showed that higher bacterial diversity in the WF period could inhibit crop morbidity. However, with the increasing bacterial diversity in the LG period, crop morbidity decreased initially and then increased.
a The ordination plots of all samples for the community structure analyzed by detrended correspondence analysis (DCA). b Analysis of similarity (ANOSIM,) of two groups based on the Bray–Curtis distances. c Shannon diversity index and its correlations with morbidity by regression analysis. d The relationship among bacterial community structure in the WF and LG periods and morbidity by partial least squares path modeling (PLS-PM). In PLS-PM, bacterial community structures are represented by the top two axes (DCA1 and DCA2) of DCA, and the bold solid line and *p < 0.05 show the relation was significant; lowercase letters a and b indicate the significance level of difference (p < 0.05) among different sampling sites in the WF period; uppercase letters A and B indicate the significance level of difference (p < 0.05) among different sampling sites in the LG period. Eight replicates are used in regression analysis
The goodness-of-fit value of PLS-PM (Fig. 5d) was 0.662, which is greater than 0.35, indicating that the results were reliable. Results showed that bacterial community structure in the WF period significantly affected bacterial community structure in the LG period (r = 0.573, p = 0.011). Morbidity was significantly influenced by bacterial community structure in the WF period (r = 0.626, p = 0.007) and showed a close correlation with bacterial community structure in the LG period (r = 0.979, p < 0.001).
Bacterial community shift from the WF period to the LG period
Successions of bacterial communities from the WF period to the LG period in different sampling sites were shown in detail at both phylum and genus levels. At the phylum level, 6 phyla (e.g., Actinobacteria) were significantly (p < 0.05) different between the two periods in the N1 group, 8 phyla (e.g., Proteobacteria) in the N4 group, 7 phyla (e.g., Chloroflexi) in the N5 group, and 15 phyla (e.g., Acidobacteria) in the N12 group (Supplemental Fig. S3).
At the genus level, 107 genera were significantly (p < 0.05) different between the two periods in the N1 group (Supplemental Fig. S4), 135 genera differed in the N4 group (Supplemental Fig. S5), 108 genera differed in the N5 group (Supplemental Fig. S6), and 132 genera differed in the N12 group (Supplemental Fig. S7). Nineteen genera, e.g., Dokdonella and Massilia, were significantly (p < 0.05) different in all sampling sites, whereas 248 genera, e.g., Acidobacteria GP2 and Lysobacter, showed no significant differences.
The relationship between the succession of genera abundances from the WF period to the LG period (the relative abundances of one genus minus the relative abundance of the same genus in the WF period) and crop morbidity is shown in Supplemental Table S4. A total of 56 genera, such as Acidobacteria GP16 (r = − 0.425), WS3 genera incertae sedis (r = − 0.477), Arthrobacter (r = − 0.431), and Massilia (r = − 0.427), showed negative correlations with morbidity, and 61 genera, such as Acidobacteria GP15 (r = 0.451) and SR1 genera incertae sedis (r = 0.431), showed positive correlations with morbidity (Supplemental Table S4).
Change of network interactions from the WF period to the LG period at different sampling sites
To discern the ecological network structure in microbial communities, microbial population data were analyzed using the RMT-based network approach. Eight networks were constructed based on the 16S rRNA gene sequencing data of four sites in the WF and LG periods, respectively. Major topological properties of the empirical MENs of microbial communities in the eight groups are shown in Table 2. With the same threshold (0.930), their correlation values were more than 0.800, indicating that the degree of distributions in both the constructed molecular ecological networks fits the power law model well. There were more nodes and links in N1 (1228 nodes and 2258 links in WF; 1339 nodes and 2387 links in LG) and N5 (1044 nodes and 6483 links in WF; 1099 nodes and 2867 links in LG) than in N12 (846 nodes and 2337 links in WF; 808 nodes and 1535 links in LG) and N4 (668 nodes and 1180 links in WF; 403 nodes and 561 links in LG). Nodes represented OTUs, and links represented possible ecological interactions among OTUs. The number of nodes and links represented the complexity of the interactions among bacteria. Crop morbidity showed no significant correlation with the number of nodes or links in the WF and LG periods (p > 0.05; data not shown). However, the decreased ratio of bacterial interaction (change of links) from the WF period to the LG period was higher, and crop morbidity was also higher; on the contrary, the decreased ratio was lower, and the crop morbidity was also lower (Fig. 6).
