Abstract
Nisin, a polycyclic antibacterial peptide produced by Lactococcus lactis, is stable at low pH. Improving the acid tolerance of L. lactis could thus enhance nisin yield. Small non-coding RNAs (sRNAs) play essential roles in acid tolerance by regulating their target mRNAs at the post-transcriptional level. In this study, a novel sRNA, s015, was identified in L. lactis F44 via the use of RNA sequencing, qRT-PCR analysis, and Northern blotting. s015 improved the acid tolerance of L. lactis and boosted nisin yield at low pH. In silico predictions enabled us to construct a library of possible s015 target mRNAs. Statistical analysis and validation suggested that s015 contains a highly conserved region (5′-GAAAAAAAC-3′) that likely encompasses the regulatory core of the sRNA. atpG, busAB, cysD, ilvB, tcsR, ung, yudD, and ywdA were verified as direct targets of s015, and the interactions between s015 and its target genes were elucidated. This work provided new insight into the adaptation mechanism of L. lactis under acid stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria are subjected to various kinds of environmental stresses, including heat/cold shock, oxidative stress, osmotic stress, and low pH conditions (Romeo et al. 2007; Wang et al. 2015, 2016b; Zere et al. 2015). In order to cope with enormous environmental fluctuations, microorganisms have evolved various mechanisms to maintain the intracellular homeostasis. Small non-coding RNAs (sRNAs) play essential roles in regulating the growth and survival via post-transcriptional control of gene expression in both eukaryotic and prokaryotic cells (Wagner and Romby 2015). Furthermore, sRNAs can be induced by environmental changes (Siqueira et al. 2016) and act as crucial regulators for stress responses (Wang et al. 2015, 2016b; Zere et al. 2015) and virulence (Bardill et al. 2011). For example, the sRNAs ArcZ, DsrA, and RprA contribute to acid tolerance in Escherichia coli, and DsrA and RprA are induced under acid stress (Bak et al. 2014). Located between and on the opposite strand of genes encoding two acid response transcriptional regulators called gadX and gadW, the sRNA gadY can form base pairs with the 3′-untranslated region of the gadX mRNA, thereby conferring increased stability and allowing for accumulation of gadX mRNA and increased expression of downstream acid resistance genes (Opdyke et al. 2004). Additionally, in Synechocystis, the expression of the sRNA NsiR4 was induced by nitrogen limitation (Klahn et al. 2015), as was NrsZ (nitrogen-regulated sRNA) (Wenner et al. 2014).
Although small non-coding RNAs represent a very recent discovery, examples of sRNAs in Gram-positive bacteria are still plentiful. In Lactococcus lactis MG1363, a recently published transcriptome landscape revealed novel hypothetical small regulatory RNAs involved in carbon uptake and metabolism. Although analysis indicated some previously undescribed small RNAs that could have a regulatory role in low pH conditions, their specific roles and regulatory mechanisms have not been corroborated (van der Meulen et al. 2016).
In Gram-positive bacteria, sRNAs are known to hybridize with the target mRNAs to inhibit or promote the translation process. The most common sRNAs are trans-encoded sRNAs, which can regulate translation initiation, RNA stability, or protein activity by forming short segments of partial nucleotide complementarity with their target genes. Translation initiation can be inhibited by several different mechanisms depending on the specific location that (a) sRNA pairs directly with the ribosome-binding site (RBS) locus to block the initiation of translation, (b) sRNA induces secondary structural changes in the RBS locus that unfold translation-inhibitory structures or hide the RBS, or (c) sRNA targets downstream of the first five codons in an area where mRNAs are generally sensitive to the antisense inhibition of translation initiation (Storz et al. 2011). Trans-encoded sRNAs have many different target mRNAs, and there is evidence that sRNA-mediated control of translation is prominent in bacteria (Boisset et al. 2007; Chunhua et al. 2012; Huntzinger et al. 2005; Morfeldt et al. 1995).
Some sRNAs are remarkably conserved, indicating that they serve critical cellular functions (Updegrove et al. 2015). In Salmonella, one example is SdsR, which is transcribed by the general stress σ-factor and employs two different regions to interact with individual targets (Frohlich et al. 2016). In Pseudomonas aeruginosa, the sRNA RgsA, which can regulate the mRNA of the global transcriptional regulator Fis and the acyl carrier protein AccP, also possesses a conserved region that acts as a regulatory core of the sRNA (Lu et al. 2016).
