Abstract
Rhamnolipids have recently emerged as promising bioactive molecules due to their novel structures, diverse and versatile biological functions, lower toxicity, higher biodegradability, as well as production from renewable resources. The advantages of rhamnolipids make them attractive targets for research in a wide variety of applications. Especially rhamnolipids are likely to possess potential applications of the future in areas such as biomedicine, therapeutics, and agriculture. The purpose of this mini review is to provide a comprehensive prospective of biosurfactant rhamnolipids as potential antimicrobials, immune modulators, and virulence factors, and anticancer agents in the field of biomedicine and agriculture that may meet the ever-increasing future pharmacological treatment and food safety needs in human health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
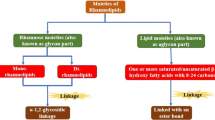
Rhamnolipids are a group of biosurfactants synthesized by Pseudomonas aeruginosa with glycolipidic structures (Soberón-Chávez et al. 2005). The initial discovery of rhamnolipids traced back to 1946 when Bergström and his colleagues (Reis et al. 2011; Müller and Hausmann 2011) reported an extracellular oily glycolipid produced by P. aeruginosa when cultivated on glucose. The glycolipid includes L-rhamnose and β-hydroxydecanoic acid moieties. Edwards and Hayashi (Edwards and Hayashi 1965) further reported that two rhamnose moieties were linked by an α-1,2-glycosidic bond. Subsequently, the rhamnolipid was named 2-O-α-1,2-L-rhamnopyranosyl-α-L-rhamnopyranosyl-β-hydroxydecanoyl-β-hydroxydecanoate. This was the first time researchers discovered that a sugar and hydroxylated fatty acid residue were linked by a glucosidic bond in the glycolipid. Subsequently, additional and novel investigations of rhamnolipids began to appear in the literature. Approximately 60 identified rhamnolipid homologs or congeners had been discovered in different Pseudomonas species and other bacteria by the year 2010 (Abdel-Mawgoud et al. 2010). More recently (2010–2017), an additional 53 novel rhamnolipid homologs or congeners had also been discovered and characterized (Table 1). These compounds showed multi-physicological functions with broad applications.
P. aeruginosa has long been considered as the primary species that produces rhamnolipids. However, many other species which do not belong to the Pseudomonadacaeae family in the taxonomical classification are also reported to produce rhamnolipids. For example, the pathogens Burkholderia pseudomallei, Burkholderia mallei and the nonpathogenic Burkholderia thailandensis (family: Burkholderia; class: Betaproteobacteria) (Toribio et al. 2010), Acinetobacter calcoaceticus (family: Moraxellaceae; class: Gammaproteobacteria) (Hošková et al. 2015), Enterobacter sp., and Pantoea sp. (family: Enterobacteriaceae; class: Gammaproteobacteria) (Hošková et al. 2015; Nitschke et al. 2005). Further, some other species that are not part of the classes Gammaproteobacteria and Betaproteobacteria are also reported to produce rhamnolipids. The most prominent examples include a Myxococcus sp. (class: Deltaproteobacteria) produces myxotyrosides A and B (Ohlendorf et al. 2009), and a Bradyrhizobium sp. (class: Alphaproteobacteria) produces rhizoleucinoside (Chen et al. 2016). Obviously, the widening rhamnolipid-producing microorganisms will facilitate researchers to screen industrially safe and nonpathogenic alternatives to human pathogen P. aeruginosa. However, it is worth noting that when a novel bacterial species is reported to synthesize rhamnolipids, in most cases only one isolate can be chosen for further studies, metabolic pathways and engineering were not sufficiently elucidated. Thus, until now, only few microorganisms can be selected as actual rhamnolipid producers. It became clear that the wide spectrum approaches will be required to develop, probably based on the presence of the rhlA, rhlB, or rhlC homologs in the biosynthetic genes of bacteria or several characterizations of rhamnolipid surfactants. We recommended to set up several standards for reporting new rhamnolipid-producing strains. For example, genes responsible for rhamnolipid production should be fully characterized based on molecular and bioinformatics tools. The absolute quantitative yields and molecular structures of these compounds should be determined (Irorere et al. 2017).
Three major biosynthetic pathways of rhamnolipids in P. aeruginosa had been fully reported according to the past literatures (Abdelmawgoud et al. 2014; Lovaglio et al. 2015), including biosynthesis of the fatty acid moiety, biosynthesis of the rhamnose moiety, and the enzymatic dimerization between the fatty acid moiety and the rhamnose moiety. These steps yield the final mono- and di-rhamnolipids. In addition to rhamnolipids biosynthesis in P. aeruginosa, the pathways are also controlled by transcriptional regulatory genes related to quorum sensing systems and key global regulators. These molecular steps are intertwined with major rhamnolipid biosynthetic networks and affect rhamnolipid production.
The precise physicochemical properties of rhamnolipids are still not adequately defined, in spite of intensive research efforts. The main functions of rhamnolipids are derived from well-known physicochemical properties of such compounds, i.e., biosurfactants (Zhu and Rock 2008; Dobler et al. 2015). Unsurprisingly, recent investigations in this field have expanded greatly due to their prospective uses in diverse fields. Recent prospective studies showed that rhamnolipids could be of potential use in developing new and promising antimicrobial drugs, anticancer drugs, and immune system modulators in agriculture and biomedicine (Bazire and Dufour 2014). This review provides an overview of rhamnolipids, covering commentary on prospective biological properties for agricultural and biomedical applications which would contribute to expansion of the understanding and utilization of these unique compounds.
Applications of rhamnolipid in agriculture and biomedicine
Although numerous rhamnolipids have been studied, there is no consensus on their exact physiological and biological roles. Many of biological roles date from well-known amphipathic related properties of rhamnolipids. Therefore, these molecules may show a variety of valuable biological characters in agriculture and biomedicine.
