Abstract
This study evaluated the effects of high-grain diets on the rumen fermentation, epithelial bacterial community, morphology of rumen epithelium, and local inflammation of goats during high-grain feeding. Twelve 8-month-old goats were randomly assigned to two different diets, a hay diet or a high-grain diet (65% grain, HG). At the end of 7 weeks of treatment, samples of rumen content and rumen epithelium were collected. Rumen pH was lower (P < 0.05), but the levels of volatile fatty acids and lipopolysaccharides were higher (P < 0.05) in the HG group than those in the hay group. The principal coordinate analysis indicated that HG diets altered the rumen epithelial bacterial community, with an increase in the proportion of genus Prevotella and a decrease in the relative abundance of the genera Shuttleworthia and Fibrobacteres. PICRUSt analysis suggested that the HG-fed group had a higher (P < 0.05) relative abundance of gene families related to energy metabolism; folding, sorting, and degradation; translation; metabolic diseases; and immune system. Furthermore, HG feeding resulted in the rumen epithelial injury and upregulated (P < 0.05) the gene expressions of IL-1β and IL-6, and the upregulations were closely related to the rumen pH, LPS level, and rumen epithelial bacteria abundance. In conclusion, our results indicated that the alterations in the rumen environment and epithelial bacterial community which were induced by HG feeding may result in the damage and local inflammation in the rumen epithelium, warranting further study of rumen microbial–host interactions in the HG feeding model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the modern goat industry, goats are often fed relatively high-grain (HG) diets to achieve maximum performance, such as the increase of meat production. However, feeding diets contained large amounts of readily fermentable carbohydrates which frequently results in subacute ruminal acidosis (SARA) (Kleen et al. 2003; O’Grady et al. 2008). During the progression of SARA, the imbalance between the rates of rumen volatile fatty acids (VFAs) production and absorption results in a dramatic decline of rumen pH, further causing a shift in the rumen microbiota. Indeed, it is well-documented that HG diets can induce a variation in the bacterial community of the rumen contents (Fernando et al. 2010; Hook et al. 2011), and meanwhile, the application of high-throughput pyrosequencing leads to a further expansion of how HG diets affect the rumen microbiota.
Rumen epithelial bacteria are directly attached to the rumen epithelium; as the biological barrier of immunity, they act as an important line of defense. As a result, compared to the microbiota in the rumen contents, the rumen epithelial bacteria are deemed important for adaptation to HG diets (Chen et al. 2011). Recently, rumen epithelial bacteria have received considerable attention (Sadet et al. 2007; Chen et al. 2011). A few studies using PCR-DGGE showed that HG feeding altered the rumen epithelial bacteria community, for example, by increasing the proportion of Bacteroidetes and decreasing the proportion of Firmicutes (Sadet-Bourgeteau et al. 2010). These results demonstrated that HG feeding can affect bacterial adhesion to and colonization of the rumen epithelium. However, due to the small number of studies and the technical limitations, it is not possible to comprehensively summarize the typical characteristics of rumen epithelial bacteria in ruminants fed an HG diet. Hence, there is still a paucity of data on how HG diets reshape the rumen epithelial bacterial community.
As the carrier of rumen epithelial bacteria, the rumen epithelium serves as the barrier between the rumen contents and the host. This barrier function mainly depends on the rumen epithelium’s multicellular structure (the stratum corneum, the stratum granulosum, the stratum spinosum, and the stratum basale) and the tight junctions that existed in the stratum granulosum (Graham and Simmons 2005). In ruminants fed HG diets, the accumulation of immunogenic compounds such as lipopolysaccharide (LPS) and the abnormal depression of pH in the rumen must have some detrimental effects on the rumen epithelium (Penner et al. 2011). Once the integrity of the rumen epithelium is impaired, these toxin compounds in the rumen or the pathogenic bacteria attached to the rumen epithelium may translocate across it, further causing inflammation (local inflammation of the rumen epithelium or systemic inflammatory response) (Khafipour et al. 2009a; Penner et al. 2011; Mani et al. 2012). However, information about the incidence of local inflammation in the rumen epithelium and the role of epithelial bacteria on the induction of inflammation during HG feeding is still scarce.
The objective of the present study was to evaluate the influence of an HG diet on rumen fermentation, epithelial bacteria, and epithelial morphology, as well as the local inflammation in the rumen epithelium of goats. Additionally, this study is expected to provide some information to support further investigation into rumen microbial–host interactions in the HG feeding model.
Methods
Animals, housing, and diets
The present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University. All experimental procedures and animal protocols conformed to the Regulations for the Administration of Affairs Concerning Experimental Animals (Chinese Science and Technology Committee 1988). This study was part of a series of studies designed to evaluate the effects of HG diets on the gastrointestinal microbiota and health of goats. Thus, a detailed description of the animal management, diets, and experimental design has been reported by Ye et al. (2016). Briefly, 12 8-month-old male goats were randomly allocated to one of two dietary treatments after 2 weeks of adaptation. Two groups of goats in the present study were fed hay (n = 6) and the HG diet (containing 65% grain, n = 6), respectively. The ingredients and nutrient composition of the two diets are presented in Table S1. At the commencement of the experiment, the differences in the body weights of the goats fed hay or the HG diet in the present study were not significant (28.10 ± 1.23 vs. 26.47 ± 0.83 kg). Animals were fed in equal allotments daily (900-g dry matter per animal per day) at 0800 hours and 1700 hours for 7 weeks.
