Abstract
Oligosaccharides are polymers with two to ten monosaccharide residues which have sweetener functions and sensory characteristics, in addition to exerting physiological effects on human health. The ones called nondigestible exhibit a prebiotic behavior being fermented by colonic microflora or stimulating the growth of beneficial bacteria, playing roles in the immune system, protecting against cancer, and preventing cardiovascular and metabolic issues. The global prebiotics market is expected to grow around 12.7% in the next 8 years, so manufacturers are developing new alternatives to obtain sustainable and efficient processes for application on a large scale. Most studied examples of biotechnological processes involve the development of new strategies for fructooligosaccharide, galactooligosaccharide, xylooligosaccharide, and mannanooligosaccharide synthesis. Among these, the use of whole cells in fermentation, synthesis of microbial enzymes (β-fructofuranosidases, β-galactosidases, xylanases, and β-mannanases), and enzymatic process development (permeabilization, immobilization, gene expression) can be highlighted, especially if the production costs are reduced by the use of agro-industrial residues or by-products such as molasses, milk whey, cotton stalks, corncobs, wheat straw, poplar wood, sugarcane bagasse, and copra meal. This review comprises recent studies to demonstrate the potential for biotechnological production of oligosaccharides, and also aspects that need more investigation for future applications in a large scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbohydrates are compounds classified according to their molecular size or degree of polymerization into monosaccharides, oligosaccharides, or polysaccharides (Mussato and Mancilha 2007). By definition, oligosaccharides are monosaccharide’s polymers with a low polymerization degree and characterized by chains with two to ten monosaccharide residues. These can be found either in a free form or in a bound form, obtained from natural sources, or produced by physical, chemical, or enzymatic processes (Neri-Numa et al. 2016; Talens-Perales et al. 2016).
Besides the sweetener functions and sensory characteristics, some oligosaccharides exert physiological effects on human health, being classified as digestible or nondigestible (NDOs) (Qiang et al. 2009; Patel and Goyal 2012). In the NDOs, the anomeric carbon atoms (C1 or C2) of the monosaccharide units (glucose, galactose, fructose, and xylose) make their glycosidic bonds nonsusceptible to the hydrolytic enzymes in the gastrointestinal tract (Mussato and Mancilha 2007; Neri-Numa et al. 2016).
NDOs exhibit a prebiotic behavior being fermented by colonic microflora or stimulating the growth of beneficial bacteria (bifid bacteria and lactobacilli) which in turn produce short-chain fatty acids (SCFAs) as acetate, propionate, and butyrate providing health benefits to the host (Hutkins et al. 2016; Gibson and Roberfroid 1995). For example, SCFA action reduces luminal pH, protecting against acid-sensitive enteropathogens. Acetate production influences butyrate formation, which is a primary substrate for growth of colonic epithelium inducing the production of immunomodulatory cytokines and promoting ammonia and amine excretion (Krumbeck et al. 2016; Wilson and Whelan 2017).
Thus, several studies have suggested that prebiotic intake plays a role in the immune system, regulating both mineral and lipidic metabolisms. Moreover, functional NDOs may protect against colon cancer and prevent cardiovascular diseases and metabolic syndromes (Di Bartolomeo et al. 2013; Lam and Cheung 2013; Slavin 2013).
Currently, inulin-based fructose oligomers, galactooligosaccharides, and lactulose are considered established prebiotics while soybean oligosaccharides and gluco-, gentio-, isomalto-, xylo-, and mannanooligosaccharides are classified as emerging ones. In addition, maltodextrin, raffinose, arabinose, arabinoxylan-oligosaccharides (AXOS), and sugar alcohols, as mannitol and sorbitol, have huge prebiotic applications (Blatchford et al. 2013; Neri-Numa et al. 2016).
Due to their beneficial effects, prebiotics have been employed for food and beverage processing, dietary supplements, and animal feed (Kothari et al. 2014). The global prebiotics market size was over U$ 2.90 billion in 2015 and has a growth expectancy around 12.7% by 2025 profiting approximately U$10.55 billion (Research and Markets 2017). Among the main prebiotics manufacturers, including Roquette America Inc., Abbott Nutrition, Friesl and Campina Domo, Clasado Ltd., and Jarrow Formulas, the company Beneo-Orafti SA is involved in production and sales of inulin and oligofructose food ingredients derived from chicory while Friesl and Campina Domo have focus on galactooligosaccharide production (Grand View Research 2016).
As NDOs can be obtained from natural sources (conventional agro-foods sources, seaweeds, and marine microalgae), manufacturers have sought more efficient, sustainable, simple, and less expensive processes for their application on a large scale (Moreno et al. 2017). This review emphasizes biotechnological processes for fructooligosaccharides, galactooligosaccharides, xylooligosaccharides, and mannanooligosaccharide production, the main founded studied examples in this emerging market.
Biotechnological oligosaccharide production
Fructooligosaccharides (FOS)
Fructooligosaccharides (FOS) belong to the prebiotic group of carbohydrates described as “nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria species already resident in the colon and thus attempt to improve host health” (Gibson and Roberfroid 1995). Effects of this prebiotic class include activation of the human immune system, maintenance of intestinal microbiota, resistance to infection, enhanced mineral absorption in the gastrointestinal tract, synthesis of B complex vitamin, reduction of serum cholesterol, and prevention of carcinogenic tumors (Gibson and Roberfroid 1995; Bruzzese et al. 2006; Delgado et al. 2010).
FOS display differences in their fructose linkage in the molecule. In the 1F type, β-2-1 linkages are found between fructosyl groups with sucrose in the molecule terminal part forming 1-kestose (GF2), nystose (GF3), and 1F fructofuranosyl nystose (GF4). In the 6G or neoFOS type, β-2-6 linkages are formed between fructosyl groups, forming 6-kestose, 6-nystose, and 6G fructofuranosyl nystose, and the fructose linkages can be with the glucose forming neokestose, neonystose, and neo fructofuranosyl nystose (Yun 1996; Straathof et al. 1986).
The major molecules are fructans presenting both 1F- and 6G-type linkages with a higher degree of polymerization (DP). FOS and fructans occur naturally in plants and have the ability to regulate plant growth, defense against pathogens, the developmental process, and carbohydrate storage (Eggleston and Côté 2003). Natural fructans have a degree of polymerization ranging from 11 to 60, but FOS have a lower mass and DP value ranging from 2 to 10 (Ritsema and Smeekens 2003).
Different fructooligosaccharides are degraded by different bacterial strains in the human colon (Gibson and Wang 1994). Van der Meulen et al. (2006) showed that Bacteroides and Bifidobaterium metabolized different types of FOS. Strains of Bifidobacterium preferentially metabolized F2, F3, F4, GF4, F5, and F6.
The extraction from natural sources or using plant enzymes is unfavorable by seasonal limitation. Only small amounts of enzyme can be produced and low FOS yields are reached. Commercially, they are being produced by the action of fructosyltransferase (EC 2.4.1) or β-fructofuranosidase (EC 3.2.1) from microbial sources (see Fig. 1), such as Aspergillus niger and Aureobasidum pullulans. Some examples are the products Neosugar™ in Japan and Rafitlose™ and Raftiline™, FOS and inulin products respectively, from Belgium (Hidaka et al. 1988; Hirayama et al. 1989; Yun 1996).
Many microorganisms are able to synthesize FOS. These include Aureobasidium pullulans, Aspergillus niger, Aspergillus oryzae, Bacillus circulans, Pseudomonas aurantiaca, Aspergillus aculeatus, Kluyveromyces marxianus, Penicillium sp., Saccharomyces cerevisiae, Rhodotorula sp., Zymomonas mobilis, and Lactobacillus reuteri (Yun 1996; Sangeeta et al. 2005; Sánchez et al. 2008; Kawamura and Matsuda 2008; Ghazi et al. 2007; Santos and Maugeri 2007).
The fructosyltransferase (FTase) enzyme reactions used to produce FOS commercially are routinely performed in high concentrations of sucrose (700 to 850 g/L). In low sucrose concentrations (200 g/L), hydrolysis activity is predominantly related to transfer the fructosyl moiety and little FOS is produced (Yun 1996; Kim et al. 1998; Atiyeh and Duvnjak 2001). The commercial production process proceeds in two stages: enzyme production by microbial fermentation followed by enzymatic reaction with a high sucrose concentration (Maiorano et al. 2008; Sangeeta et al. 2005).
FOS synthesis can be performed with whole cell microorganisms that participate in sucrose biotransformation. Enzyme production and enzymatic reactions occur in a unique fermentative process (Fernandez et al. 2004). Furthermore, this direct production avoids the need for expensive purification procedures, reducing production costs (Ning et al. 2010). Due to transfructosylation activity, this conversion depends on a high sucrose concentration, presenting the need to identify suitable strains that can tolerate high osmotic pressure. Many reports use solid-state fermentation technique with by-products in the microorganism growth for FTase production (Muñiz-Marquez et al. 2016a).
Researchers have made efforts to improve FOS production by genetic selection, improving lineages, and using process optimization strategies to achieve higher yields (Sangeeta et al. 2005). Some of these studies are summarized in Table 1.
From the studies presented in Table 1, it is possible to see that FOS synthesis is performed in most of the papers, through processes involving enzymatic catalysis with free and immobilized enzymes, from microbial sources. Few reports use microorganisms directly in the process.
Many studies have shown that FTase production and transfructosylation reaction for FOS formation are positively influenced by sucrose concentration, with values above 40% among other factors (Vega-Paulino and Zúniga-Hansen 2012, Yun 1996;Sangeeta et al. 2005, Ghazi et al. 2007). In this analysis, however, it was not possible to determine the relationship between the two groups. Enzymatic processes exhibit about 55 to 60% sucrose conversion yield in FOS whereas whole cell processes present 64% (Dominguez et al. 2012; Ning et al. 2012), indicating that both are viable processes for subsequent scale-up studies.
Galactooligosaccharides (GOS)
Prebiotic galactooligosaccharides alongside FOS have been the most studied in recent years by the scientific community (Lamsal 2012). A number of studies highlight the main reaction mechanism involving lactose biotransformation in GOS: the β-galactosidase-mediated transgalactosylation reaction (Fai and Pastore 2015; Vera et al. 2016) responsible for simultaneous lactose hydrolysis and rearrangement in larger molecules, usually in number from 2 to 5 (Lamsal 2012; Srivastava et al. 2015; Aburto et al. 2016; González-Delgado et al. 2016), reaching up 10 (Muñiz-Marquez et al. 2016b).
Enzymatic GOS synthesis has been described by several authors over the years. (Gosling et al. 2010; Torres et al. 2010; Vera et al. 2016; Yin et al. 2017). It is a series of mechanisms directly related to β-galactosidase enzyme activity using lactose as main substrate (see Fig. 2).
Transgalactosylation reaction for GOS synthesis (adapted from Vera et al. 2016)
First, covalent bonding of the lactose molecule through its galactosyl moiety occurs with the enzyme, enabling catalysis reaction. At this moment, the reaction may follow different paths depending on the selectivity degree between the enzyme and lactose concentration, directly influencing the identity of the galactosyl acceptor. In the case where the acceptor is a water molecule, hydrolysis will occur, resulting in a galactose-free molecule. When this acceptor is another sugar molecule (lactose, GOS, glucose, or galactose), it acts as both donor and acceptor of the galactosyl moiety. Synthesis of new GOS occurs, the polymerization degree depending on the enzyme affinity by these nuclei (Vera et al. 2016).
Selectivity degree, as has been reported, depends on the enzyme origin as well as lactose concentration inherent at the process (Gosling et al. 2010). Generally, transgalactosylation reaction is favored at high lactose concentrations, generating higher GOS yields (Villamiel et al. 2014; Fai and Pastore 2015; Muñiz-Marquez et al. 2016a, b; González-Delgado et al. 2016; Vera et al. 2016). However, this will depend on the lactose molecule hydrolysis degree, specific for each β-galactosidase enzyme (Fai and Pastore 2015; Muñiz-Marquez et al. 2016a, b).
Basically, processes can be classified into reactions involving whole cells in lactose biotransformation, enzyme synthesis especially β-galactosidase, and enzymatic processes for GOS production. In addition, another current trend is to employ agro-industrial by-products as raw material containing substrates essential to the GOS production process.