Rhamnolipids as antimicrobials
In earlier studies, the antimicrobial properties of rhamnolipids had been suggested, as they did show wide-ranging antimicrobial actions, including those in gram-positive and gram-negative bacteria (Itoh et al. 1971). Haba and coworkers (Haba et al. 2003) had made investigations on the antimicrobial properties of a mixture of rhamnolipids (up to 11 rhanmolipid homologs) from P. aeruginosa 47T2 using waste frying oil as carbon source. Their antimicrobial activities in vitro were evaluated by the minimum inhibitory concentration (MIC). The rhamnolipid mixture showed excellent antimicrobial properties against almost all tested gram-negative bacteria, including Klebsiella pneumoniae (MIC 0.5 μg/mL), Serratia marcescens (MIC 4 μg/mL), and Enterobacter aerogenes (MIC 8 μg/mL); additionally, good activity against the gram-positive bacteria, Bacillus subtilis (MIC 16 μg/mL), Staphylococcus epidermidis (MIC 32 μg/mL), and weak activity against Mycobacterium, Clostridium, and Arthrobacter (MIC 128 μg/mL). These molecules also showed excellent antifungal activities against Phytophthora capsici (MIC 10 μg/mL), Aspergillus niger and Gliocadium virens (MIC 16 μg/mL), Penicillium funiculosum (MIC 16 μg/mL), Botrytis cinerea (MIC 18 μg/mL), Chaetonium globusum (MIC 32 μg/mL), Penicillium crysogenum (MIC 32 μg/mL), and Aerobacidium pullulants (MIC 32 μg/mL). Sixteen different rhamnolipids produced by P. aeruginosa L2–1 also presented antimicrobial activity against other Gram-positive bacteria, including Bacillus cereus (MIC 32 μg/mL), Staphylococcus aureus (MIC 128 μg/mL), and Micrococcus luteus (MIC 32 μg/mL) (Costa et al. 2010). More recently, rhanmnolipids were described as having antifungal activity against plant pathogens, including those against Oomycetes fungi, Ascomycota fungi, and Zygomycetes (Sha et al. 2012).
Clearly, rhamnolipids can repress the growth in the bacterial exponential phase, which suggests that these compounds may have a marked influence on normal cell division (de Rienzo et al. 2016). Ulf Meyer-Hoffert (Meyer-Hoffert et al. 2011) et al. demonstrated that rhamnolipid secretion facilitates the expression of antimicrobial protein psoriasin in human’s healthy skin via flagellin. P. aeruginosa releases rhamnolipids to form vesicles or micelles and sheds flagellin. Rhamnlipids embed flagellin to penetrate healthy human skin and flagellin is subsequently released into living epidermal keratinocytes via stratum cornerum. Flagellin will activate keratinocytes to induce the expression of antimicrobial protein psoriasin, which can kill P. aeruginosa (Fig. 1). Therefore, healthy skin can prevent colonization of pathogens in the earliest phase before pathogens develop strategies to disrupt or disorder the immune defense response. The antimicrobial activity of long chain rhamnolipids has not been studied previously. Elshikh et al. (Elshikh et al. 2017) made an investigation of the antibacterial properties of long chain rhamnolipids from Burkholderia thailandensis E264 against some oral pathogens (Actinomyces naeslundii, Streptococcus sanguinis, Neisseria mucosa, and Streptococcus oralis). These studies are very important for the improvement of oral hygiene status and health globally. The viability of these bacteria will be reduced around 3–4 MIC log at different concentrations of long chain rhamnolipid congeners. In addition, Batovska and colleagues (Batovska et al. 2009) also investigated the antimicrobial activity of the close structural analogue, myristic acid, of these long chain rhamnolipids against several pathogens including Staphylococcus epidermidis and Staphylococcus aureus. Their MIC was generally around 0.5 mg/mL. These data support a requirement for the lipophilic moiety of long chain rhamnolipid congeners for the bacterial cell disruption and the biocidal effect.
Of particular note, rhamnolipids, in combination with antibiotic therapy, or antimicrobials against various pathogenic microorganisms may represent another effective antimicrobial strategy. In practical terms methylthiosulfonate and ethylthiosulfonate could be used in biomedicine as new antimicrobial agents. The presence of rhamnolipids obviously decreased minimal inhibitory and minimal bactericidal/fungicidal concentrations of methylthiosulfonate and ethylthiosulfonate against P. aeruginosa, Alcaligenes faecalis, Bacillus subtilis, and Rssula nigricans (Lazarkevich et al. 2015; Sotirova et al. 2012). The antibacterial activity of methylthiosulfonate and ethylthiosulfonate by rhamnolipids was significantly potentiated. That is because rhamnolipids provoke changes in the phospholipid’s composition of bacterial cell membrane which facilitate the access to antimicrobials or antibiotics into bacterial cells. Nisin is a commercially available antimicrobial peptide which has a well-recognized bactericidal effect against gram-positive pathogens and gram-negative pathogens, and has been widely applied for the control of pathogens in food products. The combination of rhamnolipids and nisin led to a synergistic action, improving the efficacy of antimicrobials (Magalhães and Nitschke 2013). When in combination, it could use lower concentrations to reach the same inhibitory effect compared with their individual use alone. Rhamnolipids possess anionic characters mainly due to their carboxylate groups, in solution, the negative groups of rhamnolipids tend to organize on the surface of cell lipid membranes increasing the negative charges, thus promoting the electrostatic interaction between nisin and anionic membrane, which can result in the synergistic effect. The synergistic effect of the combination with antimicrobials and rhamnolipids proved the therapeutic potential of combinations. The development of combined preparations will enable to alter the mechanism of action of antimicrobials, hence contributing to overcome drug resistance in the future. In addition, this was also the first reported on using plasma to modify the surface with rhamnolipids to produce an antimicrobial polymeric surface. The treated polypropylene surface showed considerable activity to reduce the number of pathogenic microbiology. It appears likely that the antimicrobial polypropylene film will be widely used for meeting ever-increasing needs in food and pharmacy industry as packaging materials in the future (Hajfarajollah et al. 2015).
Phytophthora sojae is the most damaging oomycete pathogen of soybean. Soltani Dashtbozorg et al. (Soltani et al. 2016) recently investigated the antimicrobial effects of rhamnolipids produced by P. aeruginosa against both Phytophthora sojae zoospores and mycelia. Of particular importance was the effectiveness against Phytophthora sojae zoospores as this species spreads infection mainly via these zoospores. Monorhamnolipids, dirhamnolipids, and rhamnolipid mixtures were similarly effective in halting the motility of all zoospores or causing their lysis; however, the mixture was more effective collectively, requiring only 8–20 mg/L. With respect to action against mycelia, the most effective rhamnolipid mixture at 1000 mg/L can inhibit the mycelial packing density by up to 30% and can completely stop its growth. This result again showed that a rhamnolipid mixture was much more effective in killing Phytophthora sojae through either anti-zoospore or anti-mycelia action. Recently, Goswami et al. (Goswami et al. 2015) demonstrated that two similar congeners Rha-Rha-C10:1 and Rha-C10 could inhibit the infection caused by the pathogenic fungus Colletotrichum falcatum to treat red rot disease in sugarcane. They exhibited stronger detrimental effects on spores of germination of Colletotrichum falcatum than mycelial growth inhibition. It was observed that more than 65% inhibition of Colletotrichum falcatum spore growth rate was obtained at only 40 μg/mL. Their antifungal activities were attributed to the disruption of fungal cell membrane. These studies suggest rhamnolipids may become a potent and environmentally friendly biopesticide against plant pathogens. However, rhamnolipids have not been explored adequately to determine either the mechanism of anti-zoospore action or for developing new anti-zoospore agrochemicals. According to Miao et al. (Miao et al. 2015), Rha-C10-C10 and Rha-Rha-C10-C10 were hydrolyzed to generate Rha-C10, Rha-Rha-C10 and the free diacids. The lowest concentrations of the four rhamnolipids and free diacids required to stop the motility of all Phytophthora sojae zoospores were around 20, 30, 40, 40, and 125 mg/L, respectively. Their results indicate that the degradation intermediates, Rha-C10 and Rha-Rha-C10, which have lost one fatty acid residue, and the free diacids from (Rha-C10-C10 and Rha-Rha-C10-C10), all have good potential anti-zoospore activity when applied to the natural environment. At present, partial rhamnolipid homologs or congeners, due to their nonmutagenicity and low mammalian toxicity, had been approved as biopesticide for vegetable, fruit, and legume crops by U. S. Environmental Protection Agency (EPA).