Sampling
On day 50, the goats were slaughtered 4 h after the morning feeding. The abdomen was opened along the midline, and the digestive organs were carefully separated and removed. During the slaughter, the rumen pH was measured immediately by collecting the representative rumen contents. Subsequently, the rumen digesta were homogenized and mixed thoroughly. Part of the homogenized digesta was harvested and then filtrated through four layers of cheesecloth. The harvested samples were divided into two sections, and each part was undergoing different pre-treatments according to different purposes. The first section of the samples was centrifuged at 6000×g for 15 min at 4 °C, and then, the supernatants were stored at −20 °C until they were analyzed for VFAs and lactate. The second section was used for the measurement of the rumen LPS. The samples were centrifuged at 13,000×g for 40 min, and then, the supernatant was filtered and harvested in the pyrogen-free tube and further boiled for 5 min before being stored at −20 °C.
Within 5 min after slaughtering, samples of the rumen epithelial tissue were harvested according to the method reported previously (Liu et al. 2013). Subsequently, the collected tissue was divided into two sections. The first section was used for RNA extracting, and the tissue was excised into small pieces using surgical scissors and then quickly stored in liquid nitrogen. The second section was scraped from the underlying tissue, snap-frozen in liquid nitrogen, and then stored at −80 °C until DNA extraction. Washed rumen papillae were deposited in 4% paraformaldehyde and 2.5% glutaraldehyde, and these samples were used for histomorphometric microscopy analysis.
Analysis of rumen fermentation parameters
The rumen pH was immediately determined with a portable pH meter (HI 9024C; HANNA Instruments, Woonsocket, RI, USA). The digesta used for VFA analysis were diluted with physiological saline (0.90% NaCl) and then were centrifuged at 12,000×g for 15 min, and the supernatants were treated with 25% (w/v) metaphosphoric acid. The VFAs were analyzed using the method described by Mao et al. (2008). Free LPS in the rumen contents was measured using chromogenic end-point Tachypleus amebocyte lysate assay kit (Xiamen Horseshoe Crab Reagent Manufactory, Xiamen, China). Lactate was detected with an LD kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Histological measurements
The rumen tissues were embedded in paraffin, sectioned (6 μm), and stained with hematoxylin and eosin (H&E). Histomorphometry analyses were performed by laboratory personnel who were blinded to treatment conditions. Lesions on the rumen epithelium were imaged using a ×40 objective lens. Three slides per goats, two images per slides, and a total of 36 replicates per measurement per group were harvested. In the present study, we used the pre-defined method which was reported in the study of Steerl et al. (2011a) to define the lesion. For the electron microscopy samples, washed tissues were quickly deposited in 2.5% glutaraldehyde (24 h), postfixed in 1% osmium (1 h), and embedded in Epon–Araldite. Semi-thin sections (0.25–0.5 μm) were cut and stained with 1% toluidine blue and 1% sodium borate. As for the ultrathin sections (70–90 nm), samples were cut and stained with uranyl acetate flowed by lead citrate. Transmission electron microscopic examination and imaging were carried out to compare the ultrastructures of the rumen epithelia obtained from different groups.
Rumen tissue RNA extraction and qPCR
Total RNA was extracted using TRIzol (Takara Bio, Otsu, Japan) according to the method described by Chomczynski and Sacchi (1987). The concentration and purity of the extracted RNA were then determined using NanoDrop (ND-1000UV-Vis; Thermo Fisher Scientific, Madison, WI, USA). The absorption ratio (260/280 nm) was determined to be 1.8–2.0, suggesting a high purity. After verifying the RNA integrity, total RNA (1 μg) was reverse-transcribed using PrimeScript® RT reagent kit with gDNA eraser (Takara Bio, Otsu, Japan).
The primers for all cytokines used in the present study were reported by Liu et al. (2013). In addition, Steerle et al. (2011a) reported that glyceraldehyde-3-phosphate dehydrogenase (GAPDH) always displayed a minor variation in the rumen epithelium samples of dairy cows, and the application in the rumen epithelium of goats had also been confirmed (Liu et al. 2013; Liu et al. 2014). Hence, GAPDH was picked and used as the housekeeping gene in our study. All the primers chosen to determine the expression of inflammation cytokines and GAPDH, as listed in Table S2, were synthesized by Invitrogen Life Technologies (Invitrogen, Shanghai, China).
Triplicate PCR reactions were conducted in a volume of 20 μL containing 4-ng complementary DNA (cDNA) in 2× SYBRGreen PCR Master Mix (Takara Bio, Otsu, Japan) and 200 nmol/L of each primer. The reactions were run in ABI 7300 real-time PCR system (Applied Biosystems, Foster, CA, USA) using the following cycling program: 95 °C for 30 s and then 40 cycles of 95 °C for 5 s and 57.5 °C (for GAPDH) or 60 °C (cytokine genes) for 31 s. Gene expressions of cytokine genes were normalized by the GAPDH level, and the data were calculated using the 2−ΔΔCt method.
Epithelial microbial DNA isolation and pyrosequencing
DNA isolation was conducted using 1 g of samples, adopting the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) and the bead-beating method. Immediately after the extraction was completed, DNA was dissolved in Tris–EDTA buffer, and its concentration and quality were determined using the NanoDrop 2000 spectrophotometer (Thermo-Fisher Scientific).
The bacteria 16S ribosomal RNA (rRNA) gene fragments (V3–V4) were amplified using the primer pairs 338F and 806R; the detailed information was as follows: 338F (5′-barcode-ACTCCTRCGGGAGGCAGCAG)-3′) and 806R (5′-GGACTACCVGGGTATCTAAT-3′). PCRs were carried out in triplicate 50-μL reactions, and the cycling programs included 95 °C for 2 min, 25 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s, and 72 °C for 5 min. PCR products were visualized using 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). An indexed sequencing library was constructed using the PCR product from each sample with the TruSeq DNA Sample Prep Kit (Illumina, Inc., San Diego, CA), and the experimental operation was in terms of the manufacturer’s instruction of the kit. The Illumina TruSeq PE Cluster Kit and the TruSeq SBS Kit reagents were used for cluster generation and library sequences, respectively.