Processes involving whole cells highlight the importance of using biomass in the lactose conversion to GOS and have demonstrated significant results. Examples of studies involving cell permeabilization, solvent recycling, and process optimization will be detailed below.
In a study performed by Petrova and Kujumdzieva (2010), the goal was selection of thermotolerant yeast strains for GOS production from dairy products such as milk, yogurt, and cheese. After growth of wild strains at 40 °C and 204 rpm in lactose and minerals medium, the biomass underwent treatment and its dry extract containing permeabilized cells was used in a medium containing 0.4 g/mL of lactose for transgalactosylation. Results as positive cell growth and bio transformation of lactose into tri- and tetragalactooligosaccharides in 15 different strains were obtained, mainly with Kluyveromyces genus, showing strong correlation between cell growth and β-galactosidase activity.
Fai et al. (2015) reported sequential optimization for GOS synthesis by Pseudozyma tsukubaensis and Pichia kluyveri, endophytic microorganisms isolated from fruits. Through the screening design methodology, followed by optimization of conditions for GOS synthesis, the researchers achieved a GOS per lactose yield of 28.35 g/100 g at 24 h and 73.71 g/L maximum GOS production, using as substrates lactose (28 g/100 mL), yeast extract (0.8 g/100 mL), and urea (91.8 g/100 mL), in a process at 30 °C and 150 rpm, being these latter two fixed parameters not statistically significant in the initial data treatment. Another relevant parameter to the process was pH, which after showing positive effect for the desired response was kept at the highest possible value, 8 in this case. Although other factors require further investigation, the strategy is feasible for a dynamic evaluation of the most important conditions inherent to the process, allowing a subsequent scale-up study.
In order to obtain a potential microorganism with transgalactosylation activity, Srivastava et al. (2015) found a yeast identified as Kluyveromyces marxianus NCIM 3551, as the most suitable biocatalyst for GOS production, and from there realized experiments with permeabilized cells. After conducting the reaction process with a mass/volume fraction of 20% lactose at 50 °C and 150 rpm for 8 h, parameters previously studied and optimized, the authors reached a maximum GOS mass fraction yield of 36%, with productivity of 24.0 g/L.h−1, high values for a whole cell system when compared to other studies. According to the authors, the optimum pH value depended on the existing β-galactosidase source, which demonstrates opportunities for adjustments to a pilot and industrial-scale process.
Sun et al. (2016) adopted a recyclable strategy for GOS production using whole cells of Kluyveromyces lactis with the intention of obtaining a considerable yield and high-purity molecules. For GOS synthesis, ethanol permeabilized cells were used, enhancing product yield. In the next stage of purification, whole cells, capable of consuming lactose and monosaccharides generated as by-products, were used, producing ethanol to be applied on cellular permeabilization of the synthesis step closing the reuse cycle. For GOS synthesis using 300 g/L lactose, temperature 35 °C, and pH 8.0, results showed a 21% yield with initial concentration of 23.6 g/L permeabilized cells in 0.5 h of reaction time. The purification step containing unconverted mass fractions of lactose (27.4%), GOS (35%), and monosaccharides (25.2% glucose and 12.4% galactose) was performed with nonpermeabilized Kluyveromyces lactis cells, resulting in a final product purity of a mass fraction 85% at different purification conditions. Ethanol was generated during the purification stage, underwent distillation, and can be reused in the cell permeabilization process. This demonstrates whole viability of the process, investing in strategies for optimization and reduction of solvent consumption.
A second approach, traditionally more studied, is the production of β-galactosidase enzyme by microorganisms, or use of the enzyme itself in GOS synthesis. Most recent studies involve these two cases showing alternatives for production improvements, such as enzymatic immobilization and process optimization.
The study performed by González-Delgado et al. (2016) aimed to optimize GOS synthesis by commercial β-galactosidase from Kluyveromyces lactis, Lactozym ™ Pure 6500 L. The analyzed parameters, in this case, temperature, enzyme concentration, pH, lactose concentration, and reaction time, were submitted to a factorial experimental design, the first two being fixed at 40 °C and 5 U/mL, respectively, in order to evaluate the yield of “desired-GOS,” trisaccharides, tetrasaccharides, and glucose/galactose ratio. Analysis led to a significant influence of the initial lactose concentration on the “desired-GOS” yield effect. The highest possible lactose concentration (250 g/L in this study) led to a maximum of 12.18% yield, at pH 7.0 and 3 h reaction. It is known that the initial lactose concentration is limited to its degree of solubility (Gänzle et al. 2008), hence the difficulty in obtaining higher GOS yields at the end of the optimized reaction.
Yin et al. (2017) studied reaction kinetics of commercial microbial β-galactosidases (Kluyveromyces lactis and Aspergillus oryzae) in comparison to the recombinant commercial Bacillus circulans β-galactosidase expressed in E. coli, as well as the GOS product profiles obtained. After incubation of 37 U/g lactose and specific reaction conditions at lactose concentration, pH, and temperature for each enzyme, results show that a specific GOS profile was obtained. Evaluation of the molecules’ production routes at certain time intervals was also studied. Yield values obtained at the end of 8 h reaction were 48.3% for Bacillus circulans β-galactosidase, 34.9% for Kluyveromyces lactis β-galactosidase, and 19.5% for Aspergillus oryzae β-galactosidase. In conclusion, the authors reported similar properties between the GOS profiles obtained for the enzymes of Kluyveromyces lactis and Aspergillus oryzae, whereas the B. circulans-derived enzyme generates different, more complex, and dynamic profiles due to its significantly higher transgalactosylation capacity.
The study developed by Aburto et al. (2016) involved simultaneous GOS synthesis and purification in order to investigate improvements in the yield and purity of the product by commercial Aspergillus oryzae β-galactosidase and Kluyveromyces marxianus, with Saccharomyces cerevisiae. This system allows for the selective removal of unwanted carbohydrates (monosaccharides and lactose). After reaction at 40 °C, pH 4.5, and 200 rpm, using lactose without additional supplements, the researchers obtained a yield of approximately 11% and 0.1 dry cell/carbohydrates ratio using the system Aspergillus oryzae β-galactosidase/Saccharomyces cerevisae at 50 IUH/glactose and 0.25 gcell biomass/gcarbohydrates. Thus, it was possible to perform a scale-up test, where the conditions previously obtained were tested at a mass fraction of 40% lactose, operated under temperature and pH control at an aeration rate of 5 vvm. According to the authors, a 40% GOS yield was obtained at 24 h reaction, corresponding to 40.3% lactose conversion, the product having 0.8% glucose and 19.2% galactose, representing according to the authors a technological advance in terms of productivity and yield. Despite not obtaining the best yield, the Aspergillus oryzae β-galactosidase/Kluyveromyces marxianus system in two sequential steps reached a purity level near 100% and carbohydrates consumption by the yeast had no interference in GOS synthesis (Gosling et al. 2010).
The following study, conducted by Satar et al. (2016), used immobilization of β-galactosidase enzyme in a graphene-linked docking method, applied on GOS production. Using Aspergillus oryzae β-galactosidase, the researchers after successful immobilization obtained a Km value almost three times greater than the solubilized enzyme, without significant modification of the reaction maximum rate. In addition, after using different lactose concentrations, the authors reached 27% GOS formed and 52% lactose conversion, a satisfactory result under the established conditions. Graphene-immobilized enzyme achieved up to 70% efficiency in the system reuse after 11 times, proving to be a good alternative in reducing process costs.
Another study with enzymatic immobilization was described by Rodriguez-Colinas et al. (2016), which used covalent binding between Bacillus circulans β-galactosidase and aldehyde-activated (glyoxal) agarose beads for GOS production in a packed-bed reactor. After successful immobilization, an enzyme activity of 97.4 U/g could be detected. The authors performed the procedure in a reactor for 213 h at 0.2 mL/min using a lactose solution at 100 g/L and 45 °C. Results show that after 14 cycles of reuse, the efficiency of the immobilized enzyme remained close to 100%, and continuous GOS production reached a maximum concentration of 24.2 g/L at a 50% lactose conversion rate, reaching a yield of approximately 25%. Although not a high yield value when compared to other works, data revealed an important alternative for the continuous process of GOS production, facilitating a future scale-up development.
The use of by-products from other industrial processes is an alternative that aims primarily to obtain environmentally sustainable and economically viable processes. In GOS production, many recent studies have reported the use of milk whey, an alternative already explored over the years in a wide variety of processes (Smithers 2015; Yadav et al. 2015). First steps with whey related to biotechnological processes involved enzyme production, such as β-galactosidase (González-Siso 1994). From that point on, other challenges have arisen, and currently, many processes involving the use of whey have been studied, such as in lactic acid production (Panesar et al. 2007; Cui et al. 2012), oil (Demir et al. 2013), ethanol (Gabardo et al. 2014; Diniz et al. 2014), and hydrogen (Romão et al. 2014). Recent studies described below are an example of whey use exclusively in GOS production.
The study by Golowczyc et al. (2013) highlights the use of whey permeate for optimization process in GOS synthesis using β-galactosidase from Aspergillus oryzae as biocatalyst, as well as preservation of Lactobacillus plantarum, recognized for its probiotic properties. At 37 °C, the procedure used 20 to 60 g solids/g suspension of whey permeate in an attempt to promote transgalactosylation with 100 IU/g lactose of enzyme, and subsequently, the GOS obtained was used in Lactobacillus plantarum inoculation performed at 37 °C and 24 h. Results indicate a maximum GOS/lactose yield of 27.3 g/100 g obtained in the initial solution of 40 g solids/100 g suspension. In addition, no significant differences about the probiotic in whey permeate with or without GOS were noted. The strategy observed in this study is relevant from the product development point of view, with probiotic and prebiotic properties, proving to be a viable alternative in future studies.
The use of cheese whey permeate to obtain GOS as well as isomerization process for lactulose synthesis was reported by Padilha et al. (2015), using transgalactosylation of Kluyveromyces lactis and Kluyveromyces marxianus β-galactosidases, strains isolated from cheeses. After reaction with 250 g/L lactose present in whey permeate at pH 6.5, 50 °C, and 6 U/mL β-galactosidase activity, authors achieved a total relation prebiotics/whey permeate of 322 g/kg. Isomerization to obtain lactulose and its derived oligosaccharides suggests a greater diversity of potential prebiotic carbohydrates present in the mixture composed of tagatose, lactulose, GOS, and oligosaccharides from lactulose (OsLu), making this method suitable to produce novel mixtures of dietary nondigestible carbohydrates.
Fischer and Kleinschmidt (2015) used β-galactosidases from Aspergillus oryzae and Kluyveromyces lactis in GOS synthesis with sweet and acid whey as substrates. By dissolving whey in order to obtain concentrations of 38 to 300 g/L lactose, the process was adjusted to 100 rpm and specific temperatures and pH values for each enzyme with 50 U/g concentration for all trials. Results indicate that Kluyveromyces lactis enzyme presented the highest yield values (33% on average) at a 200 g/L lactose concentration, but it presented higher sensitivity to cations, demonstrating that there could be differences in yield when different mineral amounts are present in sweet whey. There was no significant difference between sweet or acid whey, so the two by-products were considered suitable for GOS synthesis.
The studies shown in this topic highlight the importance of strategy development for the biotechnological GOS production. Both use of whole cells and enzymatic processes are able to obtain substantial results that can be used in future developments of continuous processes and scale-up. The use of by-products such as whey demonstrates studies focus on increasingly sustainable and economically viable processes, creating a range of diversified products and contributing to the consumer health.
Xylooligosaccharides (XOS)
Xylooligosaccharides are sugar oligomers made of xylose units linked by β(1 → 4) xylosidic bonds, which possess the molecular formula C5n H8n + 2O4n + 1, n = (2 to 6), and are obtained by hydrolysis of xylans (Kumar and Satyanarayana 2011; Moniz et al. 2014; Samanta et al. 2015). Structurally, XOS can be found in different forms depending on the number of monomers that composed their chemical structure, among them xylobiose (two monomers), xylotriose (three monomers), xylotetraose (four monomers), xylopentaose (five monomers), xylohexose (six monomers), and so on (Fig. 3) (Kumar and Satyanarayana 2011). Furthermore, some authors also suggest that molecules with xylose polymerization degree less than or equal to 20 can also be considered XOS, increasing the chemical diversity of these compounds (Mäkeläinen et al. 2010).