Rhamnolipids as immune modulators and virulence factors
The role of airway mucosa barrier is to protect against infection by enteropathogenic microorganisms. However, several enteropathogenic microorganisms can produce a variety of cytotoxins which can disrupt human airway mucosa. Zulianello et al. (Zulianello et al. 2006) investigated the effect of some virulence factors secreted by P. aeruginosa in human airway epithelium, unexpectedly finding that rhamnolipids as virulence factors could functionally disrupt human airway epithelia to promote the paracellular invasion of pathogenic microorganism. The study demonstrated that rhamnolipids can selectively alter the junction-dependent barrier of human respiratory epithelium. Furthermore, rhamnolipids have been implicated in the life-threatening ventilator-associated pneumonia. Ventilator-associated pneumonia occurred more frequently in those colonized patients during the observation period by isolates yielding high levels of rhamnolipids (Köhler et al. 2010). The effect of rhamnolipids as virulence factors on cellular viability was investigated using human skin fibroblasts as culture medium. Purified rhamnolipids can trigger a series of rapid and pronounced morphological alterations of fibroblastic cells. The addition of rhamnolipids can cause cytotoxicity in the cultured fibroblasts; this is in contrast to that of rhamnose which stimulated viability. These results demonstrated that a certain part of rhamnolipids is involved in the cytotoxicity. Ultimately, it was attributed to the lipidic chain parameters and variations rather than the carbohydrate moiety (Pantazaki and Choli-Papadopoulou 2012).
The heat-stable extracellular toxin Rha-Rha-C14-C14 from Burkholderia plantarii could stimulate immune cells to induce tumor necrosis factor α release in human mononuclear cells (Andrä et al. 2006). Obviously, such a property had not been noted in the past for rhamnolipids, the cell-stimulating activity of Rha-Rha-C14-C14 might be due to its pathophysiological activities. Like lipopolysaccharides, the cell stimulatory activity of Rha-Rha-C14-C14 could be inhibited by polymyxin B. However, the activation of immune cells by Rha-Rha-C14-C14 did not occur via receptors which were involved in lipopetide or lipopolysaccharide signaling. The structure of Rha-Rha-C14-C14 was similar or identical to other rhamnolipids previously reported from human pathogens, such as P. aeruginosa and B. pseudomallei. It was likely that other rhamnolipids also had endotoxin-like activity that induced the production of tumor necrosis factor in immune cells. Immunostimulatory properties of synthetic rhamnolipids were investigated by the secretion of tumor necrosis factor α and induction of chemiluminescence in monocytes, the results of biological activities and physicochemical parameters showed great variations depending to stereochemistry, chain length, number of rhamnoses, and number of lipid chains (Howe et al. 2006). However, lipopolysaccharides were more efficient to induce the production of tumor necrosis factor α and luminescence than synthetic rhamnolipids tested. This might relate to acyl chain length, intercalation ability, and aggregate structure. Further extended structure-activity study of synthetic rhamnolipids suggested a specific, recognition-based mode of monocyte activation with small structural variations in the synthetic rhamnolipids, leading to different immunostimulatory activities (Bauer et al. 2006). Several pathogen-associated molecular patterns of P. aeruginosa could activate human innate immune responses in epithelial cells, especially inducing the generation and release of proinflammatory cytokines such as IL-8 (Gerstel et al. 2009). The present study showed rhamnolipids were responsible for the production of flagellin from P. aeruginosa flagella, but their mechanisms of action remained unclear. Flagellin was known as a potent immunostimulatory factor. P. aeruginosa rhamnolipids altered the outer membrane composition, inducing shedding of flagellin from P. aeruginosa flagellum (Gerstel et al. 2009). In turn, epithelial cells at sites of infection recognized flagellin and caused recruitment of inflammatory cells by the release of proinflammatory cytokines. Further studies showed that P. aeruginosa rhamnolipids could significantly reduce Phorbol 12-myristate 13-acetate mediated human beta-defensin-2 release in keratinocytes to subvert the host cell innate immune response. Rhamnolipids can interfere with protein kinase C activation and calcium-regulated pathways, finally resulting in abrogation of the flagellin-induced human beta-defensin-2 expression (Döessel et al. 2012). Flagellin of P. aeruginosa can be first recognized by TLR5 (toll-like receptor 5) and aGM1 (asialoGM1) leading to the activation of PLC (phospholipase C) and the release of ATP. Because of the activation of PIP2 (phosphatidylinositol 4,5-bisphosphate), the resulting IP3 (inositol 1,4,5-triphosphate) initiates the release of Ca2+ from ER (endoplasmic reticulum). Ca2+ is combined with a classical PKC (protein kinase C) isoform resulting in activation by DAG. The activation of PKC facilitates AP1 (transcription factor AP1) in concert with NFκB (nuclear factor-κB) to induce hBD-2 expression. However, rhamnolipids suppress the flagellin-induced hBD-2 expression in keratinocytes to subvert the host innate immune response by interfering with host cell Ca2+ signaling and PKC (Fig. 2). P. aeruginosa rhamnolipids contribute to rehabilitate early compromised epithelia and skin. Once colonization of P. aeruginosa was established, rhamnolipids would further engage in a targeted attenuation of human innate immune response to protect P. aeruginosa. It is also worth mentioning that rhamnolipids can induce the release of GM-CSF (granulocyte-macrophage colony-stimulating factor), histamine from mast cells, and IL-6 from nasal epithelial cells (at noncytotoxic dose levels), and release and generation of 12-hydroxyeicosatetraenoic acid and the inflammatory mediators serotonin from human platelets (Irfan-Maqsood and Seddiq-Shams 2014; Vatsa et al. 2010). These studies will not only contribute to understand the host-pathogen interactions of rhamnolipid-producing bacteria, such as the molecular mechanisms of diseases like melipodosis, but also promote the treatment of human immune diseases.