The relationship between succession of bacterial interaction from the WF to LG periods and morbidity. The ratio of decreased links in network interactions is used to represent the succession of bacterial interaction. The ratio is calculated as follows: Links in the WF period − links in the LG period) / links in the WF period
Subnetwork interactions of Ralstonia were constructed. Ralstonia was found only in N5 WF, N4 LG, and N5 LG networks with high morbidity. In these networks, Ralstonia was positively correlated with Massilia and six unclassified genera and negatively correlated with Leptolinea, Armatimonas/Armatimonadetes GP1, Sphingomonas, Acidobacteria GP6, and two unclassified genera (Fig. 7). Subnetworks of Pasteuria, a genus negatively correlated to morbidity, were constructed. The partial network interactions of Pasteuria with the highest connections indicated that most nodes and links were in N1, followed by N12, and the least were in N5 (Supplemental Fig. S8).
Network interactions of Ralstonia at different sampling sites. Each node signified an OTU. The Ralstonia nodes were found only in networks of N5 WF, N4 LG, and N5 LG. Only this Ralstonia node and its nearest neighbors are shown. A red line represents a positive interaction between two individual nodes (OTUs), and a blue line represents a negative interaction. Purple circles represent Proteobacteria, yellow circles represent Chloroflexi, green circles represent Armatimonadetes, blue circles represent Actinobacteria, cyan circles represent Acidobacteria, and red circles represent unclassified phyla
Discussion
The interaction between the soil bacterial community and crop morbidity is an important topic in microbial ecology and biocontrol of crop disease. Previous studies have mainly focused on the impacts of species diversity and composition during the cropping period on disease morbidity (Mengesha et al. 2017). However, the species diversity and composition during the non-cropping (WF) period, as well as the soil bacterial biomass, species succession, and population interactions, are also closely associated with crop disease. Our research showed that the soil bacterial community, including soil bacterial biomass, community structure, diversity, and population interactions in the WF period, similar to those in the LG period, played a critical role in tobacco bacterial wilt incidence; furthermore, it was found that some key genera might be potential biological agents for bacterial wilt.
Soil bacterial communities in the WF period, similar to those in the LG period, were closely related to morbidity of tobacco bacterial wilt
It is reported that the soil bacterial community is not only spatially (Fierer and Jackson 2006) variable, but it is also temporally variable (Garrido et al. 2008), similar to the results of our research that bacterial communities in the WF period were significantly different from those in the LG period (Fig. 5a, b). For instance, in the sites with relatively high morbidity (N4, N5, and N12), the relative abundances of the genera Pseudomonas, Arthrobacter, and Pasteuria were significantly higher in the WF period, whereas the relative abundance of genus Ralstonia was significantly higher in the LG period (Fig. 3). However, the soil bacterial communities in the WF period were important for the formation of those in the LG period (Fig. 5d). Therefore, soil bacterial communities in the WF and LG periods should show similar relationships with morbidity of tobacco bacterial wilt.
Spence et al. (2014) reported that the soil bacterial community during the growing period was significantly related to rice blast infection. Moreover, Fu et al. (2017) reported that the consecutive application of biofertilizer affected the soil microbial community and could suppress banana Fusarium wilt disease during the cropping period. Consistent results were obtained in the present study. We found that the structure of bacterial community in the LG period significantly affected morbidity, so did that in the WF period (Fig. 5d).