Due to their physical–chemical characteristics, XOS are classified as nondigestible oligosaccharides (NDO), considered prebiotics and soluble fibers with excellent stability in a wide range of temperatures (up to 100 °C) and pH conditions (2.5 to 8.0). They can be naturally present in several food products, such as fruits and vegetables, honey, and milk, or in plant xylan-rich materials like sugarcane bagasse, bamboo, corncobs, barley straw, wheat bran, and cotton stalk (Carvalho et al. 2013; Courtin et al. 2009; Fu et al. 2012; Singh et al. 2015). Considering heat and acidity resistance, XOS exhibit better characteristics when compared to inulin and FOS, making the most advantageous as food ingredient and other applications considering the stability during several food processing such as pasteurization and autoclave sterilization at low pH (Courtin et al. 2009; Wang et al. 2009; Vasquez et al. 2000).
In the past few years, many studies described the health benefits of XOS consumption due to its selective growth stimulation and modulation of beneficial colon microbiota increasing the number of bifid bacteria and Lactobacillus by immune-stimulation, also regulation of insulin secretion from the pancreas, reduction of blood cholesterol levels, pro-carcinogenic enzyme reduction in the gastrointestinal tract, enhanced mineral absorption from the large intestine, and antioxidant and anti-inflammatory effects (Vasquez et al. 2000; Mäkeläinen et al. 2010; Chapla et al. 2012; Samanta et al. 2015; Bian et al. 2013; Kallel et al. 2015a, b; Yoshino et al. 2006).
Like other prebiotics, XOS are fermented to short-chain fatty acids (SCFA) such as acetate, propionate, butyrate, and lactate in the lower gastrointestinal tract. These compounds contribute to pH decrease and can be related to prevention of gut infection, suppression of colon cancer initiation, and improvement of intestinal health (Cummings et al. 2001; Campbell et al. 1997; Swennen et al. 2006; Topping and Clifton 2001; Singh et al. 2015).
Three main methods are employed for XOS production: (i) chemical, (ii) enzymatic, and (iii) autohydrolysis process (Samanta et al. 2015). The production of XOS by chemical process is performed using acid hydrolysis or alkali extraction at high temperature and pressure, which lead to a large xylose production, but also undesirable by-products as furfural and hydroxymethyl furfural (Xue et al. 2016). In the autohydrolysis process, biomass rich in xylan is subjected to processing with water applying specific conditions of temperature and pressure. As in the chemical process, there is an occurrence of undesirable components such as soluble lignin and a large amount of monosaccharides (Xue et al. 2016; Akpinar et al. 2010; Ho et al. 2014; Kumar and Satyanarayana 2015).
Enzymatic hydrolysis consists in the employment of a xylanolytic enzyme system composed of endoxylanase, exoxylanase, and debranching enzymes (Kumar and Satyanarayana 2015). The use of xylanases with less amount of exoxylanase or β-xylosidase activities are preferred for XOS because these hemicellulases act in solubilizing xylan (hemicellulosic component) from plant cell walls (Kumar and Satyanarayana 2015; Zhao et al. 2012; Azelee et al. 2016).
Conceptually, xylanases are defined as glycosidase (O-glycoside hydrolase, EC 3.2.1.x) able to catalyze endohydrolysis of 1,4-β-D-xylosidic linkages in xylan, considered a major structural polysaccharide in plant cells and that constituting around 30 to 35% of the lignocellulosic biomass (Collins et al. 2005). Moreover, GH10 and GH11 xylanases from the glycoside hydrolase (GH) family are important for XOS production and many studies have been focused in identification of novel microorganisms able to produce these enzymes and their application in degradation of lignocellulosic biomass or other substrates (Kim et al. 2014; Ghio et al. 2016; Ravn et al. 2017; Nieto-Dominguez et al. 2017).
In XOS enzymatic production, xylanolytic enzymes can be added directly to the reaction medium, produced in situ by xylanase producers in fermentation or immobilized inside the biomass used as substrate (Akpinar et al. 2010; Dorta et al. 2006; De Oliva-Neto and Menao 2009). The enzymatic process for xylan conversion into value-added useful products is considered promising due to the use of un-utilized and under-utilized agricultural residues as cotton stalks, corncobs, wheat straw, poplar wood, sugarcane bagasse, sunflower and tobacco stalks, as well as other lignocellulosic materials (Samanta et al. 2015; Akpinar et al. 2007; Ruzene et al. 2008; Yuan et al. 2010; Peng et al. 2009; Akpinar et al. 2009; Carvalho et al. 2013).
Table 2 shows some studies using low-cost agricultural residues in XOS production and mainly bioprocess characteristics. Thus, studies focusing on isolation of novel microbial xylanase producers, development of new strategies in lignocellulosic biomass pretreatment to obtaining xylan, and its application for XOS production can be considered an important biotechnological approach for food and nutraceutical fields.
Boonchuay et al. (2016) describe the use of pretreated corncob residues as substrate for XOS production applying an endoxylanase produced by Streptomyces thermovulgaris TISTR1948. Purified xylanase had good stability characteristics within a pH range of 4.0 to 11.5 and temperatures varying from 50 to 70 °C. Potential for total XOS production, which consisted of xylose, xylobiose (X2), xylotriose (X3), xylotetraose (X4), and xylopentaose (X5), and individual profiles of these sugars obtained during the process were compared with crude and partially purified xylanases. Results showed that final total XOS concentrations produced were similar to three preparations, about 30 mg/mL after 24 h incubation time. The main products were X2, X4, and X5 with a total mass fraction of 73.94, 69.91, and 75.2% produced (X2 + X4 + X5) at 12 h reaction time to crude, partially purified, and purified xylanase, respectively.
Other studies also showed the application of xylanases from Streptomyces strains, as S. halstedii immobilized on glyoxyl-agarose and S. matensis DW67 in bioprocess for XOS production with similar composition (Aragon et al. 2013a, b; Yan et al. 2009).
Many studies have reported the use of different xylanases from B. subtilis strains and agro-residues for XOS production. In addition, xylanases from Bacillus species have been selectively cloned and heterologous expressed in E. coli aiming reduction of costs in enzyme production (Chang et al. 2017). The use of conventional birchwood xylan and its soluble fraction as substrate applying free and immobilized recombinant xylanase (XynA) from Bacillus subtilis was recently described for XOS production (Milessi et al. 2016). The immobilized XynA was obtained in agarose activated with glyoxal groups showing high immobilization yield (100%), and when applied (1.95 UI/mL) for 24 h, the maximum XOS production reached was 2.9 and 3.2 g/L using conventional and soluble xylan at 13 mg/mL, 50 °C, and pH value of 5.5. These results were similar to those obtained using soluble XynA for hydrolysis of these substrates (3.1 mg/mL to conventional xylan and soluble xylan) under the same conditions. It is important to highlight that the XOS profile obtained in this study consisted of three major products, including X2, X3, and X4. These are considered the most interesting XOS for food industry applications.
The efficiency of a crude xylanase produced by B. subtilis KCX006 for XOS production with a low polymerization degree (mainly X2, X3, and X4) and arabinosyl- and glucoronyl-substituted XOS from ammonia-pretreated sugarcane bagasse was described by Reddy and Krishnan (2016). Relative XOS levels produced were 68.48 mg/g of X2 (50% total XOS produced, approximately), 22.42 mg/g of X3, and 4.05 mg/g of X4, while substituted XOS were produced at around 44.00 mg/g. The formation of significant xylose amounts during the biotechnological process reduces XOS yield and purity, and in this study, the xylose concentration was about 0.70 mg/g, representing 0.5% of total XOS produced, indicating economic high-pure XOS production without xylose from low-cost lignocellulosic substrate and crude xylanase mixture.
In the study performed by Kallel et al. (2015a, b), XOS was produced using xylan from garlic straw as substrate and purified xylanase from a new B. mojavensis UEB-FK strain. This xylanase exhibited high thermal and pH stability, and when applied for XOS production, the maximum yield reached after 8 h of hydrolysis was about 29%, composed mainly of X2, X3, and X4. Previously, Bragato et al. (2013) reported a biotechnological process with high XOS yield (100 mg/g) using sugarcane bagasse pretreated with glacial acetic acid and hydrogen peroxide at 60 °C for 7 to 48 h and recombinant xylanase (XynA) expressed from B. subtilis.
Recently, Heinen et al. (2017) performed the immobilization of an endoxylanase from Aspergillus tamarii Kita using ionic (carboxymethyl-cellulose; CMC) and covalent (cyanogen bromide; CnBr-agarose and glyoxyl-agarose) supports, aiming to enhance the enzymatic thermostability during XOS production. Immobilized xylanase specific activity (U/mgIP) in glyoxyl-agarose and CM-cellulose showed the best results (1945.75 and 3781.48 respectively), while immobilized xylanase in CNBr-agarose exhibited an activity around 702 U/mgIP. However, the most stable immobilized system was the glyoxyl-agarose derivative which showed the maximum activity at 65 °C and a relative activity higher than 90% in several ranges of pH (4 to 9) after 24 h, and able to hydrolyze beechwood xylan producing mainly X2, X3, X4, and X5 at 80 °C.
Similarly, Aragon et al. (2013a, b) evaluated xylanase application from A. versicolor immobilized on different supports and a packed-bed enzymatic reactor for XOS production. Different immobilization yields were obtained for four supports tested, with a high percentage when glyoxyl-agarose (88%) and glutaraldehyde-agarose (97%) were used, and lower values to monoaminoethyl-N-aminoethyl (MANAE)–agarose (34%) and polyethylenimine-agarose (19%). For XOS formation, with hydrolysis time from 0.5 to 7 h and using soluble birchwood xylan (18 mg/mL), an increase in XOS production using the glyoxyl-agarose derivative with high values of X2 and X3 was observed. After 7 h of hydrolysis, 1.87 mg/mL of X2 and 0.97 mg/mL of X3 were reached with immobilized xylanase, while 0.74 mg/mL of X2 and 0.80 mg/mL of X3 were produced with the soluble xylanase, demonstrating the efficiency of immobilization strategies for fungal xylanases.
Potential use of purified xylanase from Clostridium strain BOH3 and thermal-alkali-pretreated hardwood for XOS production was described by Rajagopalan et al. (2017). Initially, sawdust from mahogany (Swietenia sp.) and mango (Mangifera sp.) was used for xylan extraction and was pretreated separately by different methods. High XOS yields, rich in X2 and X3 monomers, were obtained for mahogany (89.5 mg/g pretreated sawdust or 527.1 mg/g xylan in pretreated sawdust) and mango (67.6 mg/g pretreated sawdust or 434.2 mg/g xylan in pretreated sawdust) using thermal pretreatment with NaOH. These oligosaccharides also exhibited prebiotic effects on several probiotic microorganisms, as Bifidobacterium animalis, Bifidobacterium adolescentis, Lactobacillus acidophilus, and Lactobacillus brevis.
Considering the increasing demand for efficient strategies for functional food ingredient production, biotechnological processes for prebiotic XOS production are promising due to the possible use of lignocellulosic biomass and controlled enzymatic hydrolysis. However, more studies are required in order to isolate novel xylanase producers, improve the xylanase performance in several temperature and pH conditions, search for suitable methods for biomass pretreatment and strategies to increase XOS production with low polymerization degree for a cost-attractive and industrially competitive bioprocess development.
Mannanooligosaccharides (MOS)
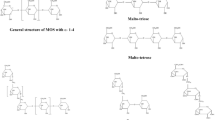
A novel class of oligosaccharide, less studied than FOS, GOS, and XOS, is the mannan-derivative oligosaccharides (MOS). Mannans are one of the major hemicellulose groups which have a role as structural polymers in plant cell walls and as storage carbohydrate in plant seeds (Moreira and Filho 2008; Mikkelson et al. 2013). They can be classified according to the main sugars in its constitution: linear mannan contains only mannopyranosyl units linked by β-1,4 linkages, while glucomannan consists of mannopyranosyl and glucopyranosyl units bounded by β-1,4 linkages. Each of these groups may also contain α-1,6 galactopyranosyl units as side groups, being called as galactomannans and galactoglucomannans respectively (Fig. 4) (Mikkelson et al. 2013; Van Zyl et al. 2010). In softwoods, the main constituent of hemicellulose is glucomannan/galactoglucomannan and galactomannan mainly found in seeds (Moreira and Filho 2008). Although MOS production from lignocellulosic biomass has several drawbacks, biomass with more than 10% dry weight of mannans, such as pine, may have potential to be employed as substrate for this reaction (Otieno and Ahring, 2012).