P. aeruginosa is the major microorganism in the infection of chronic cystic fibrosis lung disease. The persistent infection of P. aeruginosa is mainly due to individual virulence determinants such as rhamnolipids, elastase, and alginate. Rhamnolipids can elicit defense responses and protection against several cellular components of human innate immune system, e.g., rhamnolipids can surround the biofilm bacteria as an immune shield against the antimicrobial activity of polymorphonuclear neutrophilic leukocytes (PMNs) (Bjarnsholt et al. 2010; Bianconi et al. 2011). This suggests that the treatment with drugs interfering with the biosynthesis of rhamnolipids may be useful not only in the treatment of infections with P. aeruginosa but also in the treatment of other rhamnolipid-producing strains.
Rhamnolipids have been long known as virulence factors produced by human pathogen P. aeruginosa. However, several papers reported that rhamnolipids played an important role in the treatment of human diseases. Researchers had found that one or more rhamnolipids as active ingredients would become effective and useful immune modulators. In vivo data had been more targeted specifically to the treatment of autoimmune diseases, including cellular immunosuppression, immunomodulation, and immunorestoration. Several clinical data suggested one or more rhamnolipids had excellent curative therapy for dermatological autoimmune diseases of Lichen ruber planus and Psoriasis (Piljac and Piljac 1995). Rha-Rha-C10-C10 had been reported the successful treatment for chronic decubitus ulcers in an elderly when the standard therapy did not give any improvement (Piljac et al. 2008). However, its mechanism of action was still completely unclear. The partial reasons were attributed to the demonstrated biologic effects of Rha-Rha-C10-C10 in promoting wound re-epithelialization and remodeling through the interaction of human immune system. Regrettably, there are insufficient controlled clinical trials and toxicologic testings to support Rha-Rha-C10-C10 as a standard clinical agent. Rhamnolipids also exhibited broad agricultural prospects as potent stimulators of immunity. Rhamnolipids had been shown to trigger plant immune defense responses and protection against Botrytis cinerea in grapevines (Varnier et al. 2009). However, the signaling pathways of plant innate immunity elicited by rhamnolipids are still not clear. To gain more insight into rhamnolipids-induced MAMPs (microbe-associated molecular patterns)-triggered immunity, Sanchez et al. (Sanchez et al. 2012) investigated rhamnolipid-triggered immune defense responses and disease resistance against hemibiotrophic, necrotrophic, and biotrophic pathogens. The research results showed rhamnolipids triggered an immune defense response characterized by immune defense gene activation and signaling molecular accumulation. Rhamnolipid-mediated resistance was regulated by different signaling molecules depending on the type of pathogens. Jasmonic acid and ethylene were differentially required according to the pathogen lifestyle whereas salicylic acid was a central signaling molecular in overall rhamnolipid-induced resistance. Thus, the complex mechanisms involved in plant immune defense could be triggered by a series of molecules that could be perceived by plant cells. Jayanthi et al. (Jayanthi and Revathi 2016) determined the immunostimulatory effect of rhamnolipids from Pseudomonas putida strain in Labeo rohita. In this study, rhamnolipids affected the hematological components in Labeo rohita to enhance the fish immune system and reduce the number of antibiotics required to treat infectious diseases. Therefore, rhamnolipids would be recommended as dietary supplements to improve the aquaculture production of tilapia, after further evaluating cost-benefits.
Rhamnolipids as anticancers
One of the most exciting recent findings is the discovery that rhamnolipids have ability to act on cancer cells as antitumor drugs interfering with some cancer progression. For example, Thanomsub et al. (Thanomsub et al. 2006) studied the cytotoxic activities of Rha-Rha-C10-C10 and Rha-Rha-C10-C12 isolated from P. aeruginosa B189, against human breast cancer cell lines. Rha-Rha-C10-C10 inhibited the proliferation of human breast cancer MCF-7 cells (MCF-7 tumor cells), with minimum inhibitory concentration at 6.25 μg/mL, while Rha-Rha-C10-C12 had not exhibited cytotoxic activity. Moreover, Rha-Rha-C10-C10 or Rha-Rha-C10-C12 did not affect the growth of the normal cell line (Vero cell) at 50 μg/mL. This demonstrated the toxic selectivity of these rhamnolipids to different cell lines used. Furthermore, the inhibitory mechanisms of Rha-Rha-C10-C10 against MCF-7 tumor cells were as yet unclear and remain under investigation. Dirhamnolipids (major component Rha-Rha-C10-C10), produced by P. aeruginosa M14808, exhibited significant in vitro antiproliferative activity against human nonsmall lung cancer cell H460 and MCF-7 tumor cells, with a minimum inhibitory concentration at 5 and 1 μg/mL, respectively. However, monorhamnolipids (major component Rha-C10-C10) and the crude extract were not observed antitumor activity (Zhao et al. 2013). This report suggested that dirhamnolipids synthesized by P. aeruginosa M14808 could have potential anticancer applications. No anticancer activities were observed with the crude extract of this strain. This could be due to the relatively low concentrations of dirhamnolipids in the total crude extract. In same studies, Rha-Rha-C10-C10 showed cytotoxicity with MIC 6.25 μg/mL in the MCF-7 tumor cells (Thanomsub et al. 2006). This result suggested other dirhamnolipids from P. aeruginosa M14808 could better inhibit cell growth and induce differentiation in MCF-7 tumor cells as compared to Rha-Rha-C10-C10, or acted as synergist to improve the antitumor activity of Rha-Rha-C10-C10.
Jiang et al. (Jiang et al. 2014a) examined four cancer cells (human hepatocellular carcinoma HepG2 cells, human colon carcinoma cell line Caco-2, human hepatocellular liver carcinoma cells, and MCF-7 tumor cells) and two normal cells (human proximal tubular epithelial cell lines HK-2 and hepatocyte). Interestingly, rhamnolipids elicited similar sensitivities for both two cancer cells (HepG2 cell and Caco-2 cell) and a normal cell (HK-2 cell) in culture medium supplemented with or without fetal bovine serum (FBS) and the cytotoxic effect of rhamnolipids was largely attenuated due to the addition of fetal bovine serum in culture medium. Similarly, other chemical surfactants (sodium dodecyl sulfate, Tween-80, and sodium dodecyl benzene sulfonate) could also cause cytotoxicity on human HepG2 cells when its addition made the surface tension below 41 mN/m in culture medium supplemented with or without FBS. Therefore, it was found that rhamnolipids could cause cytotoxicity whenever the surface tension decreased below 41 mN/m in the culture medium. It seemed that rhamnolipids triggered cytotoxicity by decreasing surface tension of culture medium instead of changing specific structure of molecules, and therefore had no cytotoxic selectivity on different tumor cells. However, the claim on the antitumor activity of rhamanolipids was in doubt. As previously stated, a monorhamnolipid (Rha-Rha-C10-C10) could cause cytotoxicity on MCF-7 tumor cells with MIC 6.25 μg/mL cultured in serum-free medium and had no inhibition effect on normal Vero cells cultured in serum-containing medium at concentration up to 50 μg/mL (Thanomsub et al. 2006). The higher sensitive antitumor activity of rhamnolipids did not have relevant explanation. Jiang et al. (Jiang et al. 2014a) claimed that the addition of serum in medium significantly attenuated cytotoxicity during the cytotoxicity testing, the different serum content in culture medium led to wrong conclusion on the antitumor activity of rhamnolipids. However, monorhamnolipids (major component Rha-Rha-C10-C10) caused more sensitive cytotoxicity on MCF-7 tumor cells with MIC 1 μg/mL cultured in serum-containing medium (Zhao et al. 2013). In addition, MCF-7 tumor cells and normal Vero cells were not chosen to culture in the absence of fetal bovine serum and presence of fetal bovine serum to study their cytotoxicity. Taken together, Rha-Rha-C10-C10 might have cytotoxic selectivity on MCF-7 tumor cells and normal Vero cells. It is possible that the type of cancer cells and normal cells used, or dirhamnolipids and monorhamnolipids purity, will lead to different experimental results. Unquestionably, a more detailed understanding of the mechanisms of action of rhamnolipids is crucially necessary for their biological applications in antitumor drugs.