The obtained sequences from 12 samples were processed using the QIIME (v. 1.70) software package (Campbell et al. 2010) according to the standard guideline. Quality filtering was performed, and sequences were removed if their value of average quality was smaller than 20, their reads contain ambiguous characters, or their overlapped length is less than 10 bp. The high-quality sequences were then clustered into operational taxonomic units (OTUs) using UPARSE (v. 7.1 http://drive5.com/uparse/), and for chimeric sequences, the UCHIME algorithm (Edgar 2010) was adopted for identification and removal. Representative OTU sequences, which were designated on grounds of the most copious sequences within a cluster, were then assigned to the Greengenes 13.5 (DeSantis et al. 2006) using PyNAST (Caporaso et al. 2010). The alignments were then applied to generate the phylogenetic tree using FastTree (Price et al. 2009) after removing gaps and hypervariable regions using the PH Lane mask provided by QIIME. The taxonomic identity of sequences was conducted using the Ribosomal Database Project (RDP) classifier with a standard minimum support threshold of 80% (Wang et al. 2007). Diversity indices used in the present study, including the ACE, Chao 1, and Shannon index, and the rarefaction curves were performed to compare the community-level bacterial richness and diversity. A principal coordinate analysis (PCoA) based on the unweighted UniFrac distance was conducted to determine the dissimilarity (Lozupone and Knight 2005), and an unweighted distance-based analysis of molecular variance (AMOVA) was performed to evaluate the significance between samples with Mothur v.1.29.0 program (Schloss et al. 2009).
The 16S rRNA sequence information in the present study has been submitted to the NCBI Sequence Read Archive database (SRA; http://www.ncbi.nlm.nih.gov/Traces/sra/) under accession number SRP105260.
Inferred metagenomics by PICRUSt
In the present study, PICRUSt (Langille et al. 2013) analysis was conducted to predict the metagenome of rumen epithelial bacteria in all samples. By the application of PICRUSt, 16S rRNA acts as a marker gene to estimate gene abundance and to calculate the bacterial pathway activity. In the present study, functional prediction was achieved by applying pre-calculated values for genes in the database KEGG (Kanehisa and Goto 2000). Closed-reference OTU picking was performed using the sampled reads against a Greengenes reference taxonomy (Greengenes 13.5) using the pick_closed_reference_OTU.py script in QIIME (Campbell et al. 2010). To reflect the true abundances more accurately, the 16S copy number was firstly normalized; then, the metagenome functions were predicted and the data were exported into KEGG pathways (Langille et al. 2013). The Nearest Sequenced Taxon Index (NSTI, implemented in the PICRUSt) was calculated to determine the accuracy of the predictions. To estimate the similarities among bacterial functions, a principal component analysis (PCA) was also performed by the SIMCA-P (11.5) software (Umetrics, Umea, Sweden).
Statistical analyses
Statistical calculations for the data from the rumen fermentation parameters and epithelial bacteria were conducted using SPSS software (SPSS v. 16, SPSS Inc., Chicago, IL, USA). First, the Shapiro–Wilk test was adopted to check whether the distribution of the variables exhibited a normal distribution. Then, the variables that showed a normal distribution and a non-normal distribution were analyzed by the independent sample t test and the Kruskal–Wallis test, respectively. Significant differences were declared at P < 0.05.
Correlations between rumen pH, LPS, epithelial microbiota, and inflammatory cytokine expression were determined using GraphPad Prism version 5.01 (GraphPad Software, La Jolla, CA), and the Pearson correlation coefficients were calculated. Significant differences were declared at P < 0.05.
Results
The goats were healthy throughout the experiment. The goats’ heartbeat, rectal temperature, and rumen contraction were not significantly affected by HG feeding.
Rumen fermentation parameters
The fermentation parameters of the rumen are listed in Table 1. The rumen pH of the goats was significantly decreased (P < 0.001) by HG feeding. The concentrations of acetate, propionate, butyrate, isovalerate, valerate, and total VFA were greater (P < 0.05) in the HG group compared with the hay group. The levels of lactate and LPS were increased by about twofold after HG feeding (P < 0.05). However, there were no differences in isobutyrate concentration and the ratio of acetate and propionate (P > 0.05).
Rumen epithelial bacterial communities
The rumen epithelial bacterial communities were determined by sequencing 16S rRNA using the Illumina Miseq platform. In total, 778,609 reads were obtained from all samples, of which 684,121 were valid, comprising 87.86% of the raw reads. All of the epithelial bacterial reads were allocated to 21 phyla and 203 genera. At the phylum level, the relative abundances of the phyla Firmicutes and Bacteroidetes were 36.63 and 41.81%, respectively. These two were the main predominant phyla. In addition, Proteobacteria, Spirochaetae, Synergistetes, and Fibrobacteres were the secondary dominant phyla, representing 11.83, 4.20, 2.07, and 1.23%, respectively (Fig. S1). At the genus level, the dominant genera included Prevotella , Butyrivibrio, unclassified Rikenellaceae, unclassified Lachnospiraceae, unclassified Prevotellaceae, and unclassified Bacteroidales (Fig. 1). For visualization, the top 50 genera are displayed in a heat map (Fig. S2).