In order to obtain mannanooligosaccharides from biomass, enzymatic hydrolysis of linear chains into smaller oligosaccharide (two to ten) units and removal of side groups are necessary. β-Mannanases (1,4-β-D-mannan mannohydrolases, EC 3.2.1.78) attack the internal glycoside bonds of the mannan backbone chain, releasing β-1,4-mannanooligosaccharides. β-Mannosidase (1,4-β-D-mannopyranoside hydrolases, EC 3.2.1.25) releases mannose units by attacking the terminal linkage from oligosaccharides or through cleavage of mannobiose. Glucopyranose units can be removed from glucomannan and galactoglucomannan by β-glucosidases (1,4-β-D glucoside glucohydrolases, EC 3.2.1.21).
Major enzymes in side group removal are α-galactosidase (1,6-α-D-galactoside galactohydrolase, EC 3.2.1.22) and acetyl mannan esterase (EC 3.1.1.6), which release α-1,6 galactopyranosyl units from the mannan backbone and acetyl groups from galactoglucomannan respectively (Van Zyl et al. 2010). Another methodology employed for obtaining MOS is its isolation from yeast cell wall fragments. This structure is ruptured by centrifugation, washed, dried, and pasteurized to harvest the mannanooligosaccharides (Belorkar and Gupta 2016; Patel and Goyal 2012).
Mannanooligosaccharides have been reported as valuable emerging prebiotic compounds since they are able to stimulate the proliferation of normal bacterial flora and inhibit pathogenic microorganisms, contributing to the optimal gut function (Patel and Goyal 2012). In addition, MOS was proved to be beneficial due to its immune-pharmacological, therapeutic, and biomedical properties. Therefore, there is a great interest in applying this compound in food, feed, and pharmaceutical fields (Ferreira et al. 2012; Yamabhai et al. 2016; Srivastava and Kapoor 2017).
In animals, feed supplementations with MOS led to increased egg production and improved antibody production titer efficiency in aged laying hens, promoted growth, and enhanced posterior gut epithelial defense. Also, they improved growth performance of fattening pigs and modulated the immune response of the Pacific white shrimp, improving its survival rate after exposure to Vibrio harveyi (Ghasemian and Jahanian 2016; Torrecillas et al. 2013; Torrecillas et al. 2014; Torrecillas et al. 2015; Giannenas et al. 2016; Rungrassamee et al. 2014).
Microorganisms are able to produce a wide variety of enzymes, and among them, β-mannanases can be found in bacteria, yeasts, and fungi. This is an extracellular enzyme, and its production and activity are affected by parameters like temperature, pH, and carbon source, either in submerged or solid-state fermentation. Therefore, statistical methods can be used in order to optimize process conditions (Srivastava and Kapoor 2017). Also, there is the possibility of using genetic engineered microorganisms as well as heterologous gene expression for oligosaccharide production by fermentation process, contributing positively to industrial manufacturing of these prebiotics (Moreno et al. 2017). In this approach, many researchers are focusing on using microbial cells or their purified enzymes to obtain mannan-derivative oligosaccharides.
Blibech et al. (2011) reported the production of mannanooligosaccharides from locust bean gum using mannanase immobilized in chitin with glutaraldehyde by cross-linking reaction. This enzyme was obtained from Penicillium occitanis, which had already been described to produce mannotetraose, mannotriose, and mannobiose when incubated with locust bean gum and ivory nut mannan (Blibech et al. 2010). The immobilized enzyme proved to be more stable to temperature and pH than the nonimmobilized enzyme, retaining 40% of its activity after incubation at 70 °C for 30 min, while the free one retained only 7%. The optimal temperature was not altered after immobilization (70 °C), but the optimum pH shifted from 4 to a more acid range (3 to 4). In conclusion, using immobilized mannanase, it was possible to obtain mannotriose and mannotetraose to be employed as mannanooligosaccharide prebiotics (Blibech et al. 2011).
Similarly, β-mannanase from Bacillus pumilus GBSW19 was purified and tested for hydrolysis of locust bean gum. With an optimum pH of 6.5 and temperature of 65 °C, this new enzyme, named Bpman5, was shown to be thermo-, pH-, and detergent-stable, with potential application in bioconversion and fiber industries. The optimized conditions 10 U/mg enzyme, 10 mg/mL locust bean gum, and incubation at 50 °C for 24 h led to a mixture of oligosaccharides with degrees of polymerization (DP) of 2 to 6, mainly mannobiose, mannotriose, and mannopentaose, in a concentration of 1.19 mg/mL (Zang et al. 2015).
Copra meal is a residue containing approximately 60% of mannan, therefore a good substrate for MOS production. Ariandi and Meryandini (2015) optimized copra meal hydrolysis using Streptomyces sp. BF3.1 mannanase. Using a mass/volume fraction of 10% copra meal and incubation for 5 h at 30 °C, it was possible to obtain mannanooligosaccharides with DP = 4 in a concentration of 3.83 mg/mL.
Trichoderma reesei is one of the main industrial cellulolytic enzyme sources, and its genome contains about 200 glycoside hydrolase encoding genes. Therefore, three enzymes, Tr Cel5A, Tr Cel7B, and mannanase from T. reesei, were employed in konjac hydrolysis, a polysaccharide extracted from tubers of Amorphophallus konjac, for glucomannooligosaccharide production. All the enzymes studied were able to release 50% of the reducing sugars after 48 h reaction and oligosaccharides obtained have DP 2 to 6 with variable sequences (Mikkelson et al. 2013). Using response surface methodology, it was possible to identify the optimum temperature, pH, hydrolysis time, and enzyme to substrate ratio (E/S) in glucomannanooligosaccharide production from konjac. In this study, oligosaccharides produced were indicated by the concentration of direct reducing sugar (DRS) and the highest concentration (3.709 mg/mL) was obtained after a reaction time of 3.4 h, at 41 °C, pH of 7.1, and E/S of 0.49 (Chen et al. 2013).
A Bacillus subtilis YH12 strain, isolated from konjac rhizome soil, was able to produce β-mannanase when cultivated at 37 °C in a medium containing konjac powder. Enzyme content in the supernatant could be purified and exhibited high activity for hydrolysis of several substrates, such as locust bean gum, konjac powder, xanthan gum, guar gum, and fenugreek gum. When incubated with the substrates in pH 6.5 at 55 °C for 8 h, oligosaccharides with variable DP could be obtained, for example DP 1 to 7 from locust bean gum, DP 2 to 7 from konjac powder, and DP 1 to 2 from xanthan gum (Liu et al. 2015).
Jian et al. (2013) used a commercially available mannanase purified from Aspergillus niger to obtain MOS from Gleditsia sinensis (Leguminosae family) galactomannan gum. In preliminary experiments, range of pH 3 to 6, substrate concentration of 2 to 8%, enzyme amount of 5 to 9 U/g, temperature of 40 to 80 °C, and reaction time of 0 to 48 h were evaluated. Considering the enzyme optimum pH and the increase in substrate concentration, a decrease on hydrolysis yield occurred, so these two parameters were fixed in pH 4 and a fraction mass/volume of 5% respectively. Other three parameters could be optimized employing response surface methodology through a central composite design with three independent variables and five levels. It was possible to obtain oligosaccharides with DP 1 to 5 in a concentration of 29.1 g/L (yield of 75.9%) after incubation at 57.4 °C for 34.1 h and using 8.1 U/g of enzyme.
A strain of Penicillium chrysogenum QML-2 was isolated from Chinese soil samples and reported as a good producer of both xylanases and β-mannanases. In order to achieve the highest enzyme activity, parameters that affect enzyme production were optimized. Firstly, a Plackett-Burman design (PBD) was employed to screen most significant variables among wheat bran (WB), corn stover powder (CSP), (NH4)2SO4, yeast extract, NaCl, MgSO4·7H2O, KH2PO4, CaCl2, moisture content, initial pH, cultivation temperature, and inoculum size. Then, a Box–Behnken design (BBD) was applied to evaluate the significant variables moisture content, initial pH, and inoculum size. After optimization, it was possible to achieve an eightfold increase in β-mannanase activity (from 928.41 to 8479.82 U/g) when the strain was cultivated in a moisture content of 74%, initial pH 4.5, and inoculum size in a fraction mass/volume of 11.6% (Zhang and Sang 2015).
A β-mannanase-producing strain identified as Bacillus nealsonii PN-11 was isolated from landfill areas in India (Chauhan et al. 2014a), and the enzyme produced (ManPN11) was purified and characterized by Chauhan et al. (2014b). This enzyme showed optimal activity at a temperature of 65 °C and pH 8.8, which is interesting since most of the mannanases are not stable at high pH. Regarding its stability, ManPN11 retained 50 and 85% of its activity after 3 h incubation at 70 °C and pH 5 to 10 respectively. It was also shown that organic solvents did not interfere in the enzyme activity. For MOS production, 0.1% of the strain inoculum was incubated in minimal media (FeSO4·7H2O, 0.01 g/L; CaCl2·2H2O, 0.05 g/L; MgSO4·7H2O, 0.2 g/L; KH2PO4, 2.32 g/L; K2HPO4, 7.5 g/L; NH4NO3, 0.3 g/L) at 37 °C for 96 h. The mannan source used was locust bean gum (8 g/L). The MOS obtained were mainly mannotriose, mannotetraose, and mannopentaose. Prebiotic potential was proved since they enhanced the growth of Lactobacillus casei but inhibited the growth of Salmonella enterica.
Production of ManPN11 was further optimized by the “one factor at a time” method in submerged fermentation. Variables considered in this study were time (0 to 168 h), pH (4 to 9), temperature (27 to 47 °C), inoculum mass/volume fraction (0.1 to 0.5%), inoculum age (12 to 36 h), carbon and nitrogen sources, locust bean gum mass/volume fraction (0.1 to 1%), bactopeptone mass/volume fraction (0.1 to 0.5%), and agitation (100 to 200 rpm). The most influencing factors were locust bean gum, bactopeptone, and inoculum age. Under optimized conditions, it was possible to achieve a ninefold increase in enzyme yield, from 6 to 55 U/mL (Chauhan et al. 2014c).
In the biotechnological process, oftentimes, microorganisms able to produce enzymes are not suitable for industrial application. Fungi strains can produce a wide range of enzymes, but in the process for foodstuff production, there is a regulatory issue regarding that the biocatalyst employed must be recognized as safe (GRAS) and there is always the concern about mycotoxins. Moreover, bacteria and yeasts are easier to be cultivated in large-scale fermentation tanks (Ozturk et al. 2010; Zhu et al. 2014; Yang et al. 2015; Srivastava and Kapoor 2017). Therefore, several research groups have been focusing in cloning and expression of β-mannanase genes in heterologous hosts. Some of them are summarized in Table 3.
There are several studies describing isolation of β-mannanase from fungal or bacterial strains, optimization assays to obtain higher yields or enzymes activities, and even cloning of β-mannanase genes in different hosts to facilitate enzyme expression (Srivastava and Kapoor 2017). The main objective of these studies is to employ the β-mannanase for mannan hydrolysis and obtaining MOS due to its potential application as prebiotic and proved efficiency as supplement in food, feed, and medical fields. In this context, further researches are necessary aiming to overcome the drawbacks in MOS production as well as to evaluate the biosafety before availability to the consumer market.
Future perspectives
These recent studies demonstrate the great potential for biotechnological oligosaccharide production. Most used strategies point to a development of microorganisms and enzymes able to synthesize these molecules on a large scale, so the necessary advance in those studies, for example, the development of scale-up strategies.
This work also highlights the use of agro-industrial waste or by-products as raw material for oligosaccharide production, an alternative that is fully related to sustainable development and economically necessary to obtain low-cost processes. Finally, there is a need for further downstream steps studies in order to obtain desired yields and purity degrees to the final product. The complete tracking of all the biotechnological production process stages of oligosaccharides will be able to generate all the necessary data to process development in a large scale, thus feeding the productive chain of food prebiotic fibers.