Dirhamnolipids (major component Rha-Rha-C10-C10) and monorhamnolipids (major component Rha-C10-C10) produced from P. aeruginosa BN10, were also evaluated for cytotoxic effects on four human tumor cell lines (human pre-B leukemia lines, T-cell chronic lymphocytic leukemia cell lines, human promyelocytic leukemia cell lines, and human urinary bladder carcinoma cell lines) (Christova et al. 2013). This was the first time demonstrating monorhamnolipids had obvious cytotoxic activity against four human cancer cell lines when compared to dirhamnolipids. It was possible that the structure of the monorhamnolipid molecule was responsible for higher penetration in tumor cells, hence, leading to higher reduction in cell viability. Clearly, in vitro antitumor activity of monorhamnolipids was mediated by the induction of apoptosis. Monorhamnolipids-induced leukemic and epithelial human tumor cells death may be involved in multiple mechanisms due to prolonged exposure therapy, such as the activation or modification of tumor suppressor program or/and inactivation of cellular oncogenes. The apoptotic process of tumor cells appeared to be dose-independent of monorhamnolipids, which might be due to the presence and role of efflux pumps or the saturation of receptors on the cell membrane, which did not allow to more active molecules to freely permeate within the cancer cells. In addition, it also might be because higher doses of monorhamnolipids could influence the overexpression of Bcl-2 and c-myc genes in human BV-173 pre-B leukemia lines, which resulted in the antiapoptotic effects and higher proliferation rates. Nevertheless, the antiapoptotic processes of BV-173 tumor cells will be overcome after prolonged incubation (≥ 72 h) with monorhamnolipids. The Balb/c 3T3 neutral red uptake test showed monorhamnolipids had very low cytotoxicity to nontumorigenic Balb/c 3T3 mouse fibroblasts. Thus, monorhamnolipids from P. aeruginosa strain BN10 could be considered as a novel and naturally derived antitumor agents for the future research.
Additionally, Rha-Rha-C10-C10 and Rha-Rha-C10 isolated from Pseudomonas sp. strain ICTB-745 exhibited efficient cytotoxic potencies against other human tumor cell lines, such as human lung adenocarcinoma epithelial cell lines, human hepatocellular liver carcinoma cell lines, human breast adenocarcinoma cell lines, and human cervical cancer cell lines (Kamal et al. 2012). Rhizoleucinoside, a rhamnolipid-amino alcohol hybrid, isolated from Bradyrhizobium sp. BTAi1, suppressed the proliferation of BV-2 murine microglial cells with an IC50 of 6.9 μM and highly aggressive proliferating immortalized rat microglia cells with an IC50 of 22 μM (Chen et al. 2016). A novel monorhamnolipid (NDYS-4 E) produced by Streptomyces coelicoflavus NBRC (15399T) possessed moderate antitumor activity with IC50 of 88.60 μg/mL in vitro against MCF-7 tumor cells (Allada et al. 2015). Moreover, rhamnolipids were dissolved little in simulated gastric juice, thus leading to largely reduced cytotoxic effect in vivo. The mechanistic illustration of the high-level biosafety of rhamnolipids in vivo would offer a valuable guidance for further anticancer research (Jiang et al. 2014b).
Rh-AgNPs were obtained by the reaction of rhamnolipids from Pseudomonas aeruginosa JS-11 with stable silver nanoparticles. Comparative cytotoxicity data with rhamnolipids and Rh-AgNPs on cultured tumor MCF-7 cells and normal human peripheral blood mononuclear cells (normal PBMN cells) at concentration of 10 μg/mL showed both rhamnolipids and Rh-AgNPs had no significant effect on the normal PBMN cell viability, as compared to a more remarkable decrease in MCF-7 tumor cell viability treated with the same concentration of Rh-AgNPs than rhamnolipids alone treated (Dwivedi et al. 2015). The great difference might be attributed to greater endocytosis activity in tumor cells than normal cells. It might be because that the surface of cancer cells contained more negatively charged group, the cationic metal nanoparticles had a greater attachment onto the plasma membrane of MCF-7 cells, which promoted metal nanoparticles internalization through vesicular transport pathway mediated cell endocytosis. The gene expression in MCF-7 tumor cells pathway treated with Rh-AgNPs suggested the oxidative stress pathway genes induced the death of MCF-7 tumor cells. Therefore, the attachment of Rh-AgNPs to the surface of the cells might lead to the disruption of cell membrane and oxidative stress of MCF-7 tumor cells, inducing the antiproliferative activity of MCF-7 tumor cells. This suggested Rh-AgNPs would be a promising candidate as the novel cancer therapy drug.
Conclusions and future outlooks
The mini review exhibited the diversity of rhamnolipid structures, rhamnolipid-producing microorganisms, and elaborated physiochemical characterization of rhamnolipids for agricultural and medical applications. Deep insight into the physiochemical characterization and biological importance of rhamnolipids reveal their potential application values in the fields of agriculture and biomedicine, precisely in food safety, plant and animal defense, disease control and treatment, and pathogenesis. Rhamnolipids have a dual mechanism of action: they have antimicrobial properties and also elicit defense responses in plants against pathogens. This dual property will contribute to improve their efficiency and develop new biopesticides. Rhamnolipids have also been reported to be involved in inducing plant, animal and human immune defense responses and can be used to treat several immune diseases. However, studies of rhamnolipids as anticancer are still disputed, the mechanisms of actions of rhamnolipids are still understood insufficiently. A better understanding of the mechanisms of actions of rhamnolipids at the molecular level would help researchers to better learn biochemical and cellular interactions, which can serve as a guide in conducting a series of preclinical studies and clinical trials at each stage. There will be also trials to isolate purified or mixed rhamnolipid homologs or congeners to explore their physicochemical characterization for a variety of applications (Abbasi et al. 2013; Abalos et al. 2017). This may be due to their promising application values either in pure form or in mixtures. Furthermore, the structure-activity relationships studies of rhamnolipids will also help researchers to better understand their biological characterizations and predict the biological functions of different rhamnolipid mixtures.