Alterations in the rumen epithelial bacterial communities
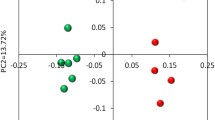
The rarefaction curves of rumen epithelial bacterial communities are displayed in Fig. S3. Results showed that the bacterial diversity in the rumen epithelium was decreased by HG feeding. Results from PCoA (Fig. 2) showed that the samples from the HG-fed group gathered together and clearly separated from the samples of the hay-fed group, indicating that different diet treatments had a significant effect on the rumen epithelial bacterial communities (AMOVA, Fs = 9.136, P < 0.001). Venn diagram analysis was conducted for further understanding of bacterial community alterations. As shown in the Venn profile, the numbers of unique OTUs assigned to the hay-fed group and the HG group were 515 and 168, respectively (Fig. S4). When the OTUs were assigned to the phyla, it was observed that the shared OTUs (878) mainly belonged to Bacteroidetes (39.60%), Firmicutes (35.29%), Proteobacteria (11.69%), and Spirochaetae (4.05%). The unique OTUs in the hay-fed group belonged to Bacteroidetes (1.64%), Firmicutes (1.79%), Proteobacteria (0.21%), and Spirochaetae (0.29%), while the unique OTUs in the HG-fed group mainly belonged to Bacteroidetes (2.79%) and Firmicutes (0.89%).
Estimators of richness and diversity (Table 2) showed that the HG diets significantly decreased the OTU numbers (P = 0.002), ACE (P = 0.002), and Chao 1 (P = 0.001), but there was no difference in the Shannon’s index (P = 0.078). At the genus level, the relative abundances of Prevotella (P = 0.030) and unclassified Clostridiales (P = 0.015) were significantly increased, while there was a corresponding decline in the abundances of Shuttleworthia (P = 0.026), unclassified Ruminococcaceae (P = 0.030), Fibrobacteres (P = 0.002), and unclassified Neisseriaceae (P = 0.006) in rumen epithelial bacterial communities after HG feeding (Table 3).
Metagenomic metabolic functions of rumen epithelial bacteria
Functional predictions were performed using PICRUSt for further understanding of rumen epithelial bacteria. As shown in Fig. S5, 37 gene families were predicted to exist in all samples. The functions of rumen epithelial bacteria were mainly focused on membrane transport, carbohydrate metabolism, replication and repair, amino acid metabolism, energy metabolism, glycan biosynthesis and metabolism, cell motility, lipid metabolism, and enzyme families. After performing a PCA analysis of the KEGG pathways, we observed that the hay and HG samples clustered differently (Fig. 3a). Compared with the hay-fed group, the HG-fed group had a higher relative abundance of gene families related to energy metabolism (P = 0.001); folding, sorting, and degradation (P = 0.030); translation (P = 0.001); metabolic diseases (P = 0.006); and immune system (P = 0.038). At the same time, the HG group had a lower relative abundance of gene families related to the nervous system (P = 0.001) and lipid metabolism (P = 0.011) (Fig. 3b).
Functional diversity of the bacterial microbiota of the rumen epithelium. a Principal component analysis of KEGG pathways encoded in the microbiota of the rumen epithelium in the hay and high-grain (HG) groups. b Influence of HG feeding on the abundance of KEGG pathways of the rumen epithelial microbiota of goats. Only the KEGG pathways that were significantly affected by dietary treatments are presented. Hay hay group, HG high-grain group
Morphology and ultrastructure of the rumen epithelium
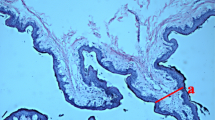
Results from hematoxylin and eosin staining revealed that the rumen epithelium from hay-fed goats showed structural integrity, whereas that from the HG-fed goats showed an obvious peeling phenomenon and severe injury (Fig. 4a, b). When comparing the rumen epithelium ultrastructure, the hay-fed goats displayed cell surface integrity (Fig. 4c), while the HG-fed goats showed irregular edges (Fig. 4d). The integrity of the cell organelles, mitochondria, and intercellular junctions were normal (Fig. 4e) in the hay-fed group. However, in the HG-fed group, numerous vacuoles were present in the cell layers. In addition, we also observed mitochondrial swelling and cellular junction erosion in the HG group (Fig. 4f).
Comparisons of rumen epithelium histology of goats from the hay group and the high-grain (HG) group. Light microscopy cross-section of rumen epithelium samples from the hay-fed group (a; scale bar = 100 μm) and the HG-fed group (b; scale bar = 100 μm). Ultrastructure of the rumen epithelium between the hay-fed goats (c; scale bar = 10 μm) and the HG-fed goats (d; scale bar = 10 μm). Comparison of junctional complex ultrastructures in the rumen epithelium of the hay-fed goats (e; scale bar = 5 μm) and the HG-fed goats (f; scale bar = 5 μm)
Changes in the relative mRNA expression of cytokines
RT-qPCR was conducted to determine the relative messenger RNA (mRNA) expression of inflammatory cytokines. As shown in Fig. 5, compared with the hay diets, IL-1β and IL-6 mRNA expressions were upregulated by 68% (P = 0.008) and 103% (P = 0.001) by the HG diet, respectively. There were no significant differences in the mRNA expressions of IL-2 (P = 0.177), IL-10 (P = 0.283), IL-12 (P = 0.970), IFN-γ (P = 0.261), and TNF-α (P = 0.164) between the hay-fed and HG-fed goats.