As an antimicrobial peptide with 34 residues, nisin is known to show a broad spectrum of antimicrobial activity against gram-positive bacteria as well as gram-negative bacteria (Rayman et al. 1981; Stevens et al. 1991; Xuanyuan et al. 2010). Previous studies have suggested that nisin could show a relatively higher stability and activity in the environment with lower pH value (Zhang et al. 2014). Improving acid tolerance of L. lactis F44 could hence enhance nisin yield (Zhang, et al. 2016).
In this study, we identified the novel sRNA s015 in L. lactis, which was found to be widely conserved across many L. lactis strains. We showed that s015 contributed to the growth and survival of L. lactis F44 subjected to acid stress. In silico analysis of the direct targets of s015 demonstrated that it interacted with its targets at a specific, conserved site. Furthermore, we verified that sRNA s015 directly bound to its targets atpG, busAB, cysD, ilvB, tcsR, ung, yudD, and ywdA by an antisense mechanism. This work revealed a new sRNA s015 that contributes to increased acid tolerance in L. lactis and could serve as a more general model for sRNA-mediated stress responses.
Materials and methods
Bacterial strains and growth conditions
The L. lactis F44 (wild type) strain used in this work was derived from L. lactis YF11 (Zhang et al. 2014, 2016). YF11 is accessible from the China General Microbiological Culture Collection Center under the accession number CGMCC7.52. Escherichia coli BL21 was used for the validation of target genes, and E. coli TG1 was used for plasmid construction. L. lactis was cultured in seed medium (1.5% yeast extract, 1.5% peptone, 2% KH2PO4, 1.5% sucrose, 0.15% NaCl, 0.015% MgSO4·7H2O) at 30 °C. E. coli BL21 and TG1 strains were cultured in LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl) at 37 °C. Micrococcus flavus ATCC 10240 was used for nisin titer assay and cultured in LB medium at 37 °C. The plasmids used in this study are shown in Supplementary Table S1.
RNA extraction and small transcript Northern blots
F44 cells were harvested during mid-log phase (OD600 3.5–4.0) by centrifugation. Total RNA was extracted using the Trizol (Invitrogen, 15596108) procedure according to the manufacturer’s instructions. RNA pellets were dissolved in DEPC-H2O. Northern blotting was performed as described with several modifications (van der Meulen et al. 2016). Briefly, at least 10 μg of total RNA was added to 7 μl RNA loading buffer (Sigma R1386 USA) and heated at 65 °C for 10 min before separation on 15% urea polyacrylamide gels. RNAs were transferred to nylon membranes (Thermo AM10100) and cross-linked at 120 J using a UV cross-linker. Membranes were dried at 80 °C. Pre-hybridization was performed at 42 °C for 30 min. The blots were then hybridized overnight at 42 °C in hybridization buffer (Sigma H7033 USA) containing a single-stranded RNA 5′ biotin-labeled probe. s015 sRNA and 5S RNA were detected by 5′ end-labeled Nbio s015 (5′-AUGGUUUUCUCGAUUCAUUUUU GUCCUUAA-3′) and Nbio 5S (5′-GGCCACUCGCCUAUCUCCCAGGGGGCAACC-3′), respectively. The membranes were washed three subsequent times with SSC wash buffers supplemented with 0.1% SDS (2×, 0.5×, and 0.1×, respectively). Finally, the hybridization signals were visualized by BIO-RAD ChemiDoc XRS.
qRT-PCR
Two micrograms of total RNA extracted from F44 cells as described above was reverse transcribed using the TIANScript RT Kit (TIANGEN) according to the manufacturer’s instructions. The resulting cDNAs were stored at −80 °C until qRT-PCR analysis. qRT-PCR was performed with Power SYBR Green PCR Master Mix (Applied Biosystems). Briefly, a 20 μl reaction solution containing 1–1000 ng of cDNA, 1 μl each of forward and reverse primers (10 mM) (see Supplementary Table S2 for a list of primers used), 10 μl of 2× Ultra SYBR Mixture (with ROX), and 3 μl of sterile water was analyzed on a LightCycler 480 Real-Time PCR System (Roche, Switzerland) according to the manufacturer’s instructions. Reactions were run in triplicate in three independent experiments for each condition. The 16S rRNA gene was used as an internal control to normalize cycle threshold (CT) values. Differences in the relative expression levels were calculated with the 2−(∆∆CT) method (Zhang et al. 2016).
Construction of an sRNA s015 deletion, complementation, and overexpression strains
The homologous double crossover recombination method was used to construct a L. lactis F44 s015 deletion mutant (Δs015). A detailed protocol has been published previously (Zhu et al. 2014).