References
Aburto C, Guerrero C, Vera C, Wilson L, Illanes A (2016) Simultaneous synthesis and purification (SSP) of galactooligosaccharides in batch operation. LWT - Food Sci Technol 72:81–89. https://doi.org/10.1016/j.lwt.2016.04.029
Akpinar O, Ozlem AK, Kavas A, Bakir U, Yilmaz L (2007) Enzymatic production of xylooligosaccharides from cotton stalks. J Agric Food Chem 55:5544–5551. https://doi.org/10.1021/jf063580d
Akpinar O, Erdogan K, Bostanci S (2009) Enzymatic production of xylooligosaccharide from selected agricultural wastes. Food Bioprod Process 87:145–151. https://doi.org/10.1016/j.fbp.2008.09.002
Akpinar O, Erdogan K, Bakir U, Yilmaz L (2010) Comparison of acid and enzymatic hydrolysis of tobacco stalk xylan for preparation of xylooligosaccharides. LWT Food Sci Technol 43:119–125. https://doi.org/10.1016/j.lwt.2009.06.025
Alvaro-Benito M, De Abreu M, Fernandez-Arrojo L, Plou FJ, Jimenez-Barbero J, Ballesteros A, Polaina J, Fernandez-Lobato M (2007) Characterization of a β-fructofuranosidase from Schwanniomyces occidentalis with transfructosylating activity yielding the prebiotic 6-kestose. J Biotechnol 132:75–81
Aragon CC, Mateo C, Ruiz-Matute AI, Corzo N, Fernandez-Lorente G, Sevillano L, Díaz M, Monti R, Santamaría RI, Guisan JM (2013a) Production of xylo-oligosaccharides by immobilized-stabilized derivatives of endo-xylanase from Streptomyces halstedii. Process Biochem 48:478–483. https://doi.org/10.1016/j.procbio.2013.01.010
Aragon CC, Santos AF, Ruiz-Matute AI, Corzo N, Guisan JM, Monti R, Mateo C (2013b) Continuous production of xylo-oligosaccharides with immobilized-stabilized biocatalysts of xylanase from Aspergillus versicolor. J Mol Catal B Enzym 98:8–14. https://doi.org/10.1016/j.molcatb.2013.09.017
Ariandi Y, Meryandini A (2015) Enzymatic hydrolysis of copra meal by mannanase from Streptomyces sp. BF3.1 for the production of mannooligosaccharides. HAYATI J Biosci 22:79–86. https://doi.org/10.4308/hjb.22.2.79
Atiyeh H, Duvnjak Z (2001) Production of fructose and ethanol from media with high sucrose concentrations by a mutant of Saccharomyces cerevisiae. J Chem Technol Biotenol 76:1017–1022. https://doi.org/10.1002/jctb.474
Azelee NIW, Jahim JM, Ismail AF, Fuzi SFZM, Rahman RA, Illias RM (2016) High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Ind Crop Prod 81:11–19. https://doi.org/10.1016/j.indcrop.2015.11.038
Belorkar SA, Gupta AK (2016) Oligosaccharides: a boon form nature’s desk. AMB Expr 6:82. https://doi.org/10.1186/s13568-016-0253-5
Bian J, Peng F, Peng XP, Peng P, Xu F, Sun RC (2013) Structural features and antioxidant activity of xylooligosaccharides enzymatically produced from sugarcane bagasse. Bioresour Technol 127:236–241. https://doi.org/10.1016/j.biortech.2012.09.112
Blatchford P, Ansell J, de Godoy MRC, Fahey G, Garcia-Mazcorro JF, Gibson GR, Goh YJ, Hotchkiss AT, Hutkins R, LaCroix C, Rastall RA, Reimer RA, Schoterman M, Van Sinderen D, Venema K, Whelan K (2013) Prebiotic mechanisms, functions and applications: a review. Int J Probiotics Prebiotics 8:109–132
Blibech M, Ghorbel RE, Fakhfakh I, Ntarima P, Piens K, Bacha AB, Chaabouni SE (2010) Purification and characterization of a low molecular weight of β-mannanase from Penicillium occitanis Pol6. Appl Biochem Biotechnol 160:1227–1240. https://doi.org/10.1007/s12010-009-8630-z
Blibech M, Chaari F, Bhiri F, Dammak I, Ghorbel RE, Chaabouni SE (2011) Production of manno-oligosaccharides from locust bean gum using immobilized Penicillium occitanis mannanase. J Mol Catal B Enzym 73:111–115. https://doi.org/10.1016/j.molcatb.2011.08.007
Boonchuay P, Takenaka S, Kuntiya A, Techapun C, Lekawasdi N, Seesuriyachan P, Chaiyaso T (2016) Purification, characterization, and molecular cloning of the xylanase from Streptomyces thermovulgaris TISTR1948 and its application to xylooligosaccharide production. J Mol Catal B Enzym 129:61–68. https://doi.org/10.1016/j.molcatb.2016.03.014
Bragato J, Segato F, Squina FM (2013) Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Ind Crop Prod 51:123–129. https://doi.org/10.1016/j.indcrop.2013.08.062
Brienzo M, Carvalho W, Milagres AMF (2010) Xylooligosaccharides production from alkali-pretreated sugarcane bagasse using xylanases from Thermoascus aurantiacus. Appl Biochem Biotechnol 162:1195–1205. https://doi.org/10.1007/s12010-009-8892-5
Bruzzese E, Volpicelli M, Squaglia M, Tartaglione A, Guarino A (2006) Impact of prebiotics on human health. Digest Liver Dis 38:S283–S287. https://doi.org/10.1016/S1590-8658(07)60011-5
Byun SH, Han WC, Kang SA, Kim CH, Jang KH (2007) Production of fructo-oligosaccharides from sucrose by two levansucrases from Pseudomonas aurantiaca and Zymomonas mobilis. J Biotechnol 131:S112–S112. https://doi.org/10.1016/j.jbiotec.2007.07.194
Cai H, Shi P, Uo H, Bai Y, Huang H, Yang P, Yao B (2011) Acidic β-mannanase from Penicillium pinophilum C1: cloning, characterization and assessment of its potential for animal feed application. J Biosci Bioeng 113:551–557. https://doi.org/10.1016/j.jbiosc.2011.08.018
Campbell JM, Fahey GC, Wolf BW (1997) Selected indigestible oligosaccharides affects large bowel mass, cecal and fecal short-chain fatty acids, pH and micro flora in rats. J Nutr 127:130–136
Carvalho AFA, Neto PO, Silva DF, Pastore GM (2013) Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int 51:75–85. https://doi.org/10.1016/j.foodres.2012.11.021
Castro CC, Nobre C, Duprez ME, De Weireld G, Hantson AL (2017) Screening and selection of potential carriers to immobilize Aureobasidium pullulans cells for fructo-oligosaccharides production. Biochem Eng J 118:82–90. https://doi.org/10.1016/j.bej.2016.11.011
Chang S, Guo Y, Wu B, He B (2017) Extracellular expression of alkali tolerant xylanase from Bacillus subtilis Lucky9 in E. coli and application for xylooligosaccharides production from agro-industrial waste. Int J Biol Macromolec 96:249–256. https://doi.org/10.1016/j.ijbiomac.2016.11.032
Chapla D, Pandit P, Shah A (2012) Production of xylooligosaccharides from corncob xylan by fungal xylanase and their utilization by probiotics. Bioresour Technol 115:215–221. https://doi.org/10.1016/j.biortech.2011.10.083
Chauhan PS, Bharadwaj A, Puri N, Gupta N (2014a) Optimization of medium composition for álcali-thermostable mannanase production by Bacillus nealsonii PN-11 in submerged fermentation. Int J Curr Microbiol App Sci 3:1033–1045
Chauhan PS, Sharma P, Puri N, Gupta N (2014b) Purification and characterization of an álcali-thermostable β-mannanase from Bacillus nealsonii PN-11 and its application in mannooligosaccharides preparation having prebiotic potential. Eur Food Res Technol 238:927–936. https://doi.org/10.1007/s00217-014-2170-7
Chauhan PS, Soni SK, Sharma P, Saini A, Gupta N (2014c) A mannanase from Bacillus nealsonii PN-11: statistical optimization of production and application in biobleaching of pulp in combination with xylanase. Int J Pharma Bio Sci 5:237–251
Chen J, Chen XM, Xu XM, Ning YW, Jin ZY, Tian YQ (2011) Biochemical characterization of an intracellular (6)G-fructofuranosidase from Xanthophyllomyces dendrorhous and its use in production of neo-fructooligosaccharides (neo-FOSs). Bioresour Technol 102:1715–1721. https://doi.org/10.1016/j.biortech.2010.08.033
Chen J, Liu D, Shi B, Wang H, Cheng Y, Zhang W (2013) Optimization of hydrolysis conditions for the production of glucomanno-oligosaccharides from konjac using β-mannanase by response surface methodology. Carbohydr Polym 93:81–88. https://doi.org/10.1016/j.carbpol.2012.05.037
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol 29:3–23. https://doi.org/10.1016/j.femsre.2004.06.005
Courtin CM, Swennen K, Verjans P, Delcour JA (2009) Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chem 112:831–837. https://doi.org/10.1016/j.foodchem.2008.06.039
Cui F, Wan C, Li Y, Liu Z, Rajashekara G (2012) Co-production of lactic acid and Lactobacillus rhamnosus cells from whey permeate with nutrient supplements. Food Bioprocess Technol 5:1278–1286. https://doi.org/10.1007/s11947-010-0426-1
Cummings JH, Macfarlane GT, Englyst HN (2001) Prebiotic digestion and fermentation. Am J Clin Nutr 73:415S–420S
De Oliva-Neto P, Menao PTP (2009) Isomaltulose production from sucrose by Protaminobacter rubrum immobilized in calcium alginate. Bioresour Technol 100:4252–4256. https://doi.org/10.1016/j.biortech.2009.03.060
Delgado GTC, Tamashiro WMSC, Pastore GM (2010) Immunomodulatory effects of fructans. Food Res Int 43:1231–1236. https://doi.org/10.1016/j.foodres.2010.04.023
Demir M, Turhan I, Kucukcetin A, Alpkentet Z (2013) Oil production by Mortierella isabellina from whey treated with lactase. Bioresour Technol 128:365–369. https://doi.org/10.1016/j.biortech.2012.10.078
Di Bartolomeo F, Startek JB, Van den Ende W (2013) Prebiotics to fight diseases: reality or fiction? Phytother Res 7:1457–1473. https://doi.org/10.1002/ptr.4901
Diniz RHS, Rodrigues MQRB, Fietto LG, Passos FML, Silveira WB (2014) Optimizing and validating the production of ethanol from cheese whey permeate by Kluyveromyces marxianus UFV-3. Biocatal Agric Biotechnol 3:111–117. https://doi.org/10.1016/j.bcab.2013.09.002
Dominguez A, Nobre C, Rodrigues LR, Peres AM, Torres D, Rocha I, Lima N, Teixeira J (2012) New improved method for fructooligosaccharides production by Aureobasidium pullulans. Carbohyd Polym 89:1174–1179. https://doi.org/10.1016/j.carbpol.2012.03.091
Dorta C, Cruz R, De Oliva-Neto P, Moura DJC (2006) Sugarcane molasses and yeast powder used in the fructooligosaccharides production by Aspergillus japonicus-FCL 119T and Aspergillus niger ATCC 20611. J Ind Microbiol Biot 33:1003–1009. https://doi.org/10.1007/s10295-006-0152-x
Eggleston G, Côté GL (2003) Oligosaccharides in food and agriculture. Acs Sym Ser 849:1–14. https://doi.org/10.1021/bk-2003-0849.ch001
Fai AEC, Pastore GM (2015) Galactooligosaccharides: production, health benefits, application to foods and perspectives. Sci Agropec 6:69–81. 10.17268/sci.agropecu.2015.01.07
Fai AEC, Simiqueli APR, Ghiselli G, Pastore GM (2015) Sequential optimization approach for prebiotic galactooligosaccharides synthesis by Pseudozyma tsukubaensis and Pichia kluyveri. LWT -Food Sci Technol 63:1214–1219. https://doi.org/10.1016/j.lwt.2015.04.064
Fernandez M, Villalonga ML, Fragoso A, Cao R, Villalonga R (2004) Effect of β-cyclodextrin-polysucrose polymer on the stability properties of soluble trypsin. Enzyme Microb Tech 34:78–82. https://doi.org/10.1016/j.enzmictec.2003.09.003