References
Abalos A, Pinazoa A, Infante MR, Casals M, García F, Manresa A (2017) Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 17(5):1367–1371. https://doi.org/10.1021/la0011735.
Abbasi H, Noghabi KA, Hamedi MM, Zahiri HS, Moosavi-Movahedi AA, Amanlou M, Teruel JA, Ortiz A (2013) Physicochemical characterization of a monorhamnolipid secreted by Pseudomonas aeruginosa MA01 in aqueous media. An experimental and molecular dynamics study. Colloids Surf B Biointerfaces 101(1):256–265. https://doi.org/10.1016/j.colsurfb.2012.06.035.
Abdel-Mawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86(5):1323–1336. https://doi.org/10.1007/s00253-010-2498-2
Abdelmawgoud AM, Lépine F, Déziel E (2014) A stereospecific pathway diverts β-oxidation intermediates to the biosynthesis of rhamnolipid biosurfactants. Chem Biol 21(1):156–164. https://doi.org/10.1016/j.chembiol.2013.11.010
Allada LTK, Guntuku GS, Muthyala MKK, Duddu MK, Golla NS (2015) Characterization of bioactive compound from Streptomyces coelicoflavus NBRC (15399T) and its anticancer activity. Int J Chem Pharm Anal 2(4):1–13
Andrä J, Rademann J, Howe J, Koch MH, Heine H, Zähringer U, Brandenburg K (2006) Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: immune cell stimulation and biophysical characterization. Biol Chem 300(3):301–310. https://doi.org/10.1515/BC.2006.040
Batovska DI, Todorova IT, Tsvetkova IV, Najdenski HM (2009) Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol J Microbiol 58(1):43–47
Bauer J, Brandenburg K, Zähringer U, Rademann J (2006) Chemical synthesis of a glycolipid library by a solid-phase strategy allows elucidation of the structural specificity of immunostimulation by rhamnolipids. Chem Eur J 12(27):7116–7124. https://doi.org/10.1002/chem.200600482
Bazire A, Dufour A (2014) The Pseudomonas aeruginosa rhlG and rhlAB genes are inversely regulated and Rhl G is not required for rhamnolipid synthesis. BMC Microbiol 14(1):1–9. https://doi.org/10.1186/1471-2180-14-160
Behrens B, Baune M, Jungkeit J, Tiso T, Blank LM, Hayen H (2016a) High performance liquid chromatography-charged aerosol detection applying an inverse gradient for quantification of rhamnolipid biosurfactants. J Chromatogr A 1455:125–132. https://doi.org/10.1016/j.chroma.2016.05.079
Behrens B, Helmer PO, Tiso T, Blank LM, Hayen H (2016c) Rhamnolipid biosurfactantanalysis using online turbulent flow chromatography-liquid chromatography-tandem mass spectrometry. J Chromatogr A 1465:90–97. https://doi.org/10.1016/j.chroma.2016.08.044
Behrens B, Engelen J, Tiso T, Blank LM, Hayen H (2016b) Characterization of rhamnolipids by liquid chromatography/mass spectrometry after solid-phase extraction. Anal Bioanal Chem 408(10):2505–2514. https://doi.org/10.1007/s00216-016-9353-y
Bianconi I, Milani A, Cigana C, Paroni M, Levesque RC, Bertoni G, Bragonzi A (2011) Positive signature-tagged mutagenesis in Pseudomonas aeruginosa: tracking patho-adaptive mutations promoting airways chronic infection. PLoS Pathog 7(2):e1001270. https://doi.org/10.1371/journal.ppat.1001270
Bjarnsholt T, Jensen PØ, Jakobsen TH, Phipps R, Nielsen AK, Rybtke MT, Tolker-Nielsen T, Givskov M, Høiby N, Ciofu O (2010) Quorum sensing and virulence of Pseudomonas aeruginosa during lung infection of cystic fibrosis patients. PLoS One 5(4):e10115. https://doi.org/10.1371/journal.pone.0010115
Chen JW, Sun JW, Deering RW, DaSilva N, Seeram NP, Wang H, Rowley DC (2016) Rhizoleucinoside, a Rhamnolipid–Amino Alcohol Hybrid from the Rhizobial Symbiont Bradyrhizobium sp. BTAi1. Org Lett 18(6):1490–1493. https://doi.org/10.1021/acs.orglett.6b00461
Costa SG, Nitschke M, Lepine F, Deziel E, Contiero J (2010) Structure, properties and applications of rhamnolipids produced by Pseudomonas aeruginosa L2-1 from cassava wastewater. Process Biochem 45(9):1511–1516. https://doi.org/10.1016/j.procbio.2010.05.033
Christova N, Tuleva B, Kril A, Georgieva M, Konstantinov S, Terziyski I, Nikolova B, Stoineva I (2013) Chemical structure and in vitro antitumor activity of rhamnolipids from Pseudomonas aeruginosa BN10. Appl Biochem Biotechnol 170(3):676–689. https://doi.org/10.1007/s12010-013-0225-z
de Rienzo MAD, Stevenson P, Marchant R, Banat IM (2016) Antibacterial properties of biosurfactants against selected gram-positive and -negative bacteria. FEMS Microbiol Lett 363(2):fnv224. https://doi.org/10.1093/femsle/fnv224
de SantanaFilho AP, Camiliosneto D, de Souza LM, Sassaki GL, Mitchell DA, Krieger N (2015) Evaluation of the structural composition and surface properties of rhamnolipid mixtures produced by Pseudomonas aeruginosa UFPEDA 614 in different cultivation periods. Appl Biochem Biotechnol 175(2):988–995. https://doi.org/10.1007/s12010-014-1343-y
Dobler L, Vilela LF, Almeida RV, Neves BC (2015) Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnol 33(1):123–135. https://doi.org/10.1016/j.nbt.2015.09.005
Döessel J, Meyer-Hoffert U, Schröder JM, Gerstel U (2012) Pseudomonas aeruginosa-derived rhamnolipids subvert the host innate immune response through manipulation of the human beta-defensin-2 expression. Cellular Microbial 14(9):1364–1375. https://doi.org/10.1111/j.1462-5822.2012.01801.x
Dwivedi S, Saquib Q, Al-Khedhairy AA, Ahmad J, Siddiqui MA, Musarrat J (2015) Rhamnolipids functionalized AgNPs-induced oxidative stress and modulation of toxicity pathway genes in cultured MCF-7 cells. Colloids Surf B Biointerfaces 132:290–298
Edwards JR, Hayashi JA (1965) Structure of a rhamnolipid from Pseudomonas aeruginosa. Arch Biochem Biophys 111(2):415–421. https://doi.org/10.1016/0003-9861(65)90204-3
Elshikh M, Funston S, Chebbi A, Ahmed S, Marchant R, Banat IM (2017) Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol 36:26–36. https://doi.org/10.1016/j.nbt.2016.12.009
Gerstel U, Czapp M, Bartels J, Schröder JM (2009) Rhamnolipid-induced shedding of flagellin from Pseudomonas aeruginosa provokes hBD-2 and IL-8 response in human keratinocytes. Cell Microbiol 11(5):842–853. https://doi.org/10.1111/j.1462-5822.2009.01299.x
Goswami D, Borah SN, Lahkar J, Handique PJ, Deka S (2015) Antifungal properties of rhamnolipid produced by Pseudomonas aeruginosa DS9 against Colletotrichum falcatum. J Basic Microbiol 55(11):1265–1274. https://doi.org/10.1002/jobm.201500220