Interestingly, we also found that the high soil bacterial diversity in the non-cropping WF period, similar to that in the LG period, had a weak but significant relationship with bacterial wilt morbidity. It has been previously reported that crop diversity could also suppress the density of individual species, and the transmission of many infectious agents is inhibited (Lacroix et al. 2013; Johnson et al. 2012), which has been applied to factual farming, e.g., rotation cropping and intercropping. Many studies were focused on the soil microbial diversity–pathogen interaction. Microbial diversity was also found to be inversely correlated with the invasibility of the wheat rhizosphere by Pseudomonas aeruginosa (Matos et al. 2005) and also affected the ability of R. solanacearum to induce wilting disease in tomato (Yoshiko et al. 2006). van Elsas and Salles (2012) found a negative correlation between the diversity of the soil microbiota and the survival of the invading E. coli. Therefore, soil bacterial diversity can exert a positive effect on the decline of this plant pathogen, which is consistent with our result. Because several functions of the soil microbial community are a key to soil functioning (van Elsas et al. 1997), the considerable functional redundancy has been thought to overwhelm any type of diversity–function relationship (Franklin and Mills 2006). Communities with high diversity may thus consist of a variety of species that have wide and complementary functions. Thus, a greater variety of potential BCAs in the WF period is of benefit to the control of plant pathogens. However, the relationship between morbidity and soil bacterial diversity in the LG period was not consistent with the positive correlation. This may be because the soil bacterial community in the LG period was not the cause, but the result of interactions between the pathogen and crop.
Compared with the site with low morbidity (N1), biomass was low in other sites during the LG period, whereas biomass showed no significant differences during the WF period. This might be because climate was the key factor to influence microbe growth during the WF period. However, during the LG period, more pathogens competed with indigenous bacteria for nutrients (e.g., soil carbon, nitrogen, mineral elements, and water) and thus impaired their growth, resulting in lower biomass in soil. Besides, lower biomass might be a cause that fewer antibiotics or exudates were produced to resist the pathogen, leading to higher morbidity.
Bacterial community succession from the WF period to LG period might be closely related to interactions between bacterial communities and pathogens. Therefore, we further explored the soil bacterial interactions during the WF to LG periods using the RMT, which is powerful for identifying molecule ecological networks in microbial communities and has previously been employed to analyze the co-occurrence/interaction among different microbial populations (Zhou et al. 2010; Deng et al. 2012a, b). Results showed that the change of bacterial interactions (the number of decreased links in network; Table 2, Fig. 6) was positively correlated with morbidity. This suggested that the decreased interaction of the bacterial community might prompt pathogen growth. Pathogens should compete with other microorganisms for nutrients. The close interactions among microorganisms were decreased, meaning that the ability of the plant to resist the pathogen was weakened, resulting in high morbidity and yield loss.
Some key genera might be potential biological agents for bacterial wilt
It is commonly accepted that the soil microbiome is important in crop production, and many studies have been conducted to assess microorganisms for the biocontrol of plant disease (Silo-Suh et al. 1994). Using statistical analysis, the key genera which may be closely related to crop disease can be found (Franke-Whittle et al. 2015; She et al. 2016; Yang et al. 2016). However, it is unreasonable if we just determined the relationships between morbidity and genera (the 16S rRNA sequencing data of the soil bacterial community) in the LG period. That is, to find the potential BCAs, it is necessary to compare the relationships between morbidity and genera/genera succession in different periods. In the present study, 104 genera, e.g., Acidobacteria GP6 and Pasteuria, during the LG period were closely correlated with tobacco bacterial wilt incidence (Figs. 5c and 3e, f), and 56 genera, such as Arthrobacter, Massilia, and Acidobacteria GP6, were also significantly correlated with morbidity (Supplemental Table S4). Besides, it was found that morbidity was correlated with successions of 117 genera from the WF to LG periods (Supplemental Table S4). Therefore, statistically meaningful specific bioassays should be conducted to evaluate the potential use of biocontrol strains.