The sRNA expression plasmid pLEB-sRNA was constructed from the pLEB124 plasmid backbone with some modifications. The p45-promoter-MCS-terminator fragments including EcoRI/BglII restriction sites were amplified using non-template PCR. The EcoRI/BglII-digested PCR fragments were cloned into the EcoRI/BglII-digested pLEB124 plasmid. The sRNA sequences were amplified from L. lactis F44 and cloned into the sRNA expression vector pLEB-sRNA using the homologous recombination method with EasyGeno Kit (TIANGEN). The overexpression and complementation strains (F44-ps015 and F44-cs015, respectively) were obtained by electroporation (2.45 kV) of the pLEB-sRNA-s015 vector into F44 and F44-Δs015, respectively.
The accession number of s015 is KY985350, and the transcriptome sequencing raw data has been submitted to sequence read archive (SRA): SRP105011–PRJNA383925, SRS2139720-sRNA.
Acid tolerance assays
The F44, F44-Δs015, F44-ps015, and F44-cs015 L. lactis strains were incubated for three generations before being used in acid tolerance assays. Bacteria of the youngest generation were grown to early logarithmic phase (OD600 5.0–5.5) at 30 °C. Cells were harvested by centrifugation (8000 r/s, 8 min, 4 °C) and re-suspended in the same volume of 0.9% NaCl. Cells were then exposed to tryptone aqueous solution (2% tryptone, 0.5% NaCl) at different pH levels (2.0, 3.0, 4.0, 5.0, or 7.0) for 2 h. After treatment, cells were diluted and plated on seed medium. Colony-forming units (CFUs) before and after stress treatment were determined by counting colonies after 24 h of incubation. The strain survival was calculated as the ratio of the CFU at the different sampling times normalized to the ratio obtained at pH 7.0.
Cell growth assay and nisin titer
The F44, F44-Δs015, F44-ps015, and F44-cs015 L. lactis strains were incubated for three generations and cultured in fermentation broth (1.5% peptone, 1.5% yeast extract, 1.5% sucrose, 2.0% KH2PO4, 0.15% NaCl, 0.3% corn steep liquor, 0.26% cysteine, and 0.015% MgSO4·7H2O). Optical density (OD) was measured at 600 nm every 2 h with a TU-1810 spectrophotometer to monitor cell growth. The nisin titer assay was performed as described previously (Zhang et al. 2016).
In silico analysis of RNA structures
The mfold web server was used to predict the structures of folded RNAs, including both sRNAs and target mRNAs (Waugh et al. 2002; Zuker 2003; Zuker and Jacobson 1998). Default folding conditions were used except for temperature, which was set to 30 °C.
sRNA target prediction
Target predictions for s015 were obtained using three different online programs: sTarPicker (sRNATarBase) (Wang et al. 2016a), CopraRNA (Comparative prediction algorithm for small RNA targets) (Busch et al. 2008; Wright et al. 2014) and Interacting RNA (IntaRNA) (Pain et al. 2015), and Target RNA 2.0 (Kery et al. 2014). These programs are available at http://ccb.bmi.ac.cn/starpicker/, http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp, and http://cs.wellesley.edu/~btjaden/TargetRNA2/, respectively.
Validation of sRNA targets using reporter fusion
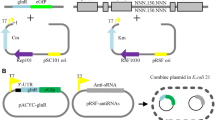
Plasmids for these experiments were constructed as described (Urban and Vogel 2007). Briefly, all sRNA plasmids were constructed from the pRSF-Dute-1 plasmid backbone. First, the s015 sequence and a nonsense control rrfB (gi 49175990) sequence were amplified from the L. lactis F44 and E. coli DH5α genomes, respectively. BamHI and HindIII restriction sites were added to the sequences during amplification. The BamHI/HindIII-digested PCR fragments were cloned into the BamHI/HindIII-digested pRSF-Dute plasmid. For LacZ-target fragment-eGfp fusion cloning, the pACYC Dute-1 plasmid was used as the backbone. First, the residues spanning codons 2–59 of the lacZ gene were amplified. Next, the region spanning from the last 30–40 codons of the upstream C-terminal region to the first 10–15 codons of a potential target gene were fused to the second codon of eGfp. Meanwhile, the two fragments above were fused to construct lacZ-target fragment-eGfp. NcoI and XhoI restriction sites were added to the fusion DNA fragment, which was then digested and cloned into the NcoI/XhoI-digested pACYC plasmid. This plasmid was called pACYC-target (Fig. S1 A). Each pACYC-target plasmid was transformed along with an sRNA plasmid (either s015 or the nonsense control rrfB) into E. coli BL21 (Fig. S1 B).