Haba E, Pinazo A, Jauregui O, Espuny M, Infante MR, Manresa A (2003) Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol Bioeng 81(3):316–322. https://doi.org/10.1002/bit.10474
Hajfarajollah H, Mehvari S, Habibian M, Mokhtarani B, Noghabi KA (2015) Rhamnolipid biosurfactant adsorption on a plasma-treated polypropylene surface to induce antimicrobial and antiadhesive properties. RSC Adv 5(42):33089–33097. https://doi.org/10.1039/C5RA01233C
Hošková M, Ježdík R, Schreiberová O, Chudoba J, Šir M, Čejkov á A, Masák J, Jirků V, Řezanka T (2015) Structural and physiochemical characterization of rhamnolipids produced by Acinetobacter calcoaceticus, Enterobacter asburiae and Pseudomonas aeruginosa in single strain and mixed cultures. J Biotechnol 193:45–51. https://doi.org/10.1016/j.jbiotec.2014.11.014
Howe J, Bauer J, Andrä J, Schromm AB, Ernst M, Rössle M, Zähringer U, Rademann J, Brandenburg K (2006) Biophysical characterization of synthetic rhamnolipids. FEBS J 273(22):5101–5112. https://doi.org/10.1111/j.1742-4658.2006.05507.x
Irfan-Maqsood M, Seddiq-Shams M (2014) Rhamnolipids: well-characterized glycolipids with potential broad applicability as biosurfactants. Ind Biotechnol 10(4):285–291. https://doi.org/10.1089/ind.2014.0003
Irorere VU, Tripathi L, Marchant R, Mcclean S, Banat IM (2017) Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl Microbiol Biot 101(10):3941–3951. https://doi.org/10.1007/s00253-017-8262-0
Itoh S, Honda H, Tomita F, Suzuki T (1971) Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C 12, C 13 and C 14 fractions). J Antibiot 24(12):855–859
Jayanthi C, Revathi K (2016) Immunostimulation by rhamnolipid biosurfactant fromPseudomonas putida in Labeo rohita. Int J Res Biol Sci 3:179–183
Jiang L, Shen C, Long X, Zhang G, Meng Q (2014b) Rhamnolipids elicit the same cytotoxic sensitivity between cancer cell and normal cell by reducing surface tension of culture medium. Appl Microbiol Biotechnol 98(24):10187–10196. https://doi.org/10.1007/s00253-014-6065-0
Jiang L, Shen C, Long X, Meng Q (2014a) Mechanistic studies of differential cytotoxicities of rhamnolipids between in vitro and in vivo. Abstr 14AlChE Annual Meeting, abstr 599at
Kamal A, Shaik AB, Kumar CG, Mongolla P, Rani PU, Krishna KV, Mamidyala SK, Joseph J (2012) Metabolic profiling and biological activities of bioactive compounds produced by Pseudomonas sp. strain ICTB-745 isolated from Ladakh, India. J Microbiol Biotechnol 22(1):69–79. https://doi.org/10.4014/jmb.1105.05008
Köhler T, Guanella R, Carlet J, Delden CV (2010) Quorum sensing-dependent virulence during Pseudomonas aeruginosa colonisation and pneumonia in mechanically ventilated patients. Thorax 65(8):703–710. https://doi.org/10.1136/thx.2009.133082
Lazarkevich I, Sotirova A, Avramova T, Galabova D (2015) Comparative study on antibacterial activity of synthetic analogues of biologically active compounds and their combination with rhamnolipid-biosurfactant. J Biosci Biotechnol SE/ONLINE:55–62
Liu JF, Wu G, Yang SZ, BZ M (2014) Structural characterization of rhamnolipid produced by Pseudonomas aeruginosa strain FIN2 isolated from oil reservoir water. World J Microbiol Biotechnol 30(5):1473–1484. https://doi.org/10.1007/s11274-013-1565-0
Lovaglio RB, Silva VL, Ferreira H, Hausmann R, Contiero J (2015) Rhamnolipids know-how: looking for strategies for its industrial dissemination. Biotechnol Adv 33(8):1715–1726. https://doi.org/10.1016/j.biotechadv.2015.09.002
Magalhães L, Nitschke M (2013) Antimicrobial activity of rhamnolipids against Listeria monocytogenes and their synergistic interaction with nisin. Food Control 29(1):138–142. https://doi.org/10.1016/j.foodcont.2012.06.009
Meyer-Hoffert U, Zimmermann A, Czapp M, Bartels J, Koblyakova Y, Gläser R, Schröder JM, Gerstel U (2011) Flagellin delivery by Pseudomonas aeruginosa rhamnolipids induces the antimicrobial protein psoriasin in human skin. PLoS One 6(1):e16433. https://doi.org/10.1371/journal.pone.0016433
Miao S, Dashtbozorg SS, Callow NV, LK J (2015) Rhamnolipids as platform molecules for production of potential anti-zoospore agrochemicals. J Agri Food Chem 63(13):3367–3376. https://doi.org/10.1021/acs.jafc.5b00033
Müller MM, Hausmann R (2011) Regulatory and metabolic network of rhamnolipid biosynthesis: traditional and advanced engineering towards biotechnological production. Appl Microbiol Biotechnol 91(2):251–264. https://doi.org/10.1007/s00253-011-3368-2
Nalini S, Parthasarathi R (2014) Production and characterization of rhamnolipids produced by Serratia rubidaea SNAU02 under solid-state fermentation and its application biocontrol agent. Bioresour Technol 173:231–238. https://doi.org/10.1016/j.biortech.2014.09.051
Nie M, Yin X, Ren C, Wang Y, Xu F, Shen Q (2010) Novel rhamnolipid biosurfactants produced by a polycyclic aromatic hydrocarbon-degrading bacterium Pseudomonas aeruginosa strain NY3. Biotechnol Adv 28(5):635–643. https://doi.org/10.1016/j.biotechadv.2010.05.013
Nott K, Richard G, Laurent P, Jérôme C, Blecker C, Wathelet JP, Paquot M, Deleu M (2013) Enzymatic synthesis and surface properties of novel rhamnolipids. Process Biochem 48(1):133–143. https://doi.org/10.1016/j.procbio.2012.11.019
Nitschke M, Costa SG, Contiero J (2005) Rhamnolipid surfactants: an update on the general aspects of these remarkable biomolecules. Biotechnol Prog 21(6):1593–1600. https://doi.org/10.1021/bp050239p
Ohlendorf B, Lorenzen W, Kehraus S, Krick A, Bode HB, König GM (2009) Myxotyrosides A and B, unusual rhamnosides from Myxococcus sp. J Nat Prod 72(1):82–86. https://doi.org/10.1021/np8005875
Pantazaki A, Choli-Papadopoulou T (2012) On the Thermus thermophilus HB8 potential pathogenicity triggered from rhamnolipids secretion: morphological alterations and cytotoxicity induced on fibroblastic cell line. Amino Acids 42(5):1913–1926. https://doi.org/10.1007/s00726-011-0917-z
Piljac A, Stipcević T, Piljac-Zegarac J, Piljac G (2008) Successful treatment of chronic decubitus ulcer with 0.1% dirhamnolipid ointment. J Cutan Med Surg 12(3):142–146. https://doi.org/10.2310/7750.2008.07052
Piljac G, Piljac V. November 1995. Immunological activity of rhamnolipids. US patent 5466675.