Pal and Gardener (2006) reported that some bacteria, such as Bacillus, Agrobacterium, Arthrobacter, Pseudomonas, Erwinia, Flavobacterium, Pasteuria, Rhizobium, and Xanthomonas, were employed as potential BCAs against plant pathogens. In this study, Agrobacterium and Erwinia were not detected in soil samples. Pearson correlation analysis showed that Bacillus (0–0.26%), Flavobacterium (0.005–6.86%), Rhizobium (0.03–1.18%), and Xanthomonas (0–0.08%) had no significant relationship with bacterial wilt morbidity in both the WF and LG periods (Supplemental Table S4). Arthrobacter (0.02–4.97%) and Pseudomonas (0.03–18.13%) were positively correlated with morbidity; however, the succession of relative abundance from the WF to LG periods was negatively correlated with morbidity (Supplemental Table S4), suggesting that an increase of these genera from the WF to LG periods might control or inhibit bacterial wilt incidence. Pasteuria (0–1.73%) had a negative relationship with morbidity in both the WF and LG periods (Supplemental Table S4). These analyses inferred that Arthrobacter, Pasteuria, and Pseudomonas might be potential BCAs for controlling bacterial wilt.
We also found some other genera closely correlated with morbidity, e.g., Acidobacteria GP4, Acidobacteria GP6, Acidobacteria GP16, Lysobacter, Massilia, and Spartobacteria genera incertae sedis. Even though our study provided no evidence directly demonstrating that they were potential BCAs, previous studies have already suggested their potential role in the biocontrol of plant pathogens. For example, some previous reports (Franke-Whittle et al. 2015; She et al. 2016; Yang et al. 2016) found that Acidobacteria GP4, Acidobacteria GP6, and Acidobacteria GP16 decreased morbidity. Although no evidence relating these subgroups of phylum Acidobacteria to crop disease or disease suppression exists in previous research, Yin et al. (2013) suggested Acidobacteria may act as probiotic bacteria and can promote plant growth. It was also reported that Acidobacteria were preponderant in soil (Liu et al. 2016) and higher abundances of the three subgroups were often found in disease-suppressive soils, rather than in disease-conducive soils (Shen et al. 2015; Yin et al. 2013). Ward et al. (2009) reported that polyketide synthase and macrolide glycosylation genes, known for their roles in the synthesis of antibacterial compounds, were found in three genomes of phylum Acidobacteria, which can provide insight into the functions of Acidobacteria in soil. It has also been reported that Lysobacter enzymogenes C3 secreted an antifungal compound (dihydromaltophilin), and this strain could effectively protect crops from fungal disease (Lou et al. 2011). Ji et al. (2008) reported that Lysobacter antibioticus 13-1 could significantly inhibit the growth of various phytopathogenic bacteria and fungi, especially the bacterial blight pathogen Xanthomonas oryzae pv. oryzae, and their research suggested that antibiotics produced by strain 13-1 might be involved in the biological control of rice bacterial blight. These antibiotics might also be effective for controlling tobacco bacterial wilt disease.
Some genera during the WF, or the LG, period, or the succession process, showed similar relationships with morbidity; however, some showed different relationships. Due to the complex interactions among pathogens, soil bacterial community, plants, soil nutrients, and climate, some of the correlations of the bacterial community with morbidity might be the results rather than the cause of plant disease. For example, some of the bacterial genera exploit the dying plants as saprophytes, which can also explain the positive correlation. In other words, it is possible that the correlations may be stochastic (Zhou et al. 2014). Therefore, we were unable to confirm whether these genera were potential BCAs, or whether they were of benefit to R. solanacearum growth; however, these abundant relationships provide a reference for further research. The primary challenge is to isolate the pure cultures of these bacteria to test their functions of biological control.