Results
Identification of s015, a novel small non-coding RNA upregulated under acid stress
To study the role of sRNAs in regulating the response to acid stress in L. lactis F44, the transcriptome at both pH 5.0 and pH 7.0 was sequenced using the Illumina HiSeq 2000 sequencing platform, and transcript levels were compared between the two conditions (sample SRS2139720—sRNA). The RNA-sequencing results revealed that several sRNAs were upregulated under acid stress, including s015, which is located in an intergenic region. To confirm the results of the transcriptome sequencing data, we identified sRNA s015 (KY985350) by Northern blot analysis (Fig. 1a) and qRT-PCR (Fig. 1b), showing that it was upregulated in low pH conditions.
a Northern blot of s015 in L. lactis F44 at pH 7.0 and pH 5.0. Grayscale analysis indicated a 1.42-fold IOD value (pH 5.0, 31,228.207 vs. pH 7.0, 22,011.945). b qRT-PCR of s015 at pH 7.0 and pH 5.0. Values are normalized to pH 7.0. The error bars represent +1 standard deviation. Statistical differences between each group were analyzed using Student’s t test; ***p < 0.0005
High conservation of s015 across L. lactis strains would suggest an important function in this species. We found that the s015 gene is indeed conserved in other 11 related L. lactis strains, and the most highly conserved regions, namely nucleotides 12–33 and 63–108, are shown in Fig. 2.
s015 facilitates L. lactis F44 acid tolerance and nisin production
To explore the function of sRNA s015, we constructed the s015 deletion strain F44-Δs015 as well as an s015 complemented strain (F44-cs015) and an s015 overexpression strain (F44-ps015). As the fermentation process progresses, the bacterial cells suffer increasing levels of acidic stress. In order to evaluate the effects of s015 under varying degrees of acid stress, L. lactis F44, F44-Δs015, F44-ps015, and F44-cs015 were exposed to pH conditions ranging from 5.0 to 2.0 for 2 h. Results indicated that the F44-Δs015 strain displayed a lower survival ratio in acidic conditions compared to the wild type, while the F44-ps015 strain showed a higher survival ratio. The survival ratio of F44-cs015 fluctuated around that of the wild-type strain (Fig. 3a and Table 1). We also compared the growth of the four strains in the fermentation broth and found that the wild-type strain grew faster than the F44-Δs015 strain. However, compared with F44-Δs015 and L. lactis F44, little difference can be observed on the growth of F44-cs015 and F44-ps015, respectively (Fig. 3b). Previous studies have suggested that improving acid tolerance of L. lactis F44 could enhance nisin yield (Zhang et al. 2016). Thus, we suspected that the F44-Δs015 deletion strain might produce less nisin compared to the wild-type one, as it displays reduced acid tolerance. Accordingly, we hypothesized that F44-ps015 would have improved nisin yields due to its increased acid tolerance and that F44-cs015 would have a similar nisin yield to the wild-type F44. To test this, we measured nisin production using a fermentation assay. As expected, compared to the wild type, the F44-Δs015 strain produced less nisin and the F44-ps015 strain had a slightly increased nisin yield. The F44-cs015 strain had a lower nisin yield compared to the wild-type strain (Fig. 3c). Nisin production is generally growth dependent. To account for the impact of biomass on our results, we calculated the nisin yield per unit biomass. Figure 3d shows that the thus-normalized nisin yield of the F44-ps015 strain is the highest, while that of the F44-Δs015 strain is the lowest. No significant difference was observed between the normalized nisin yields of F44 and F44-cs015. Together, these data suggested that s015 plays a crucial role in maintaining the growth of L. lactis F44 and improving its nisin yield in acidic conditions.