Reis RS, Pereira AG, Neves BC, Freire DM (2011) Gene regulation of rhamnolipid production in Pseudomonas aeruginosa—a review. Bioresour Technol 102(11): 6377–6384. doi : https://doi.org/10.1016/j.biortech.2011.03.074.
Rezanka T, Siristova L, Sigler K (2011) Rhamnolipid-producing thermophilic bacteria of species Thermus and Meiothermus. Extremophiles 15(6):697–709. https://doi.org/10.1007/s00792-011-0400-5
Sanchez L, Courteaux B, Hubert J, Kauffmann S, Renault JH, Clément C, Baillieul F, Dorey S (2012) Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol 160(3):1630–1641. https://doi.org/10.1104/pp.112.201913
Schaffer S, Wessel M, Thiessenhusen A, Apirl SN (2015) Cells and methods for producing rhamnolipids. US patent 20130130319
Soberón-Chávez G, Lépine F, Déziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68(6):718–725. https://doi.org/10.1007/s00253-005-0150-3
Sotirova A, Avramova T, Stoitsova S, Lazarkevich I, Lubenets V, Karpenko E, Galabova D (2012) The importance of rhamnolipid-biosurfactant-induced changes in bacterial membrane lipids of Bacillus subtilis for the antimicrobial activity of thiosulfonates. Curr Microbiol 65(5):534–541. https://doi.org/10.1007/s00284-012-0191-7
Soltani Dashtbozorg S, Miao S, LK J (2016) Rhamnolipids as environmentally friendly biopesticide against plant pathogen Phytophthora sojae. Environ Prog Sustain Energy 35(1):169–173. https://doi.org/10.1002/ep.12187
Sha R, Jiang L, Meng Q, Zhang G, Song Z (2012) Producing cell-free culture broth of rhamnolipids as a cost-effective fungicide against plant pathogens. J Basic Microbiol 52(4):458–466. https://doi.org/10.1128/JB.186.10.2936-2945.2004.
Tavares LF, Silva PM, Junqueira M, Mariano DC, Nogueira FC, Domont GB, Freire DM, Neves BC (2013) Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl Microbiol Biotechnol 97(5):1909–1921. https://doi.org/10.1007/s00253-012-4454-9
Thanomsub B, Pumeechockchai W, Limtrakul A, Arunrattiyakorn P, Petchleelaha W, Nitoda T, Kanzaki H (2006) Chemical structures and biological activities of rhamnolipids produced by Pseudomonas aeruginosa B189 isolated from milk factory waste. Bioresour Technol 97(18):2457–2461. https://doi.org/10.1016/j.biortech.2005.10.029
Toribio J, Escalante AE, Soberón-Chávez G (2010) Rhamnolipids: Production in bacteria other thanPseudomonas aeruginosa. E J Lipid Sci Tech 112(10):1082-1087. https://doi.org/10.1002/ejlt.200900256
Varnier AL, Sanchez L, Vatsa P, Boudesocque L, Garcia-Brugger A, Rabenoelina F, Sorokin A, Renault JH, Kauffmann S, Pugin A, Clement C, Baillieul F, Dorey S (2009) Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ 32(2):178–193. https://doi.org/10.1111/j.1365-3040.2008.01911.x
Vatsa P, Sanchez L, Clement C, Baillieul F, Dorey S (2010) Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int J Mol Sci 11(12):5095–5108. https://doi.org/10.3390/ijms11125095
Yin XH, Nie MQ, Shen QR (December 2011) Rhamnolipid biosurfactant from Pseudomonas Aeruginosa strain NY3 and methods of use. US patent 20110306569
Zeng YB, Wang H, Zuo WJ, Zheng B, Yang T, Dai HF, Mei WL (2012) A fatty acid glycoside from a marine-derived fungus isolated from mangrove plant scyphiphora hydrophyllacea. Mar Drugs 10(3):598–603. https://doi.org/10.3390/md10030598
Zhu K, Rock CO (2008) RhlA converts β-hydroxyacyl-acyl carrier protein intermediates in fatty acid synthesis to the β-hydroxydecanoyl-β-hydroxydecanoate component of rhamnolipids in Pseudomonas aeruginosa. J Bacteriol 190(9):3147–3154. https://doi.org/10.1128/JB.00080-08
Zhao J, Wu Y, Alfred AT, Xin X, Yang S (2013) Chemical structures and biological activities of rhamnolipid biosurfactants produced by Pseudomonas aeruginosa M14808. J Chem Pharm Res 5(12):177–182
Zulianello L, Canard C, Köhler T, Caille D, Lacroix JS, Meda P (2006) Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect Immun 74(6):3134–3147. https://doi.org/10.1128/IAI.01772-05
Acknowledgments
The authors thank Michael J. Murphy, Ph.D., for his suggestions in this manuscript.
Funding
This project was supported by National Natural Science Foundation of China (No. 81773628 and No. 41776139), National Natural Science Foundation of China (Key Program) (No. 21337005), and Zhejiang Provincial Natural Science Foundation (No. LY16H300008 and No. LY16H300007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, J., Wu, Q., Hua, Y. et al. Potential applications of biosurfactant rhamnolipids in agriculture and biomedicine. Appl Microbiol Biotechnol 101, 8309–8319 (2017). https://doi.org/10.1007/s00253-017-8554-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8554-4