In conclusion, the soil bacterial community was obviously different between the WF and LG periods and there was an obvious relationship between tobacco bacterial wilt disease and soil bacterial community in the WF and LG periods. Although studies have mainly focused on the effects on the crop yield, the effects of pathogens on soil indigenous bacterial community (including biomass, bacterial interaction, bacterial diversity, and composition) might determine the soil quality for further planting, because of the close correlation between the soil microbial community and crop morbidity in different periods. Therefore, to control or decrease plant disease incidence, the improvement of the soil microbial community, such as increase of microbial diversity and abundances of potential BCAs, should not be ignored.
References
Ahimou F, Deleu MJP (2000) Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzym Microb Technol 27(10):749–754. https://doi.org/10.1016/S0141-0229(00)00295-7
Barberán A, Ramirez KS, Leff JW, Bradford MA, Wall DH, Fierer N (2014) Why are some microbes more ubiquitous than others? Predicting the habitat breadth of soil bacteria. Ecol Lett 17(7):794–802. https://doi.org/10.1111/ele.12282
Berkovitch YA (1996) Instrumentation for plant health and growth in space. Adv Space Res 18(4–5):157–162. https://doi.org/10.1016/0273-1177(95)00872-C
Bramley R, Ellis N, Nable RO, Garside AL (1996) Changes in soil chemical properties under long-term sugar cane monoculture and their possible role in sugar yield decline. Aust J Soil Res 34(6):967–984. https://doi.org/10.1071/SR9960967
Cui P, Fan F, Yin C, Song A, Huang P, Tang Y, Zhu P, Peng C, Li T, Wakelin SA, Liang Y (2016) Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol Biochem 93(131–141. https://doi.org/10.1016/j.soilbio.2015.11.005
Defoirdt T, Boon N, Sorgeloos P, Verstraete W, Bossier P (2007) Alternatives to antibiotics to control bacterial infections: luminescent vibriosis in aquaculture as an example. Trends Biotechnol 25(10):472–479. https://doi.org/10.1128/AEM.01610-13
Deng Y, He Z, Xu M, Qin Y, Van Nostrand JD, Wu L, Roe BA, Wiley G, Hobbie SE, Reich PB (2012a) Elevated carbon dioxide alters the structure of soil microbial communities. Appl Environ Microb 78(8):2991–2995. https://doi.org/10.1128/AEM.06924-11
Deng Y, Jiang YH, Yang Y, He Z, Luo F, Zhou J (2012b) Molecular ecological network analyses. BMC Bioinformatics 13(1):113. https://doi.org/10.1186/1471-2105-13-113
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27(16):2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Nat Acad Sci USA 103(3):626–631. https://doi.org/10.1073/pnas.0507535103
Franke-Whittle IH, Manici LM, Insam H, Stres B (2015) Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 395(1):317–333. https://doi.org/10.1007/s11104-015-2562-x
Franklin RB, Mills AL (2006) Structural and functional responses of a sewage microbial community to dilution-induced reductions in diversity. Microbial Ecol 52(2):280–288. https://doi.org/10.1007/s00248-006-9033-0
Fu L, Penton CR, Ruan Y, Shen Z, Xue C, Li R, Shen Q (2017) Inducing the rhizosphere microbiome by biofertilizer application to suppress banana Fusarium wilt disease. Soil Biol Biochem 104:39–48. https://doi.org/10.1016/j.soilbio.2016.10.008
Garrido P, Gonzalez-Toril E, Garcia-Moyano A, Moreno-Paz M, Amils R, Parro V (2008) An oligonucleotide prokaryotic acidophile microarray: its validation and its use to monitor seasonal variations in extreme acidic environments with total environmental RNA. Environ Microbiol 10(4):836–850. https://doi.org/10.1111/j.1462-2920.2008.01477.x