Effect of s015 deletion in L. lactis F44. a The survival rate of L. lactis F44, F44-Δs015, F44-ps015, and F44-cs015 at various pH levels compared to survival rate at pH 7.0. The gray bars indicate fold changes in CFU (calculated as means from acid tolerance experiments) relative to pH 7.0. b Growth characteristics of L. lactis F44, F44-Δs015, F44-ps015, and F44-cs015. c Nisin yield of L. lactis F44, F44-Δs015, F44-ps015, and F44-cs015. d The unit nisin production of L. lactis F44, F44-Δs015, F44-ps015, and F44-cs015
Prediction of target mRNAs of s015 in L. lactis F44 via computational analysis
To further characterize sRNA s015 in L. lactis F44, the secondary structure of s015 was analyzed using the mfold web server (Zuker 2003). Four stem-loop structures were predicted, as shown in Fig. 4a. The first stem-loop and the second one are rich of A–U pairs, indicating an unstable region to bind to target mRNAs. Potential targets of s015 were predicted by three software programs: sTarPicker, CopraRNA, and IntaRNA, and Target RNA 2.0; results are shown in Table S3. The target genes identified by each program are summarized in a Venn diagram in Fig. 4b and c. A region located between the first and the second stem-loop structure of s015 was identified as the possible regulatory core. The predicted interaction regions of s015 controlled 74 candidate targets (Fig. 5a–c). Twelve predicted target genes, atpG, busAB, cysD, ilvB, tcsR, ung, yudD, ywdA, yrbI, ftsW, tcsK, and SufS, were selected for further validation.
Validation of s015 target genes by qRT-PCR and reporter fusions
To investigate the effect of s015 on 12 target genes, the transcription levels of these target genes in L. lactis F44 and F44-Δs015 were analyzed by qRT-PCR. As shown in Fig. 6a, at pH 7.0, the mRNA levels of atpG, busAB, and yudD were higher in F44-Δs015 compared to F44, while the mRNA levels of cysD, tcsR, and ung were lower. ilvB and ywdA mRNA levels displayed little difference between two strains. At pH 5.0, the mRNA levels of atpG, busAB, cysD, yudD, and ywdA were higher in F44-Δs015 compared to F44, while the mRNA levels of ilvB, tcsR, and ung were noticeably lower (as shown in Table 2). But the mRNA level of yrbI, ftsW, tcsK, and SufS showed little change in F44-Δs015 compared to F44 at both pH 7.0 and pH 5.0 (data not shown). Taken together, these results suggested that s015 is responsible for the inhibition of atpG, busAB, cysD, yudD, and ywdA as well as upregulation of ilvB, tcsR, and ung at pH 5.0.
a mRNA expression levels of candidate genes in L. lactis F44-Δs015 compared to the wild type at pH 7.0 and 5.0. b eGfp levels of candidate genes and c a negative control as measured using reporter plasmids. Error bars represent the SD. Statistical differences between each group were analyzed using Student’s t test; *p < 0.05, **p < 0.005, ***p < 0.0005
To further assess the effect of s015 on its target genes, we designed fusion constructs containing a lacZ gene fragment in front of the binding sites of each target gene followed by a green fluorescent protein (GFP) driven by a constitutive promoter. The eGfp levels were then assayed in the presence of either a nonsense sRNA or sRNA s015 after co-transforming along with the target gene::eGfp fusion construct into E. coli BL21 (Fig. S1). Compared to the nonsense sRNA, s015 repressed atpG::eGfp, busAB::eGfp, cysD::eGfp, ilvB::eGfp, yudD::eGfp, and ywdA::eGfp by approximately 2.56-fold, 2.50-fold, 2.17-fold, 1.61-fold, 1.61-fold, and 1.67-fold, respectively. In contrast, it increased tcsR::eGfp and ung::eGfp by 1.85-fold and 1.31-fold, respectively (Fig. 6b). A negative control, yidC::eGfp, showed almost no difference in eGfp level between the nonsense control and sRNA s015 (Fig. 6c). Collectively, these results demonstrated that s015 could either inhibit or activate its target genes at the post-transcriptional level.