Correlation analysis
This study evaluated the correlative relationships among epithelial cytokines, rumen pH, LPS level, and rumen epithelial dominant genera (Fig. 6). Rumen pH correlated negatively with the expressions of IL-1β (r = −6.209; P = 0.031) and IL-6 (r = −0.716; P = 0.009), whereas the LPS level correlated positively with the expression of IL-1β (r = 0.745; P = 0.005). In addition, the abundance of seven taxa [four negative: Desulfovibrio (r = −0.656; P = 0.021), Fibrobacter (r = −0.629; P = 0.028), Comamonas (r = −0.654; P = 0.021), and unclassified Neisseriaceae (r = −0.614; P = 0.034); three positive: Treponema (r = 0.635; P = 0.027), unclassified Succinivibrionaceae (r = 0.785; P = 0.003), and unclassified Erysipelotrichaceae (r = 0.808; P = 0.002)] was correlated with IL-1β expression. The proportion of three genera [two positive: Prevotella (r = 0.614; P = 0.034) and unclassified Clostridiales (r = 0.624; P = 0.030); one negative: unclassified Neisseriaceae (r = −0.655; P = 0.021)] was correlated with the IL-6 expression. Three taxa [Treponema (r = 0.700; P = 0.011), unclassified Succinivibrionaceae (r = 0.608; P = 0.036), and unclassified Erysipelotrichaceae (r = 0.578; P = 0.049)] correlated positively with the IL-2 expression, and Desulfobulbus correlated negatively with the IL-12 expression. No significant correlations (P < 0.05) were found between the abundance of predominant epithelial bacterial populations and IL-10 and TNF-α expressions.
Correlation analyses between the rumen fermentation characteristics and epithelial microbiota (at the genus level) and the levels of epithelial inflammatory cytokine expression. Based on the Pearson correlation coefficients, cells in the figure were filled with different colors. Among them, the red color represented a significant positive correlation (P < 0.05), the blue color represented a significant negative correlation (P < 0.05), and the green color represented a non-significant correlation (P > 0.05) (color figure online)
Discussion
When cattle are fed diets containing a high proportion of cereal grain, the pH of the rumen contents often falls to low levels (pH < 6) (Zebeli et al. 2008). This decreases the efficiency by which feed is converted to VFA and microbial protein for animal production (Zebeli et al. 2008). The drop in pH is often associated with the accumulation of VFA, which can lead to SARA (Krause and Oetzel 2006; Gozho et al. 2006). Therefore, maintaining a high rumen pH (pH > 5.8) is crucial for the prevention of SARA and the maintenance of rumen health (Zebeli et al. 2008). In the present study, the average rumen pH and total VFA level in the hay-fed goats were 6.67 and 68.28 μmol/g, respectively. However, when compared with the hay diets, HG diets resulted in decreased rumen pH (5.68) and increased concentration of total VFA (112.12 μmol/g) and LPS (71,318 EU/g). These results were consistent with previous reports and suggested that the high readily fermented carbohydrates in HG diets led to the rapid accumulation of VFAs and hence a sharp reduction in rumen pH (Krause and Oetzel 2006; Gozho et al. 2006). It has been suggested that rumen LPS is produced by gram-negative bacteria, and the significant correlations between rumen LPS and the amount of gram-negative bacteria have been demonstrated (Khafipour et al. 2009b; Metzler-Zebeli et al. 2013).
Our results revealed a comprehensive structure of rumen epithelial bacteria. At the phylum level, Firmicutes and Bacteroidites dominated, followed by Proteobacteria, Spirochaetae, Synergistetes, and Fibrobacteres. At the genus level, the dominant genera included Butyrivibrio, unclassified Rikenellaceae, unclassified Lachnospiraceae, unclassified Prevotellaceae, and unclassified Bacteroidales. These results confirmed the previous reports that the phyla Firmicutes, Bacteroidites, and Proteobacteria were the core microbiomes of the rumen epimural community (Li et al. 2012). In addition, Firmicutes and Bacteroidites were also the most predominant phyla in the rumen content, but Proteobacteria was proportionally less represented (Sadet-Bourgeteau et al. 2010). These results may imply some differences between the role of rumen content bacteria and rumen epithelial bacteria and support further investigation into the functions of rumen epithelial bacteria.
The normal rumen function is extremely important for ruminants. Rumen bacteria digest the cellulose (hay) and starch (grains) to the fatty acid spectrum (such as acetate, propionate, and butyrate), and the rumen epithelium successively absorbs these energy compounds and then transports and utilizes them as carbon and energy source for life. The structure and composition of rumen bacteria are affected by the host animal’s age, diet, and antibiotic regime, as well as the animal’s care and management (Stewart et al. 1997). Among these factors, diet can be the primary influence on bacterial structure and function (Tajima et al. 2000; Zhou et al. 2010). In the present study, PCoA showed that the composition of the rumen epithelial bacterial community in the HG group was significantly different from the hay group. In addition, we observed a reduction in bacterial richness in goats fed an HG diet with decreased values of ACE and Chao 1. However, the bacterial diversity was not affected in both the hay and HG groups. A previous study reported that rumen epithelial bacteria were relatively more stable than rumen content bacteria (Sadet-Bourgeteau et al. 2010), and this study also showed that there was no significant difference between rumen epithelial bacterial communities from the hay and HG groups. However, Chen et al. (2011) found that when beef cows were fed HG diets, the bacterial diversity in rumen epithelial tissue was altered. This discrepancy may be explained by the fact that the two studies used different experimental designs, the diets contained different components, and most importantly, there may have been different host genetic effects from the animal species used in the experiment (Chen et al. 2011).