Ferreira SA, Oslakovic C, Cukalevski R, Frohm B, Dahlbäck B, Linse S, Gama FM, Cedervall T (2012) Biocompatibility of mannan nanogel-safe interaction with plasma proteins. Biochim Biophys Acta 1820:1043–1051. https://doi.org/10.1016/j.bbagen.2012.04.015
Fischer C, Kleinschmidt T (2015) Synthesis of galactooligosaccharides using sweet and acid whey as a substrate. Int Dairy J 48:15–22. https://doi.org/10.1016/j.idairyj.2015.01.003
Fu A, Lee AW, Nishi S, Case IL, Cho SS (2012) Efficacy and safety of xylooligosaccharides. In: Dietary fiber and health. Ed, Cho SS and Almeida N, pp 497–518. https://doi.org/10.1201/b12156-37
Gabardo S, Rech R, Rosa CA, Ayub MAS (2014) Dynamics of ethanol production from whey and whey permeate by immobilized strains of Kluyveromyces marxianus in batch and continuous bioreactors. Renew Energ 69:89–96. https://doi.org/10.1016/j.renene.2014.03.023
Ganaie MA, Gupta US, Kango N (2013) Screening of biocatalysts for transformation of sucrose to fructooligosaccharides. J Mol Catal B-Enzym 97:12–17. https://doi.org/10.1016/j.molcatb.2013.07.008
Ganaie MA, Soni H, Naikoo GA, Oliveira LTS, Rawat HK, Mehta PK, Narain N (2017) Screening of low cost agricultural wastes to maximize the fructosyltransferase production and its applicability in generation of fructooligosaccharides by solid state fermentation. Int Biodeter Biodegr 118:19–26. https://doi.org/10.1016/j.ibiod.2017.01.006
Gänzle MG, Haase G, Jelen P (2008) Lactose: crystallization, hydrolysis and value-added derivatives. Int Dairy J 18:685–694. https://doi.org/10.1016/j.idairyj.2008.03.003
Ghasemian M, Jahanian R (2016) Dietary mannan-oligosaccharides supplementation could affect performance, immunocompetence, serum lipid metabolites, intestinal bacterial populations, and ileal nutrient digestibility in aged laying hens. Anim Feed Sci Technol 213:81–89. https://doi.org/10.1016/j.anifeedsci.2015.12.012
Ghazi I, Fernandez-Arrojo L, Garcia-Arellano H, Ferrer M, Ballesteros A, Plou FJ (2007) Purification and kinetic characterization of a fructosyltransferase from Aspergillus aculeatus. J Biotechnol 128:204–211. https://doi.org/10.1016/j.jbiotec.2006.09.017
Ghio S, Insani EM, Piccinni FE, Talia PM, Grasso DH, Campos E (2016) GH10 XynA is the main xylanase identified in the crude enzymatic extract of Paenibacillus sp. A59 when grown on xylan or lignocellulosic biomass. Microbiol Res 186-187:16–26. https://doi.org/10.1016/j.micres.2016.02.006
Giannenas I, Doukas D, Karamoutsios A, Tzora A, Bonos E, Skoufos I, Tsinas A, Christaki E, Tontis D, Florou-Paneri P (2016) Effects of Enterococcus faecium, mannan oligosaccharide, benzoic acid and their mixture on growth performance, intestinal microbiota, intestinal morphology and blood lymphocyte subpopulations of fattening pigs. Anim Feed Sci Technol 220:159–167. https://doi.org/10.1016/j.anifeedsci.2016.08.003
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412. https://doi.org/10.1079/nrr200479
Gibson GR, Wang X (1994) Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol 77:412–420. https://doi.org/10.1111/j.1365-2672.1994.tb03443.x
Golowczyc M, Vera C, Santos M, Guerrero C, Carasi P, Illanes A, Gómez-Zavaglia A, Tymczyszyn E (2013) Use of whey permeate containing in situ synthesized galacto-oligosaccharides for the growth and preservation of Lactobacillus plantarum. J Dairy Res 80:374–381. https://doi.org/10.1017/S0022029913000356
González-Delgado I, Lopez-Muñoz M, Morales G, Segura Y (2016) Optimization of the synthesis of high galacto-oligosaccharides (GOS) from lactose with β-galactosidase from Kluyveromyces lactis. Int Dairy J 61:211–219. https://doi.org/10.1016/j.idairyj.2016.06.007
González-Siso MI (1994) β-Galactosidase production by Kluyveromyces lactis on milk whey: batch versus fed-batch cultures. Process Biochem 29:565–568. https://doi.org/10.1016/0032-9592(94)80019-7
Gosling A, Stevens GW, Barber AR, Kentish SE, Gras SL (2010) Recent advances refining galactooligosaccharide production from lactose. Food Chem 121:307–318. https://doi.org/10.1016/j.foodchem.2009.12.063
Gowdhaman D, Ponnusami V (2015) Production and optimization of xylooligosaccharides from corncob by Bacillus aerophilus KGJ2 xylanase and its antioxidant potential. Int J Biol Macromolec 79:595–600. https://doi.org/10.1016/j.ijbiomac.2015.05.046
Grand View Research (2016) Prebiotics market analysis by ingredient (FOS, inulin, GOS, MOS), by application (food & beverages, animal feed, dietary supplements) and segment forecasts to 2024. Grand View Research, Inc. http://www.grandviewresearch.com/industry-analysis/prebiotics-market. Accessed 07 Apr 2017
Heinen PR, Pereira MG, Rechia CGV, Almeida PZ, Monteiro LMO, Pasin TM, Messias JM, Cereia M, Kadowaki MK, Jorge JA, Polizeli MLTM (2017) Immobilized endo-xylanase of Aspergillus tamarii Kita: an interesting biological tool for production of xylooligosaccharides at high temperatures. Process Biochem 53:145–152. https://doi.org/10.1016/j.procbio.2016.11.021
Hidaka H, Hirayama M, Sumi N (1988) A fructooligosaccharide-producing enzyme from Aspergillus niger ATCC 20611. Agr Biol Chem Tokyo 52:1181–1187. https://doi.org/10.1271/bbb1961.52.1181
Hirayama M, Sumi N, Hidaka H (1989) Purification and properties of a fructooligosaccharide-producing beta-fructofuranosidase from Aspergillus niger ATCC 20611. Agr Biol Chem Tokyo 53:667–673. https://doi.org/10.1080/00021369.1989.10869350
Ho AL, Carvalheiro F, Duarte LC, Roseiro LB, Charalampopoulos D, Rastall RA (2014) Production and purification of xylooligosaccharides from oil palm empty fruit bunch fibre by a non-isothermal process. Bioresour Technol 152:526–529. https://doi.org/10.1016/j.biortech.2013.10.114
Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G, Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME (2016) Prebiotics: why definitions matter. Curr Opin Biotechnol 37:1–7. https://doi.org/10.1016/j.copbio.2015.09.001
Jian HL, Zhu LW, Zhang WM, Sun DF, Jiang JX (2013) Enzymatic production and charactereization of manno-oligosaccharides from Gleditsia sinensis galactomannan gum. Int J Biol Macromol 55:282–288. https://doi.org/10.1016/j.ijbiomac.2013.01.025
Kaira GS, Panwar D, Kapoor M (2016) Recombinant endo-mannanase (ManB-1601) production using agro-industrial residues: development of economical medium and application in oil extraction from copra. Bioresour Technol 209:220–227. https://doi.org/10.1016/j.biortech.2016.02.133
Kallel F, Driss D, Bouaziz F, Neifer M, Ghorbel R, Chaabouni SE (2015a) Production of xylooligosaccharides from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK and their in vitro evaluation as prebiotics. Food Bioprod Process 94:536–546. https://doi.org/10.1016/j.fbp.2014.07.012
Kallel F, Driss D, Chaabouni SE, Ghorbel R (2015b) Biological activities of xylooligosaccharides generated from garlic straw xylan by purified xylanase from Bacillus mojavensis UEB-FK. Appl Biochem Biotechnol 175:950–964. https://doi.org/10.1007/s12010-014-1308-1
Katapodis P, Kalogeris E, Kekos D, Christakopoulos P (2004) Biosynthesis of fructo-oligosaccharides by Sporotrichum thermophile during submerged batch cultivation in high sucrose media. Appl Microbiol Biotechnol 63:378–382. https://doi.org/10.1007/s00253-003-1348-x
Katrolia P, Zhou P, Zhang P, Yan Q, Li Y, Jiang Z, Xu H (2012) High level expression of a novel β-mannanase from Chartomium sp. exhibiting efficient manna hydrolysis. Carbohydr Polym 87:480–490. https://doi.org/10.1016/j.carbpol.2011.08.008
Kawamura M, Matsuda N (2008) Synthesis of a series of fructooligosaccharides with sucrose and cycloinulohexaose extending over ten degrees of polymerization using cycloinulooligosaccharide fructanotransferase from Bacillus circulans OKUMZ 31B. Biosci Biotechnol Biochem 72:1119–1121. https://doi.org/10.1271/bbb.70758
Kim BW, Choi JW, Yun JW (1998) Selective production of GF(4)-fructooligosaccharide from sucrose by a new transfructosylating enzyme. Biotechnol Lett 20:1031–1034. https://doi.org/10.1023/A:1005465829053
Kim DY, Ham SJ, Lee HJ, Cho HY, Kim JH, Kim YJ, Shin DH, Rhee YH, Son KH, Park HY (2011) Cloning and characterization of a modular GH5 β-1,4-mannanase with high specific activity from the fibrolytic bacterium Cellulosimicrobium sp. strain HY-13. Bioresour Technol 102:9185–9192. https://doi.org/10.1016/j.biortech.2011.06.073
Kim HM, Lee KH, Kim KH, Lee DS, Nguyen QA, Bae HJ (2014) Efficient function and characterization of GH10 xylanase (Xyl10g) from Gloeophyllum trabeum in lignocellulose degradation. J Biotechnol 172:38–45. https://doi.org/10.1016/j.jbiotec.2013.12.013
Kothari D, Patel S, Goyal A (2014) Therapeutic spectrum of nondigestible oligosaccharides: overview of current state and prospect. J Food Sci 79:R1491–R1498. https://doi.org/10.1111/1750-3841.12536
Krumbeck JA, Maldonado-Gomez MX, Ramer-Tait AE, Hutkins RW (2016) Prebiotics and synbiotics. Curr Opin Gastroenterol 32:110–119. https://doi.org/10.1097/mog.0000000000000249
Kumar V, Satyanarayana T (2011) Applicability of thermos-alkali-stable and cellulose free xylanase from a novel thermos-halo-alkaliphilic Bacillus haloduransin producing xyloligosaccharides. Biotechnol Lett 33:2279–2285. https://doi.org/10.1007/s10529-011-0698-1
Kumar V, Satyanarayana T (2015) Generation of xylooligosaccharides from microwave irradiated agroresidues using recombinant thermos-alkali-stable endoxylanase of the olyextremophilic bacterium Bacillus halodurans expressed in Pichia pastoris. Bioresour Technol 179:382–389. https://doi.org/10.1016/j.biortech.2014.12.049
Lam KL, Cheung PCK (2013) Non-digestible long chain beta-glucans as novel prebiotics. Bioact Carbohydr Dietary Fibre 2:45–64. https://doi.org/10.1016/j.bcdf.2013.09.001
Lamsal BP (2012) Production, health aspects and potential food uses of dairy prebiotic galactooligosaccharides. J Sci Food Agric 92:2020–2028. https://doi.org/10.1002/jsfa.5712
Liao H, Li S, Zheng H, Wei Z, Liu D, Raza W, Shen Q, Xu Y (2014) A new acidophilic thermostable endo-1,4-β-mannanase from Penicillium oxalicum GZ-2: cloning expression in Pichia pastoris. BMC Biotechnol 14:90. https://doi.org/10.1186/s12896-014-0090-z
Liu HX, Gong JS, Li H, Lu ZM, Li H, Qian JY, Xu ZH, Shi JS (2015) Biochemical characterization and cloning of an endo-1,4-β-mannanase from Bacillus subtilis YH12 with unusually broad substrate profile. Process Biochem 50:712–721. https://doi.org/10.1016/j.procbio.2015.02.011