The conserved region of sRNA s015 directly inhibits/activates target gene expression by an antisense mechanism
Bioinformatics analysis suggested that the predicted targets, atpG, busAB, cysD, ilvB, tcsR, ung, yudD, and ywdA, are direct binding partners of s015 (Fig. 7a–h). To experimentally assess the putative base-pairing interactions, mutations of nucleotides within the predicted interaction regions were introduced. Specifically, s015-mut-1 involved mutations in nucleotides 18–26 (GAAAAAAAC → CTTTTTTTG) (Fig. 7b, c, d, e, g, h), and s015-mut-2 involved mutations in nucleotides 99–104 (TATTCC → ATAAGG) (Fig. 7a). Our experiments showed that both mutated sRNAs failed to regulate target mRNAs in the manner of the wild-type s015 (Table 3). We observed the repression decreased moderately in eGFP levels in the strain with s015-mut-1 compared to wild-type s015 for atpG::eGfp (~1.19-fold for s015-mut-1 vs. ~2.56-fold for wild-type s015, relative to the nonsense control), cysD::eGfp (1.02-fold vs. ~2.17-fold), ilvB::eGfp (~1.22-fold vs. ~1.61-fold), and yudD::eGfp (~1.16-fold vs. ~1.61-fold). The eGfp level of tcsR::eGfp was repressed by 1.25-fold when transformed with s015-mut-1, while it was activated by 1.85-fold when transformed with s015 (Table 3). And ung::eGfp was activated by 1.74-fold for s015-mut-1 while by 1.28-fold for wild-type s015 relative to the nonsense control. The eGfp level of ywdA::eGfp was not significantly different for s015-mut1 compared to the wild-type s015 because the predicted interaction region for this gene was not located in the mutated region (Fig. 8a). Because busAB is the only one predicted to bind to s015-mut-2 region, while the other genes are all predicted to bind to the first region, s015-mut-2 was only applied to busAB. For s015-mut-2, the eGfp level of busAB was repressed ~1.15-fold compared with ~2.50-fold for wild-type sRNA s015 (Fig. 8b). These results verified that s015 regulates many of its targets, atpG, cysD, ilvB, tcsR, ung, and yudD, using the conserved regions we identified.
Discussion
In this study, we demonstrated that the novel sRNA s015 plays a critical role in the response to acid stress in L. lactis and employs an unstable region to regulate target genes involved in multiple pathways. Here, sRNA s015 was identified as a novel trans-encoded sRNA in L. lactis F44, and its homologs are conserved in other 11 L. lactis strains.
In L. lactis F44, it has been shown that nisin yield can be enhanced by improving acid tolerance (Zhang et al. 2016). Here, we showed that s015 was highly transcribed under acid stress. The growth and relative survival in acidic conditions of the F44-Δs015, F44-ps015, and F44-cs015 strains compared to the wild-type L. lactis F44 strain indicate that s015 can improve acid tolerance in L. Lactis F44. Accordingly, one unit biomass of the s015 overexpression strain L. lactis F44-ps015 produced a higher yield of nisin compared to the same biomass of the wild-type F44, F44-cs015, and F44-Δs015 strains. We could thus infer that s015 enhanced nisin yield by improving the acid tolerance of L. lactis F44.
The secondary structure of sRNAs determines their target specificity. Specific regions are responsible for maintaining stability and binding to the RBS and/or coding sequence (CDS) of target mRNAs. Bioinformatics and statistical analysis of predicted s015 target genes revealed that s015 employs a conserved region to interact with individual targets. In Salmonella, two conserved regions of SdsR, one located in the distal sequence of the first stem-loop and the other located downstream of an RNaseE-dependent cleavage site in the center of sRNA SdsR molecule, could regulate target genes (Frohlich et al. 2016). In P. aeruginosa, sRNA RgsA also possess a conserved region that acts as the regulatory core of the sRNA to inhibit its targets (Lu et al. 2016). In this vein, our work indicated that s015 has a highly conserved single-stranded region (18–26, GAAAAAAAC) that was able to bind to several target mRNAs, as this region is not stable and can easily cross-link with specific target mRNAs, including atpG, cysD, ilvB, tcsR, ung, yudD. In contrast, the gene ywdA was not predicted to interact at the conserved single-stranded region, and accordingly, we showed that the s015-mut-1 effected no change in eGfp levels compared to the wild-type s015. From our data, we inferred that the region of s015 encompassing nucleotides 18–26 (GAAAAAAAC) is the interaction region responsible for the regulation of many specific target genes (Fig. 9).
Targeting deeper into the CDS (i.e., downstream of the first five codons in an area where mRNAs are generally sensitive to antisense inhibition of translation initiation) has been reported as a common mechanism of sRNA-mediated regulation (Frohlich et al. 2012). In this work, the region of s015 encompassing nucleotides 10–32 was shown to bind to the deeper CDS (codons 5–13) of atpG, and the eGfp validation experiment showed that atpG was repressed by s015. These results suggested that s015 might repress the expression of atpG through the translation blockage as occurs in P. aeruginosa with regulation of fis by the sRNA RgsA (Lu et al. 2016).