At the genus level, the proportions of Prevotella and unclassified Clostridiales in rumen epithelial bacterial communities were increased after HG feeding. However, the proportions of Shuttleworthia, unclassified Ruminococcaceae, Fibrobacter, and unclassified Neisseriaceae were correspondingly decreased. Previous studies showed that the members of Prevotella exhibited high functional diversity, and the fermented substrates were very extensive, including various hemicelluloses, pectin, starch, and protein (Bekele et al. 2010; Purushe et al. 2010). Thus, the increase in the relative abundance of Prevotella in the HG-fed group may be related to higher starch content in an HG diet. Fibrobacter, which is the major cellulose-degrading bacteria in the herbivore gut (Ransom-Jones et al. 2012), is the only genus belonging to the phylum Fibrobacteres. In our study, the decreased proportion of Fibrobacter may be related to the low pH of the rumen contents caused by HG feeding, since most rumen cellulolytic bacteria are pH-sensitive (Hu et al. 2005). Information about the genus Shuttleworthia is limited; therefore, its functions are unknown. Collectively, the altered rumen epithelial bacteria during HG feeding may disrupt the balance between the potentially protective and pathogenic bacteria, which may have detrimental effects on the host.
To further understand the functional roles of rumen epithelial bacteria, PICRUSt was carried out in our study to predict the potential functions of bacteria associated with rumen epithelium. The results of PICRUSt here showed that rumen epithelial bacteria had multiple and diverse functions, especially for membrane transport. The system of membrane transport exists in all living organisms and is important and necessary for the communications with the environment or other tissues, such as importing molecules into cells and exporting waste from the cells (Konishi et al. 2015). Thus, our data demonstrated the previous concept that rumen epithelial bacteria were involved in host–microbial interactions (Chen et al. 2011) and that this process may be achieved by the membrane transport system existing in both the bacteria and the rumen epithelium. Rumen epithelial bacteria supported their growth and reproduction by capturing nutrients and secreted functional proteins or substances (such as virulence and toxins) and then regulated the function of the rumen epithelium (Cheng and Wallace 1979; Petri et al. 2013). Hence, it is not a surprise that our data revealed that rumen epithelial bacteria also participated in some metabolic functions, such as carbohydrate metabolism, amino acid metabolism, energy metabolism, and lipid metabolism. In addition, rumen epithelial bacteria were predicted to have greater capabilities for replication and repair, and this may be due to the rapid turnover rate of rumen epithelial bacteria.
Diets could reshape the rumen epithelial bacterial community, and the functions of rumen epithelial bacteria may be altered along with their changes. In the present study, when compared with the hay-fed group, the relative abundance of gene families related to energy metabolism; folding, sorting, and degradation; translation; metabolic diseases; and the immune system was higher in the HG-fed group, while the relative abundance of gene families related to the nervous system and lipid metabolism was lower. The increased abundance of genes related to energy metabolism suggested that the energy intake ability of the bacterial communities may be enhanced after adapting HG diets. In addition, the abundance of genes related to the immune system was increased after HG diets, indicating that HG diets may affect the immune functions of rumen epithelial bacteria. However, due to the complexity of the bacterial community and the current technical limitations, future research should be conducted to explore and validate the functions of rumen epithelial bacteria.
The rumen epithelium has a multicellular structure (the strata basale, granulosum, spinosum, and corneum layers) and junctional complexes located in the stratum granulosum. It acts as a physical protective barrier between the rumen contents and the host, preventing the translocation of toxic compounds (such as LPS), and also plays an important role in the absorption of nutrients (such as VFAs) (Steele et al. 2011b). In the rumen, rumen epithelial bacteria attach to the epithelial tissue of the rumen wall, so the alteration in its composition may affect the function of the rumen epithelium. In the present study, the results from the ultrastructure revealed that the rumen epithelium from hay-fed goats exhibited the integrity of the cell surface, cell organelles, and the tight intercellular junctions. As expected, the present study showed that HG feeding disrupted the integrity of the cell surface and organelles and resulted in the intercellular junction erosion in the rumen epithelium, and a possible explanation is that the alterations in rumen fermentation and in the composition and function of rumen epithelial bacteria caused by HG feeding affected the integrity and function of the rumen epithelium. Indeed, earlier studies have shown that HG diets can result in rumen epithelial injuries in goats or dairy cows (Steele et al. 2011b; Liu et al. 2013). Therefore, our results were consistent with these earlier reports and further suggested that HG diets could result in damage to rumen epithelial ultrastructure, manifested as cell surface shedding, fracture of the tight junction, and the larger intracellular spaces. Meanwhile, the expressions of inflammatory cytokines IL-1β and IL-6 were upregulated after HG feeding. Under normal physiological conditions, the barrier function of the intact rumen epithelium prevents the paracellular transport of toxic compounds across the epithelium (Steele et al. 2011b). Lines of evidence in human studies suggested that the cytokines IL-1β and IL-6 impaired intestinal barrier function by decreasing tight junction protein expression (Al-Sadi and Ma 2007; Suzuki et al. 2011). If IL-1β and IL-6 have a similar function in the rumen epithelium, they may contribute to increasing the permeability and reducing the rumen epithelium barrier function of goats fed an HG diet.
An observation from this study that is both intriguing and worth pursuing is the consistency of functional changes in the rumen epithelium and the rumen epithelial bacteria. As mentioned above, results of PICRUSt suggested that the abundance of genes related to the immune system was increased in the rumen epithelial bacteria after the HG diets. Correspondingly, the immune responses that occurred in the rumen epithelium, such as a local inflammation and elevated expression of Toll-like receptor genes, have also been reported by previous studies (Chen et al. 2012; Dionissopoulos et al. 2012). Similarly, our results also indicated that the HG-fed goats had a higher relative abundance of genes from the energy metabolism family. This alteration corresponded to the upregulation of energy-demanding or energy-metabolizing events recorded in the rumen epithelium during HG feeding, such as rumen papillae proliferation, enhanced VFA absorption, and increased expression of vH+-ATPase (Shen et al. 2004; Penner et al. 2009; Kuzinski et al. 2012). These findings further demonstrated the close relationship between the rumen epithelial bacteria and the rumen epithelium. We can reasonably speculate that there is a synergistic effect between the functions of rumen epithelial bacteria and the rumen epithelium and also provide more direct evidence about the rumen epithelial bacteria contributing to the alterations in the rumen epithelium during HG feeding. However, without a doubt, the mechanisms behind these host–microbial interactions need further study.