Ma R, Bai YG, Huang HQ, Luo HY, Chen SF, Fan YL, Cai L, Yao B (2017) Utility of thermostable xylanases of Mycothermus thermophilus in generating prebiotic xylooligosaccharides. J Agric Food Chem 65:1139–1145. https://doi.org/10.1021/acs.jafc.6b05183
Maiorano AE, Piccoli RM, da Silva ES, Rodrigues MFD (2008) Microbial production of fructosyltransferases for synthesis of pre-biotics. Biotechol Lett 30:1867–1877. https://doi.org/10.1007/s10529-008-9793-3
Mäkeläinen H, Forssten S, Saarinen M, Stowell J, Rautonen N, Ouwehand AC (2010) Xylo-oligosaccharides enhance the growth of bifidobacteria and Bifidobacterium lactis in a simulated colon model. Benefic Microbes 1:81–91. https://doi.org/10.3920/BM2009.0025
Markosyan AA, Abelyan LA, Adamyan MO, Ekazhev ZD, Akopyan ZI, Abelyan VA (2007) Production of fructooligosaccharide syrup from sucrose in combination with palatinose and trehalose. Appl Biochem Micro+ 43:383–389. https://doi.org/10.1134/S0003683807040047
Mikkelson A, Maaheimo H, Hakala TK (2013) Hydrolysis of kinjac glucomannan by Trichoderma reesei mannanase and endoglucanases Cel7B and Cel5A for the production of glucomannooligosaccharides. Carbohydr Res 372:60–68. https://doi.org/10.1016/j.carres.2013.02.012
Milessi TSS, Kopp W, Rojas MJ, Manrich A, Baptista-Neto A, Tardioli PW, Giordano RC, Fernandez-Lafuente R, Guisan JM (2016) Immobilization and stabilization of an endoxylanase from Bacillus subtilis (XynA) for xylooligosaccharides (XOS) production. Catal Today 259:130–139. https://doi.org/10.1016/j.cattod.2015.05.032
Moniz P, Pereira H, Duarte LC, Carvalheiro F (2014) Hydrothermal production and gel filtration purification of xylo-oligosaccharides from rice straw. Ind Crop Prod 62:460–465. https://doi.org/10.1016/j.indcrop.2014.09.020
Moreira LRS, Filho EXF (2008) An overview of manna structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178. https://doi.org/10.1007/s00253-008-1423-4
Moreno FJ, Corzo N, Montilla A, Villamiel M, Olano A (2017) Current state and latest advances in the concept, production and functionality of prebiotic oligosaccharides. Curr Opin Food Sci 13:50–55. https://doi.org/10.1016/j.cofs.2017.02.009
Muñiz-Marquez DB, Contreras JC, Rodríguez R, Mussatto SI, Teixeira JA, Aguilar CN (2016a) Enhancement of fructosyltransferase and fructooligosaccharides production by A. oryzae DIA-MF in solid-state fermentation using aguamiel as culture medium. Bioresour Technol 213:276–282. https://doi.org/10.1016/j.biortech.2016.03.022
Muñiz-Marquez DB, Contreras JC, Rodríguez R, Mussatto SI, Teixeira JA, Aguilar CN (2016b) Biotechnological production of oligosaccharides: advances and challenges. In: Ravishankar RV (ed) Advances in food biotechnology, 1st edn. Wiley, New York, pp 381–390
Mussato SI, Mancilha IM (2007) Non-digestible oligosaccharides: a review. Carbohydr Polym 68:587–597. https://doi.org/10.1016/j.carbpol.2006.12.011
Mussato SI, Aguilar CN, Rodrigues LR, Teixeira JA (2009) Fructooligosaccharides and beta-fructofuranosidase production by Aspergillus japonicus immobilized on lignocellulosic materials. J Mol Catal B-Enzym 59:76–81. https://doi.org/10.1016/j.molcatb.2009.01.005
Nascimento AKC, Nobre C, Cavalcanti MTH, Teixeira JA, Porto ALF (2016) Screening of fungi from the genus Penicillium for production of β-fructofuranosidase and enzymatic synthesis of fructooligosaccharides. J Mol Catal B-Enzym 134:70–78. https://doi.org/10.1016/j.molcatb.2016.09.005
Neri-Numa IA, Paulino BN, Pessoa MG, Abrahão MRE, Bution ML, Molina G, Pastore GM (2016) Industrial additives obtained through microbial biotechnology: biosurfactants and prebiotic carbohydrates. In: Gupta VK, Sharma GD, Tuohy MG, Gaur R (eds) The handbook of microbial bioresources. CABI, Oxfordshire, pp 528–548. https://doi.org/10.1079/9781780645216.0528
Nieto-Dominguez M, Eugenio LI, York-Durán MJ, Rodríguez-Colinas B, Plou FJ, Chenoll E, Pardo E, Codoñer F, Martínez MJ (2017) Prebiotic effect of xylooligosaccharides produced from birchwood xylan by a novel fungal G11 xylanase. Food Chem. https://doi.org/10.1016/j.foodchem.2017.03.149
Ning YW, Wang JP, Chen J, Yang N, Jin ZY, Xu XM (2010) Production of neo-fructooligosaccharides using free-whole-cell biotransformation by Xanthophyllomyces dendrorhous. Bioresour Technol 101:7472–7478. https://doi.org/10.1016/j.biortech.2010.04.026
Ning YW, Li Q, Chen F, Yang N, Jin ZY, Xu XM (2012) Low-cost production of 6G-fructofuranosidase with high value-added astaxanthin by Xanthophyllomyces dendrorhous. Bioresour Technol 104:660–667. https://doi.org/10.1016/j.biortech.2011.10.098
Nobre C, Castro CC, Hantson AL, Teixeira JA, De Weireld G, Rodrigues LR (2016) Strategies for the production of high-content fructo-oligosaccharides through the removal of small saccharides by co-culture or successive fermentation with yeast. Carbohyd Polym 136:274–281. https://doi.org/10.1016/j.carbpol.2015.08.088
Otieno DO, Ahring BK (2012) The potential for oligosaccharide production from the hemicellulose fraction of biomass through pretreatment processes: xylooligosaccharides (XOS), arabinooligosaccharides (AOS), and mannooligosaccharides (MOS). Carbohydr Res 360:84–92. https://doi.org/10.1016/j.carres.2012.07.017
Ozturk B, Cekmecelioglu D, Ogel ZB (2010) Optimal conditions for enhanced β-mannanase production by recombinant Aspergillus sojae. J Mol Catal B Enzym 64:135–139. https://doi.org/10.1016/j.molcatb.2010.02.009
Padilha B, Frau F, Ruiz-Matute AI, Montilla A, Belloch C, Manzanares P, Corzo N (2015) Production of lactulose oligosaccharides by isomerisation of transgalactosylated cheese whey permeate obtained by β-galactosidases from dairy Kluyveromyces. J Dairy Res 82:356–364. https://doi.org/10.1017/S0022029915000217
Panesar PS, Kennedy JF, Gandhi DN, Bunko K (2007) Bioutilisation of whey for lactic acid production. Food Chem 105:1–14. https://doi.org/10.1016/j.foodchem.2007.03.035
Patel S, Goyal A (2012) The current trends and future perspectives of prebiotics research: a review. 3. Biotech 2:115–125. https://doi.org/10.1007/s13205-012-0044-x
Peng F, Ren JL, Xu F, Bian J, Peng P, Sun RC (2009) Comparative study of hemicelluloses obtained by graded ethanol precipitation from sugarcane bagasse. J Agric Food Chem 47:32–39. https://doi.org/10.1021/jf900986b
Petrova VY, Kujumdzieva AV (2010) Thermotolerant yeast strains producers of galacto-oligosaccharides. Biotechnol Biotec Eq 24:1612–1619. https://doi.org/10.2478/V10133-010-0014-6
Qiang X, YongLie C, QianBing W (2009) Health benefit application of functional oligosaccharides. Carbohy Polym 77:435–441. https://doi.org/10.1016/j.carbpol.2009.03.016
Rajagopalan G, Shanmugavelu K, Yang KL (2017) Production of prebiotic-xylooligosaccharides from alkali pretreated mahogany and mango wood sawdust by using purified xylanase of Clostridium strain BOH3. Carbohyd Polym 167:158–166. https://doi.org/10.1016/j.carbopol.2017.03.021
Ravn JL, Thogersen JC, Eklof J, Petterson D, Ducatelle R, van Immerseel F, Pedersen NR (2017) GH11 xylanase increases prebiotic oligosaccharides from wheat bran favouring butyrate-producing bacteria in vitro. Anim Feed Sci Tech 226:113–123. https://doi.org/10.1016/j.anifeedsci.2017.02.011
Reddy SS, Krishnan C (2016) Production of high-pure xylooligosaccharides from sugarcane bagasse using crude β-xylosidase-free xylanase of Bacillus subtilis KCX006 and their bifidogenic function. LWT - Food Sci Technol 65:237–245. https://doi.org/10.1016/j.lwt.2015.08.013
Research and Markets (2017) Global prebiotic ingredients market analysis & trends—industry forecast to 2025. Research and Markets. http://www.researchandmarkets.com/research/bcrhhf/global_prebiotic. Accessed 07 Apr 2017
Ritsema T, Smeekens S (2003) Fructans: beneficial for plants and humans. Curr Opin Plant Biol 6:223–230. https://doi.org/10.1016/S1369-5266(03)00034-7
Rodriguez-Colinas B, Fernandez-Arrojo L, Santos-Moriano P, Ballesteros AO, Plou FJ (2016) Continuous packed bed reactor with immobilized β-galactosidase for production of galactooligosaccharides (GOS). Catalogue 6:1–12. https://doi.org/10.3390/catal6120189
Romão BB, Batista FRX, Ferreira JS, Costa HCB, Resende MM, Cardoso VL (2014) Biohydrogen production through dark fermentation by a microbial consortium using whey permeate as substrate. Appl Biochem Biotechnol 172:3670–3685. https://doi.org/10.1007/s12010-014-0778-5
Rozen R, Steinberg D, Bachrach G (2004) Streptococcus mutans fructosyltransferase interactions with glucans. FEMS Microbiol Lett 232:39–43. https://doi.org/10.1016/S0378-1097(04)00065-5
Rungrassamee W, Kingcha Y, Srimarut Y, Maibunkaew S, Karoonuthaisiri N, Visessanguan W (2014) Mannooligosaccharides from copra meal improves survival of the Pacific white shrimp (Litopenaeus vannamei) after exposure to Vibrio harveyi. Aquaculture 434:403–410. https://doi.org/10.1016/j.aquaculture.2014.08.032
Ruzene DS, Silva PD, Vicente AA, Gonçalves AR, Teixeira JA (2008) An alternative application to the Portuguese agro industrial residue: wheat straw. Appl Biochem Biotechnol 147:85–96. https://doi.org/10.1007/978-1-60327-526-2_43
Samanta AK, Jayapal N, Jayaram C, Roy S, Kolte AP, Senani S, Sridhar M (2015) Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioactive Carbohydrates and Dietary Fibre 5:62–71. https://doi.org/10.1016/j.bcdf.2014.12.003
Sánchez O, Guio F, Garcia D, Silua E, Caiced L (2008) Fructooligosaccharides production by Aspergillus sp. N74 in a mechanically agitated airlift reactor. Food Bioprod Process 86:109–115. https://doi.org/10.1016/j.fbp.2008.02.003
Sangeeta PT, Ramesh MN, Prapulla SG (2004) Production of fructo-oligosaccharides by fructosyl transferase from Aspergillus oryzae CFR 202 and Aureobasidium pullulans CFR 77. Process Biochem 39:753–758. https://doi.org/10.1016/S0032-9592(03)00186-9
Sangeeta PT, Ramesh MN, Prapulla SG (2005) Recent trends in the microbial production, analysis and application of fructooligosaccharides. Trends Food Sci Tech 16:442–457. https://doi.org/10.1016/j.tifs.2005.05.003
Santos AMP, Maugeri F (2007) Synthesis of fructooligosaccharides from sucrose using inulinase from Kluyveromyces marxianus. Food Technol. Biotech 45:181–186
Satar R, Ismail SA, Rehan M, Ansari SA (2016) Elucidating the binding efficacy of β-galactosidase on graphene by docking approach and its potential application in galacto-oligosaccharide production. Bioprocess Biosyst Eng 39:807–814. https://doi.org/10.1007/s00449-016-1560-6