Granér G, Persson P, Meijer J, Alström S (2003) A study on microbial diversity in different cultivars of Brassica napus in relation to its wilt pathogen, Verticillium longisporum. FEMS Microbiol Lett 224(2):269–276. https://doi.org/10.1016/S0378-1097(03)00449-X
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119(4):917–935. https://doi.org/10.1111/jam.12886
Handelsman J, Nesmith WC, Raffel SJ (1991) Microassay for biological and chemical control of infection of tobacco by Phytophthora parasitica var. nicotianae. Curr Microbiol 22(5):317–319. https://doi.org/10.1007/BF02091961
Handelsman J, Raffel S, Mester EH, Wunderlich L, Grau CR (1990) Biological control of damping-off of alfalfa seedlings with Bacillus cereus UW85. Appl Environ Microb 56(3):713–718
Hu Y, Wu K, Liu N, Chen H, Jia X (2004) Studies on microbial population dynamics in the cucumber rhizospheres at different developmental stages. Sci Agri Sinica 37(10):1521–1526
Huo Q, Zhang S, Wang R (2007) Advance and control of tobacco bacterial wilt disease. Chin Agri Sci Bull:364–368
Ji G, Wei L, He Y, Wu Y, Bai X (2008) Biological control of rice bacterial blight by Lysobacter antibioticus strain 13-1. Biol Control 45(3):288–296. https://doi.org/10.1016/j.biocontrol.2008.01.004
Johnson PTJ, Preston DL, Hoverman JT, Henderson JS, Paull SH, Richgels KLD, Redmond MD (2012) Species diversity reduces parasite infection through cross-generational effects on host abundance. Ecology 93(1):56–64. https://doi.org/10.1890/11-0636.1
Kailasapathy K (2002) Microencapsulation of probiotic bacteria: technology and potential applications. Curr Iss Intest Microbiol 3(2):39–48
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274(1):1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Kloepper JW, Rodríguez-Kábana R, Zehnder AW, Murphy JF, Sikora E, Fernández C (1999) Plant root-bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Australas Plant Path 28(1):21–26. https://doi.org/10.1071/AP99003
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Pseudomonas siderophores: a mechanism explaining disease-suppressive soils. Curr Microbiol 4(5):317–320. https://doi.org/10.1007/BF02602840
Kyselková M, Kopecký J, Frapolli M, Défago G, Ságová-Marečková M, Grundmann GL, Moënne-Loccoz Y (2009) Comparison of rhizobacterial community composition in soil suppressive or conducive to tobacco black root rot disease. ISME J 3(10):1127–1138. https://doi.org/10.1038/ismej.2009.61
Lacroix C, Jolles A, Seabloom EW, Power AG, Mitchell CE, Borer ET (2013) Non-random biodiversity loss underlies predictable increases in viral disease prevalence. J Roy Soc Int 11(92):20130947. https://doi.org/10.1098/rsif.2013.0947
Leifert C, Li H, Chidburee S, Hampson S, Workman S, Sigee D, Epton HAS, Harbour A (1995) Antibiotic production and biocontrol activity by Bacillus subtilis CL27 and Bacillus pumilus CL45. J Appl Bacteriol 78(2):97–108. https://doi.org/10.1111/j.1365-2672.1995.tb02829.x
Liu J, Sui Y, Yu Z, Yao Q, Shi Y, Chu H, Jin J, Liu X, Wang G (2016) Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Soil Biol Biochem 95:212–222. https://doi.org/10.1016/j.soilbio.2015.12.021
Lou L, Qian G, Xie Y, Hang J, Chen H, Zaleta-Rivera K, Li Y, Shen Y, Dussault PH, Liu F (2011) Biosynthesis of HSAF, a tetramic acid-containing macrolactam from Lysobacter enzymogenes. J Am Chem Soc 133(4):643–645. https://doi.org/10.1021/ja105732c
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63(63):541–556. https://doi.org/10.1146/annurev.micro.62.081307.162918