Base-pairing between an sRNA and its target mRNA usually leads to mRNA degradation, repression of translation, or both (Storz et al. 2011; Waters and Storz 2009). A wealth of sRNAs bind to the RBS of their target genes to repress translation and decrease mRNA levels. For example, in P. aeruginosa, RgsA interacts with a region within the acpP RBS to form a “kissing complex” (Lu et al. 2016). Here, we found that s015 could bind around the RBS of busAB, cysD, and ywdA, leading to the repression of transcript levels through translational inhibition. In Enterobacteria, the MicC sRNA binds to the coding sequence of the ompD mRNA and directly promotes its degradation (Pfeiffer et al. 2009). Here, yudD mRNA was degraded due to secondary structure changes induced by binding of s015. The 5′ UTR of target mRNAs may fold into a hairpin that sequesters the RBS into a double-stranded secondary structure, inhibiting translation initiation. The sRNAs RprA, DsrA, and ArcZ can bind to a specific site within the rpoS 5′ UTR and sequester sequences that would otherwise participate in forming the translation-inhibitory structure, thereby relieving translational inhibition (Mika and Hengge 2014; Soper and Woodson 2008). In a similar vein, our data suggested that s015 could relieve the RBS blocks of tcsR and ung mRNAs by binding to the base-pair sequence that formed a double-stranded hairpin. For ilvB, validation by qRT-PCR and reporter plasmids yielded conflicting results. Further study indicated that a possible binding site of ilvB was located upstream of the start codon. This might indicate the competition between the two binding targets and needs to be investigated further.
Located in intergenic regions and shown to act on targets elsewhere in the genome, trans-encoded sRNAs control multiple target mRNAs via imperfect base-pairing, and there is evidence that this mechanism is prominent in bacteria (Boisset et al. 2007; Chunhua et al. 2012). The sRNA s015 is present in the intergenic region between two ORFs, as is shown in Fig. S3. Due to the distance between s015 and its flanking ORFs, it is likely that it does not interact directly with these ORFs; rather, it is more plausible that its targets are located elsewhere in the genome.
To summarize, as a non-coding regulator, s015 assisted L. lactis F44 in surviving acidic conditions. Although not all predicted s015 target candidates were validated in this work, we have identified several target genes (atpG, busAB, cysD, ilvB, tcsR, ung, yudD, and ywdA) and characterized their interactions with s015. Taken together, our data indicate that the sRNA s015 has vital roles in regulating genes involved in responding to acid stress. Our study could assist us better understand how L. lactis responds to various environmental stress conditions. These findings also enhance our knowledge of the mechanisms that s015 directly inhibit or activate target mRNAs.
References
Bak G, Han K, Kim D, Lee Y (2014) Roles of rpoS-activating small RNAs in pathways leading to acid resistance of Escherichia coli. Microbiology 3:15–28. doi:10.1002/mbo3.143
Bardill JP, Zhao X, Hammer BK (2011) The Vibrio cholerae quorum sensing response is mediated by Hfq-dependent sRNA/mRNA base pairing interactions. Mol Microbiol 80:1381–1394. doi:10.1111/j.1365-2958.2011.07655.x
Boisset S, Geissmann T, Huntzinger E, Fechter P, Bendridi N, Possedko M, Chevalier C, Helfer AC, Benito Y, Jacquier A, Gaspin C, Vandenesch F, Romby P (2007) Staphylococcus aureus RNAIII coordinately represses the synthesis of virulence factors and the transcription regulator rot by an antisense mechanism. Genes Dev 21:1353–1366. doi:10.1101/gad.423507
Busch A, Richter AS, Backofen R (2008) IntaRNA: efficient prediction of bacterial sRNA targets incorporating target site accessibility and seed regions. Bioinformatics 24:2849–2856. doi:10.1093/bioinformatics/btn544
Chunhua M, Yu L, Yaping G, Jie D, Qiang L, Xiaorong T, Guang Y (2012) The expression of LytM is down-regulated by RNAIII in Staphylococcus aureus. J Basic Microbiol 52:636–641. doi:10.1002/jobm.201100426
Frohlich KS, Haneke K, Papenfort K, Vogel J (2016) The target spectrum of SdsR small RNA in Salmonella. Nucleic Acids Res. doi:10.1093/nar/gkw632