Sheu DC, Lio PJ, Chen ST, Lin CT, Duan KJ (2001) Production of fructooligosaccharides in high yield using a mixed enzyme system of β-fructofuranosidase and glucose oxidase. Biotechnol Lett 23:1499–1503. https://doi.org/10.1023/A:1011689531625
Singh RD, Banerjee J, Arora A (2015) Prebiotic potential of oligosaccharides: a focus on xylan derived oligosaccharides. Bioactive Carbohydrates and Dietay Fibre 5:19–30. https://doi.org/10.1016/j.bcdf.2014.11.003
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5:1417–1435. https://doi.org/10.3390/nu5041417
Smithers GW (2015) Whey-ing up the options—yesterday, today and tomorrow. Int Dairy J 48:2–14. https://doi.org/10.1016/j.idairyj.2015.01.011
Srivastava PK, Kapoor M (2017) Productin, properties, and applications of endo-β-mannanases. Biotechnol Adv 35:1–9. https://doi.org/10.1016/j.biotechadv.2016.11.001
Srivastava A, Mishra S, Chand S (2015) Transgalactosylation of lactose for synthesis of galacto-oligosaccharides using Kluyveromyces marxianus NCIM 3551. New Biotechnol 32:412–418. https://doi.org/10.1016/j.nbt.2015.04.004
Srivastava PK, Rao ARA, Kapoor M (2016) Metal-dependent thermal stability of recombinant endo-mannanase (ManB-1601) belonging to family GH 26 from Bacillus sp. CFR1601. Enzym Microb Technol 84:41–49
Straathof AJJ, Kieboom APG, Vanbekkum H (1986) Invertase-catalyzed fructosyl transfer in concentrated-solutions of sucrose. Carbohydr Res 146:154–159. https://doi.org/10.1016/0008-6215(86)85033-9
Sun MZ, Zheng HC, Meng LC, Sun JS, Song H, Bao YJ, Pei HS, Yan Z, Zhang XQ, Zhang JS, Liu YH, FP L (2015) Direct cloning, expression of a thermostable xylanase gene from the metagenomic DNA of cow dung compost and enzymatic production of xylooligosaccharides from corncob. Biotechnol Lett 37:1877–1886. https://doi.org/10.1007/s10529-015-1857-6
Sun H, You S, Wang M, Qi W, Su R, He Z (2016) Recyclable strategy for the production of high-purity galactooligosaccharides by Kluyveromyces lactis. J Agr Food Chem 64:5679–5685. https://doi.org/10.1021/acs.jafc.6b01531
Swennen K, Courtin CM, Delcour JA (2006) Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci Nutr 46:459–471. https://doi.org/10.1080/10408390500215746
Talens-Perales D, Polaina J, Marín-Navarro J (2016) Enzyme engineering for oligosaccharide biosynthesis. In: Shukla P (ed) Frontier discoveries and innovations in interdisiplinary microbiology. Springer, New York, pp 9–31. https://doi.org/10.1007/978-81-322-2610-9_2
Topping DL, Clifton PM (2001) Short chain fatty acids and human colonic functions: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81:1031–1064
Torrecillas S, Makol A, Betancor MB, Montero D, Caballero MJ, Sweetman J, Izquierdo M (2013) Enhanced intestinal epithelial barrier health status on European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol 34:1485–1495. https://doi.org/10.1016/j.fsi.2013.03.351
Torrecillas S, Montero D, Izquierdo M (2014) Improved health and growth of fish fed mannan oligosaccharides: potential mode of action. Fish Shellfish Immunol 36:525–544. https://doi.org/10.1016/j.fsi.2013.12.029
Torrecillas S, Montero D, Caballero MJ, Robaina L, Zamorano MJ, Sweetman J, Izquierdo M (2015) Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles. Fish Shellfish Immunol 42:508–516. https://doi.org/10.1016/j.fsi.2014.11.033
Torres DPM, Gonçalves MPF, Teixeira JA, Rodrigues LR (2010) Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci F 9:438–454. https://doi.org/10.1111/j.1541-4337.2010.00119.x
Van Der Meulen R, Makras L, Verbrugghe K, Adriany T, De Vuyst L (2006) In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl Environ Microb 72:1006–1012. https://doi.org/10.1128/AEM.72.2.1006-1012.2006
Van Zyl WH, Rose SH, Trollope K, Görgens JF (2010) Fungal β-mannanases: mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem 45:1203–1213. https://doi.org/10.1016/j.procbio.2010.05.011
Vasquez MJ, Alonso JL, Dominguez H, Parajo JC (2000) Xylo-oligosaccharides: manufacture and applications. Trends Food Sci Technol 11:387–393
Vega-Paulino RJ, Zúniga-Hansen ME (2012) Potential application of commercial enzyme preparations for industrial production of short-chain fructooligosaccharides. J Mol Catal B-Enzym 76:44–51. https://doi.org/10.1016/j.molcatb.2011.12.007
Vera C, Córdova A, Aburto C, Guerrero C, Suárez S, Illanes A (2016) Synthesis and purification of galacto-oligosaccharides: state of the art. World J Microbiol Biotechnol 32:1–20. https://doi.org/10.1007/s11274-016-2159-4
Villamiel M, Montilla A, Olano A, Corzo N (2014) Production and bioactivity of oligosaccharides derived from lactose. In: Moreno J, Sanz ML (eds) Food oligosaccharides: production, analysis and bioactivity, 1st edn. Wiley-Blackwell, New York, pp 137–167
Wang J, Sun B, Cao Y, Tian Y, Wang C (2009) Enzymatic preparation of wheat bran xylooligosaccharides and their stability during pasteurization and autoclave sterilization at low pH. Carbohyd Polym 77:816–821. https://doi.org/10.1016/j.carbpol.2009.03.005
Wang Y, Shi P, Luo H, Bai Y, Huang H, Yang P, Xiong H, Yao B (2012) Cloning, over-expression and characterization of an alkali-tolerant endo-β-1,4-mannanase from Penicillium freii F63. J Biosci Bioeng 113:710–714. https://doi.org/10.1016/j.jbiosc.2012.02.005
Wilson B, Whelan K (2017) Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol 32:64–68. https://doi.org/10.1111/jgh.13700
Xie YJ, Zhou HX, Liu CX, Zhang J, Li N, Zhao ZL, Sun GY, Zhong YH (2017) A molasses habitat-derived fungus Aspergillus tubingensis XG21 with high beta-fructofuranosidase activity and its potential use for fructooligosaccharides production. AMB Expr 7:128. https://doi.org/10.1186/s13568-017-0428-8
Xu X, Liu M, Huo W, Dai X (2016) Obtaining a mutant of Bacillus amyloliquefaciens xylanase A with improved catalytic activity by directed evolution. Enzym Microb Technol 86:59–66. https://doi.org/10.1016/j.enzmictec.2016.02.001
Xue JL, Zhao S, Liang RM, Yin X, Jiang SX, LH S, Yang Q, Duan CJ, Liu JL, Feng JX (2016) A biotechnological process efficiently co-produces two high value-added products, glucose and xylooligosaccharides, from sugarcane bagasse. Bioresour Technol 204:130–138. https://doi.org/10.1016/j.biortech.2015.12.082
Yadav JSS, Yana S, Pilli S, Kumar L, Tyagi RD, Surampalli RY (2015) Cheese whey: a potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol Adv 33:756–774. https://doi.org/10.1016/j.biotechadv.2015.07.002
Yamabhai M, Sak-Ubol S, Srila W, Haltrich D (2016) Mannan biotechnology: from biofuels to health. Crit Rev Biotechnol 36:32–42. https://doi.org/10.3109/07388551.2014.923372
Yan Q, Hao S, Jiang Z, Zhai Q, Chen W (2009) Properties of a xylanase from Streptomyces matensis being suitable for xylooligosaccharides production. J Mol Catal B Enzym 58:72–77. https://doi.org/10.1016/j.molcatb.2008.11.010
Yang H, Shi P, Lu H, Wang H, Luo H, Huang H, Yang P, Yao B (2015) A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem 173:283–289. https://doi.org/10.1016/j.foodchem.2014.10.022
Yin H, Bultema JB, Dijkhuizen L, Leeuwen SS (2017) Reaction kinetics and galactooligosaccharide product profiles of the β-galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chem 225:230–238. https://doi.org/10.1016/j.foodchem.2017.01.030
Yoshikawa J, Amachi S, Shinoyama H, Fujii T (2008) Production of fructooligosaccharides by crude enzyme preparations of b-fructofuranosidase from Aureobasidium pullulans. Biotechnol Lett 30:535–539. https://doi.org/10.1007/s10529-007-9568-2
Yoshino K, Higashi N, Koga K (2006) Inhibitory effects of acidic xylooligosaccharide on stress-induced gastric inflammation in mice. J Food Hyg Soc Japan 47:284–287. https://doi.org/10.3358/shokueishi.47.284
Yuan TQ, Xu F, He J, Sun RC (2010) Structural and physiochemical characterization of hemicellulose from ultrasound assisted extraction of partially delignified fast growing poplar wood through organic solvents and alkaline solutions. Biotechnol Adv 28:583–593. https://doi.org/10.1016/j.biotechadv.2010.05.016
Yun JW (1996) Fructooligosaccharides-occurence, preparation, and application. Enzyme Microb Tech 19:107–117. https://doi.org/10.1016/0141-0229(95)00188-3
Zang H, Xie S, Wu H, Wang W, Shao X, Wu L, Rajer FU, Gao X (2015) A novel thermostable GH5_7 β-mannanase from Bacillus pumilus GBSW19 and its application in manno-oligosaccharides (MOS) production. Enzym Microb Technol 78:1–9. https://doi.org/10.1016/j.enzmictec.2015.06.007
Zhang H, Sang Q (2015) Production and extraction optimization of xylanase and β-mannanase by Penicillium chryogenum QML-2 and primary application in saccharification of corn cob. Biochem Eng J 97:101–110. https://doi.org/10.1016/j.bej.2015.02.014
Zhang J, Liu C, Xie Y, Li N, Ning Z, Du N, Huang X, Zhong Y (2017) Enhancing fructooligosaccharides production by genetic improvement of the industrial fungus Aspergillus niger ATCC 20611. J Biotechnol 249:25–33. https://doi.org/10.1016/j.jbiotec.2017.03.021
Zhao H, Hua X, Yang R, Zhao L, Zhao W, Zhang Z (2012) Diafiltration process on xylooligosaccharides syrup using nanofiltration and its modeling. Int J Food Sci Technol 47:32–39. https://doi.org/10.1111/j.1365-2621.2011.02803.x
Zheng J, Zhao W, Gui N, Lin F, Tian J, Wu L, Zhou H (2012) Development of an intrustrial medium and a novel fed-batch strategy for high expression of recombinant β-mananase by Pichia pastoris. Bioresour Technol 118:257–264. https://doi.org/10.1016/j.biortech.2012.05.065
Zhu T, Sun H, Li P, Xue Y, Li Y, Ma Y (2014) Constitutive expression of alkaline β-mannanase in recombinant Pichia pastoris. Process Biochem 49:2025–2029. https://doi.org/10.1016/j.procbio.2014.08.014
Acknowledgements
The authors acknowledge the funding agencies Fundação de Amparo a Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes).
Funding
This study was funded by the agencies Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP - Process number 2015/50333-1) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - Process number 140512/2016-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mano, M.C.R., Neri-Numa, I.A., da Silva, J.B. et al. Oligosaccharide biotechnology: an approach of prebiotic revolution on the industry. Appl Microbiol Biotechnol 102, 17–37 (2018). https://doi.org/10.1007/s00253-017-8564-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8564-2