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald P, Dow M, Verdier V, Beer SV, Machado MA (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13(6):614–629. https://doi.org/10.1111/j.1364-3703.2012.00804.x
Matos A, Kerkhof L, Garland JL (2005) Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microbial Ecol 49(2):257–264. https://doi.org/10.1007/s00248-004-0179-3
Mazurier S, Corberand T, Lemanceau P, Raaijmakers JM (2009) Phenazine antibiotics produced by fluorescent pseudomonads contribute to natural soil suppressiveness to Fusarium wilt. ISME J 3(8):977–991. https://doi.org/10.1038/ismej.2009.33
Mengesha WK, Powell SM, Evans KJ, Barry KM (2017) Diverse microbial communities in non-aerated compost teas suppress bacterial wilt. W J Microb Biot 33(3):49. https://doi.org/10.1007/s11274-017-2212-y
Moyne AL, Cleveland TE, Tuzun S (2004) Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett 234(1):43–49. https://doi.org/10.1111/j.1574-6968.2004.tb09511.x
Niu J, Chao J, Xiao Y, Chen W, Zhang C, Liu X, Rang Z, Yin H, Dai L (2017) Insight into the effects of different cropping systems on soil bacterial community and tobacco bacterial wilt rate. J Basic Microbiol 57(1):3–11. https://doi.org/10.1002/jobm.201600222
Pal KK, Gardener MS (2006) Biological control of plant pathogens. Plant Health Instructor 202:147
Schmidt HP, Kammann C, Niggli C, Evangelou MWH, Mackie KA, Abiven S (2014) Biochar and biochar-compost as soil amendments to a vineyard soil: influences on plant growth, nutrient uptake, plant health and grape quality. Agri Ecosyst Environ 191(117–123. https://doi.org/10.1016/j.agee.2014.04.001
Sharifi-Tehrani A, Zala M, Natsch A, Moënne-Loccoz Y, Défago G (1998) Biocontrol of soil-borne fungal plant diseases by 2,4-diacetylphloroglucinol-producing fluorescent pseudomonads with different restriction profiles of amplified 16S rDNA. Eur J Plant Pathol 104(7):631–643. https://doi.org/10.1023/A:1008672104562
She S, Niu J, Zhang C, Xiao Y, Chen W, Dai L, Liu X, Yin H (2016) Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch Microbiol 199(2):267–275. https://doi.org/10.1007/s00203-016-1301-x
Shen Z, Ruan Y, Xue C, Zhong S, Li R, Shen Q (2015) Soils naturally suppressive to banana Fusarium wilt disease harbor unique bacterial communities. Plant Soil 393(1):1–13. https://doi.org/10.1007/s11104-015-2474-9
Shi S, O Callaghan M, Jones EE, Richardson AE, Walter C, Stewart A, Condron L (2012) Investigation of organic anions in tree root exudates and rhizosphere microbial communities using in situ and destructive sampling techniques. Plant Soil 359(359):149–163. https://doi.org/10.1007/s11104-012-1198-3
Shiomi Y, Nishiyama M, Onizuka T, Marumoto T (1999) Comparison of bacterial community structures in the rhizoplane of tomato plants grown in soils suppressive and conducive towards bacterial wilt. Appl Environ Microb 65(9):3996–4001
Silo-Suh LA, Lethbridge BJ, Raffel SJ, He H, Clardy J, Handelsman J (1994) Biological activities of two fungistatic antibiotics produced by Bacillus cereus UW85. Appl Environ Microb 60(6):2023–2030
Smith KP, Havey MJ, Handelsman J (1993) Suppression of cottony leak of cucumber with Bacillus cereus strain UW85. Plant Dis 77(2):139. https://doi.org/10.1094/PD-77-0139
Spence C, Alff E, Johnson C, Ramos C, Donofrio N, Sundaresan V, Bais H (2014) Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol 14(1):130. https://doi.org/10.1186/1471-2229-14-130
van Elsas JD, Jansson J, Trevors JT (1997) Modern soil microbiology. Marcel Dekker, New York