The exact mechanisms responsible for local inflammation are not clear. In this study, our results showed that pH was negatively correlated with the increased mRNA abundances of IL-1β and IL-6, while the concentration of LPS was significantly correlated with IL-1β. These results suggested that rumen pH and LPS level may play an important role in inducing local inflammation. Indeed, in human medical studies, the effects of high acidity and LPS accumulation on intestinal inflammation are well-documented (Mortensen and Clausen 1996; Maes et al. 2008). Thus, the decreased pH and elevated LPS concentration in rumen content from HG-fed goats may contribute to the occurrence of local inflammation, accompanying the secretion of pro-inflammatory cytokines in the rumen epithelium.
In conclusion, feeding goats HG diets resulted in an increase in the concentrations of VFAs and LPS and a decrease in rumen pH. Changes in rumen fermentation characteristics further led to a shift in rumen epithelial bacterial communities, which eventually resulted in rumen epithelial injuries.
References
Al-Sadi RM, Ma TY (2007) IL-1β causes an increase in intestinal epithelial tight junction permeability. J Immunol 178:4641–4649
Bekele AZ, Koike S, Kobayashi Y (2010) Genetic diversity and diet specificity of ruminal Prevotella revealed by 16S rRNA gene-based analysis. FEMS Microbiol Lett 305:49–57
Campbell BJ, Polson SW, Hanson TE, Mack MC, Schuur EA (2010) The effect of nutrient deposition on bacterial communities in Arctic tundra soil. Environ Microbiol 12:1842–1854
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Chen Y, Penner GB, Li M, Oba M, Guan LL (2011) The epithelial tissue associated bacterial diversity changes in the rumen of beef cattle during dietary transition to high grain diets. Appl Environ Microbiol 77:5770–5781
Chen Y, Oba M, Guan LL (2012) Variation of bacterial communities and expression of Toll-like receptor genes in the rumen of steers differing in susceptibility to subacute ruminal acidosis. Vet Microbiol 159:451–459
Cheng KJ, Wallace RJ (1979) The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacteria in the urea flux. Br J Nutr 42:553–557
Chinese Science and Technology Committee (1988) Regulations for the Administration of Affairs Concerning Experimental Animals. Beijing, China
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72:5069–5072
Dionissopoulos L, Steele M, AlZahal O, McBride B (2012) Adaptation to high grain diets proceeds through minimal immune system stimulation and differences in extracellular matrix protein expression in a model of subacute ruminal acidosis in nonlactating dairy cows. Am J Anim Vet Sci 7:84–91
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Fernando SC, Purvis HT, Najar FZ, Sukharnikov LO, Krehbiel CR, Nagaraja TG, Roe BA, DeSilva U (2010) Rumen microbial population dynamics during adaptation to a high-grain diet. Appl Environ Microbiol 76:7482-7490
Gozho GN, Krause DO, Plaizier JC (2006) Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J Dairy Sci 89:4404–4413
Graham C, Simmons NL (2005) Functional organization of the bovine rumen epithelium. Am J Physiol Regul Integr Comp Physiol 288:R173-R181
Hook SE, Steele MA, Northwood KS, Dijkstra J, France J, Wright AD, McBride BW (2011) Impact of subacute ruminal acidosis (SARA) adaptation and recovery on the density and diversity of bacteria in the rumen of dairy cows. FEMS Microbiol Ecol 78:275-284
Hu ZH, Yu HQ, Zhu RF (2005) Influence of particle size and pH on anaerobic degradation of cellulose by ruminal microbes. Int Biodeter Biodegr 55:233–238
Kanehisa M, Goto S (2000) KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28:27–30
Khafipour E, Krause DO, Plaizier JC (2009a) A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J Dairy Sci 92:1060–1070
Khafipour E, Li S, Plaizier JC, Krause DO (2009b) Rumen microbiome composition determined using two nutritional models of subacute ruminal acidosis. Appl Environ Microbiol 75:7115–7124
Kleen JL, Hooijer GA, Rehage J, Noordhuizen JP (2003) Subacute ruminal acidosis (SARA): a review. J Vet Med A 50:406-414
Konishi H, Fujiya M, Kohgo Y (2015) Host–microbe interactions via membrane transport systems. Environ Microbiol 17:931–937
Krause KM, Oetzel GR (2006) Understanding and preventing subacute ruminal acidosis in dairy herds: a review. Anim Feed Sci Tech 126:215–236
Kuzinski J, Zitnan R, Albrecht E, Viergutz T, Schweigel-Röntgen M (2012) Modulation of vH+-ATPase is part of the functional adaptation of sheep rumen epithelium to high-energy diet. Am J Physiol Regul Integr Comp Physiol 303:R909–R920
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotech 31:814–821
Li MJ, Zhou M, Adamowicz E, Basarab JA, Guan LL (2012) Characterization of bovine ruminal epithelial bacterial communities using 16S rRNA sequencing, PCR-DGGE, and qRT-PCR analysis. Vet Microbiol 155:72–80
Liu JH, Xu TT, Liu YJ, Zhu WY, Mao SY (2013) A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am J Physiol Regul Integr Comp Physiol 305:R232–R241
Liu J, Xu T, Zhu W, Mao S (2014) High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br J Nutr 112:416–427
Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Maes M, Kubera M, Leunis JC (2008) The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett 29:117–124
Mani V, Weber TE, Baumgard LH, Gabler NK (2012) Endotoxin, inflammation and intestinal function in livestock. J Anim Sci 90:1452–1465
Mao SY, Zhang G, Zhu WY (2008) Effect of disodium fumarate on ruminal metabolism and rumen bacterial communities as revealed by denaturing gradient gel electrophoresis analysis of 16S ribosomal DNA. Anim Feed Sci Tech 140:293-306
Metzler-Zebeli BU, Schmitz-Esser S, Klevenhusen F, Podstatzky-Lichtenstein L, Wagner M, Zebeli Q (2013) Grain-rich diets differently alter ruminal and colonic abundance of microbial populations and lipopolysaccharide in goats. Anaerobe 20:65–73
Mortensen PB, Clausen MR (1996) Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scand J Gastroenterol Suppl 216:132–148
O’Grady L, Doherty ML, Mulligan FJ (2008) Subacute ruminal acidosis (SARA) in grazing Irish dairy cows. Vet J 176:44–49
Penner GB, Aschenbach JR, Gäbel G, Rackwitz R, Oba M (2009) Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J Nutr 139:1714–1720
Penner GB, Steele MA, Aschenbach JR, McBride BW (2011) Ruminant nutrition symposium: molecular adaptation of ruminal epithelia to highly fermentable diets. J Anim Sci 89:1108-1119
Petri RM, Schwaiger T, Penner GB, Beauchemin KA, Forster RJ, McKinnon JJ, McAllister TA (2013) Changes in the rumen epimural bacterial diversity of beef cattle as affected by diet and induced ruminal acidosis. Appl Environ Microbiol 79:3744-3755
Price MN, Dehal PS, Arkin AP (2009) FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650
Purushe J, Fouts DE, Morrison M, White BA, Mackie RI, Coutinho PM, Nelson KE (2010) Comparative genome analysis of Prevotella ruminicola and Prevotella bryantii: insights into their environmental niche. Microbial Ecol 60:721–729
Ransom-Jones E, Jones DL, McCarthy AJ, McDonald JE (2012) The Fibrobacteres: an important phylum of cellulose-degrading bacteria. Microb Ecol 63:267–281
Sadet S, Martin C, Meunier B, Morgavi DP (2007) PCR-DGGE analysis reveals a distinct diversity in the bacterial population attached to the rumen epithelium. Animal 1:939-944
Sadet-Bourgeteau S, Martin C, Morgavi DP (2010) Bacterial diversity dynamics in rumen epithelium of wethers fed forage and mixed concentrate forage diets. Vet Microbiol 146:98-104
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Shen Z, Seyfert HM, Löhrke B, Schneider F, Zitnan R, Chudy A, Kuhla S, Hammon HM, Blum JW, Martens H, Hagemeister H, Voigt J (2004) An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J Nutr 134:11-17
Steerl MA, Vandervoort G, AlZahal O, Hook SE, Matthews JC, McBride BW (2011a) Rumen epithelial adaptation to high-grain diets involves the coordinated regulation of genes involved in cholesterol homeostasis. Physiol Genomics 43:308–316
Steele MA, Croom J, Kahler M, AlZahal O, Hook SE, Plaizier K, McBride BW (2011b) Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am J Physiol Regul Integr Comp Physiol 300:R1515–R1523
Stewart CS, Flint HJ, Bryant MP (1997) The rumen bacteria. In: Hobson PN, Stewart CS (eds) The rumen microbial ecosystem. Springer Netherlands, Dordrecht, pp 10–72
Suzuki T, Yoshinaga N, Tanabe S (2011) Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J Biol Chem 286:31263–31271
Tajima K, Arai S, Ogata K, Nagamine T, Matsui H, Nakamura M, Aminov RI, Benno Y (2000) Rumen bacterial community transition during adaptation to high-grain diet. Anaerobe 6:273–284
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Ye H, Liu J, Feng P, Zhu W, Mao S (2016) Grain-rich diets altered the colonic fermentation and mucosa-associated bacterial communities and induced mucosal injuries in goats. Sci Rep 6:20329
Zebeli Q, Dijkstra J, Tafaj M, Steingass H, Ametaj BN, Drochner W (2008) Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J Dairy Sci 91:2046-2066
Zhou M, Hernandez-Sanabria E, Guan LL (2010) Characterization of variation in rumen methanogenic communities under different dietary and host feed efficiency conditions, as determined by PCR-denaturing gradient gel electrophoresis analysis. Appl Environ Microbiol 76:3776–3786
Acknowledgements
SM, JL, and RZ designed the study; RZ and HY conducted the study and collected the data; RZ, HY, and SM analyzed the data; JL helped with the manuscript writing; RZ, HY, and SM wrote the paper; and SM had primary responsibility for the final content. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by the Natural Science Foundation of China (31372339) and the Natural Science Foundation of Jiangsu Province of China (BK20151431).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The present study was approved by the Animal Care and Use Committee of Nanjing Agricultural University. All experimental procedures and animal protocol conformed to the Regulations for the Administration of Affairs Concerning Experimental Animals (Chinese Science and Technology Committee, 1988).
Electronic supplementary material
ESM 1
(PDF 665 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Ye, H., Liu, J. et al. High-grain diets altered rumen fermentation and epithelial bacterial community and resulted in rumen epithelial injuries of goats. Appl Microbiol Biotechnol 101, 6981–6992 (2017). https://doi.org/10.1007/s00253-017-8427-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8427-x