van Elsas JD, Garbeva P, Salles J (2002) Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 13(1):29–40
van Elsas JD, Salles JF (2012) Microbial diversity determines the invasion of soil by a bacterial pathogen. P Nat Acad Sci USA 109(4):1159–1164. https://doi.org/10.1073/pnas.1109326109
Voisard C, Keel C, Haas D, Dèfago G (1989) Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J 8(2):351–358
Wagg C, Bender SF, Widmer F, van der Heijden MGA (2014) Soil biodiversity and soil community composition determine ecosystem multifunctionality. P Nat Acad Sci 111(14):5266–5270. https://doi.org/10.1073/pnas.1320054111
Ward NL, Challacombe JF, Janssen PH, Bernard H, Coutinho PM, Martin W, Gary X, Haft DH, Michelle S, Jonathan B (2009) Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl Environ Microb 75(7):2046–2056. https://doi.org/10.1128/AEM.02294-08
Wu L, Wen C, Qin Y, Yin H, Tu Q, Nostrand JDV, Yuan T, Yuan M, Ye D, Zhou J (2015) Phasing amplicon sequencing on Illumina Miseq for robust environmental microbial community analysis. BMC Microbiol 15(1):1–12. https://doi.org/10.1186/s12866-015-0450-4
Yang H, Niu J, Tao J, Gu Y, Zhang C, She S, Chen W, Yang H, Yin H (2016) The impacts of different green manure on soil microbial communities and crop health. Preprints. doi: https://doi.org/10.20944/Preprints 2016:2016090056
Yin C, Hulbert SH, Schroeder KL (2013) Role of bacterial communities in the natural suppression of rhizoctonia solani bare patch disease of wheat (Triticum aestivum L.) Appl Environ Microb 79(23):7428–7438. https://doi.org/10.1128/AEM.01610-13
Yin H, Niu J, Ren Y, Cong J, Zhang X, Fan F, Xiao Y, Zhang X, Deng J, Xie M (2015) An integrated insight into the response of sedimentary microbial communities to heavy metal contamination. Sci Rep 5(1):93–102. https://doi.org/10.1038/srep14266
Yoshiko I, Masaya N, Shigeto O, Keishi S (2006) Rhizobacterial community-level, sole carbon source utilization pattern affects the delay in the bacterial wilt of tomato grown in rhizobacterial community model system. Appl Soil Ecol 34(1):27–32. https://doi.org/10.1016/j.apsoil.2005.12.003
Zhan F, Lu Y, Guan G (2005) Community structures of microorganisms and their dynamics in the rhizosphere of flue-cured tobacco. Acta Pedol Sin 42(3):488–494
Zhou J, Deng Y, Luo F, He Z, Tu Q, Zhi X (2010) Functional molecular ecological networks. MBio 1(4):e00169-10–e00169-19. https://doi.org/10.1128/mBio.00169-10
Zhou J, Deng Y, Zhang P, Xue K, Liang Y, Van Nostrand JD, Yang Y, He Z, Wu L, Stahl DA (2014) Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. P Nat Acad Sci USA 111(9):836–845. https://doi.org/10.1073/pnas.1324044111
Funding
The study was supported by Key Project of Science and Technology of Hunan Branch of China National Tobacco Corporation (16-19Aa02) and the Graduate Student Research Innovation Project in Central South University (No. 2016zzts105).
Author information
Authors and Affiliations
Contributions
H. Y. and J. L. conceived of the experiment. Y. X. and H. Y. performed the experiment. Y. X. analyzed the data and wrote the manuscript. X. L., D. M., J. T., and Y. G. participated in the discussions.
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
ESM 1
(PDF 2523 kb)
Rights and permissions
About this article
Cite this article
Xiao, Y., Liu, X., Meng, D. et al. The role of soil bacterial community during winter fallow period in the incidence of tobacco bacterial wilt disease. Appl Microbiol Biotechnol 102, 2399–2412 (2018). https://doi.org/10.1007/s00253-018-8757-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-8757-3