Frohlich KS, Papenfort K, Berger AA, Vogel J (2012) A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD. Nucleic Acids Res 40:3623–3640. doi:10.1093/nar/gkr1156
Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, Namane A, Lina G, Etienne J, Ehresmann B, Ehresmann C, Jacquier A, Vandenesch F, Romby P (2005) Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. EMBO J 24:824–835. doi:10.1038/sj.emboj.7600572
Kery MB, Feldman M, Livny J, Tjaden B (2014) TargetRNA2: identifying targets of small regulatory RNAs in bacteria. Nucleic Acids Res 42:W124–W129. doi:10.1093/nar/gku317
Klahn S, Schaal C, Georg J, Baumgartner D, Knippen G, Hagemann M, Muro-Pastor AM, Hess WR (2015) The sRNA NsiR4 is involved in nitrogen assimilation control in cyanobacteria by targeting glutamine synthetase inactivating factor IF7. Proc Natl Acad Sci U S A 112:E6243–E6252. doi:10.1073/pnas.1508412112
Lu P, Wang Y, Zhang Y, Hu Y, Thompson KM, Chen S (2016) RpoS-dependent sRNA RgsA regulates Fis and AcpP in Pseudomonas aeruginosa. Mol Microbiol 102:244–259. doi:10.1111/mmi.13458
Mika F, Hengge R (2014) Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol 11:494–507. doi:10.4161/rna.28867
Morfeldt E, Taylor D, von Gabain A, Arvidson S (1995) Activation of alpha-toxin translation in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J 14:4569–4577
Opdyke JA, Kang JG, Storz G (2004) GadY, a small-RNA regulator of acid response genes in Escherichia coli. J Bacteriol 186:6698–6705. doi:10.1128/jb.186.20.6698-6705.2004
Pain A, Ott A, Amine H, Rochat T, Bouloc P, Gautheret D (2015) An assessment of bacterial small RNA target prediction programs. RNA Biol 12:509–513. doi:10.1080/15476286.2015.1020269
Pfeiffer V, Papenfort K, Lucchini S, Hinton JC, Vogel J (2009) Coding sequence targeting by MicC RNA reveals bacterial mRNA silencing downstream of translational initiation. Nat Struct Mol Biol 16:840–846. doi:10.1038/nsmb.1631
Rayman MK, Aris B, Hurst A (1981) Nisin: a possible alternative or adjunct to nitrite in the preservation of meats. Appl Environ Microbiol 41:375–380
Romeo Y, Bouvier J, Gutierrez C (2007) Osmotic regulation of transcription in Lactococcus lactis: ionic strength-dependent binding of the BusR repressor to the busA promoter. FEBS Lett 581:3387–3390. doi:10.1016/j.febslet.2007.06.037
Siqueira FM, de Morais GL, Higashi S, Beier LS, Breyer GM, de Sa Godinho CP, Sagot MF, Schrank IS, Zaha A, de Vasconcelos AT (2016) Mycoplasma non-coding RNA: identification of small RNAs and targets. BMC Genomics 17:743. doi:10.1186/s12864-016-3061-z
Soper TJ, Woodson SA (2008) The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA 14:1907–1917. doi:10.1261/rna.1110608
Stevens KA, Sheldon BW, Klapes NA, Klaenhammer TR (1991) Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol 57:3613–3615
Storz G, Vogel J, Wassarman KM (2011) Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891. doi:10.1016/j.molcel.2011.08.022
Updegrove TB, Shabalina SA, Storz G (2015) How do base-pairing small RNAs evolve? FEMS Microbiol Rev 39:379–391. doi:10.1093/femsre/fuv014
Urban JH, Vogel J (2007) Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res 35:1018–1037. doi:10.1093/nar/gkl1040
van der Meulen SB, de Jong A, Kok J (2016) Transcriptome landscape of Lactococcus lactis reveals many novel RNAs including a small regulatory RNA involved in carbon uptake and metabolism. RNA Biol 13:353–366. doi:10.1080/15476286.2016.1146855
Wagner EG, Romby P (2015) Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv Genet 90:133–208. doi:10.1016/bs.adgen.2015.05.001
Wang J, Liu T, Zhao B, Lu Q, Wang Z, Cao Y, Li W (2016a) sRNATarBase 3.0: an updated database for sRNA-target interactions in bacteria. Nucleic Acids Res 44:D248–D253. doi:10.1093/nar/gkv1127
Wang L, Wang W, Li F, Zhang J, Wu J, Gong Q, Shi Y (2015) Structural insights into the recognition of the internal A-rich linker from OxyS sRNA by Escherichia coli Hfq. Nucleic Acids Res 43:2400–2411. doi:10.1093/nar/gkv072
Wang L, Yang G, Qi L, Li X, Jia L, Xie J, Qiu S, Li P, Hao R, Wu Z, Du X, Li W, Song H (2016b) A novel small RNA regulates tolerance and virulence in Shigella flexneri by responding to acidic environmental changes. Front Cell Infect Microbiol 6:24. doi:10.3389/fcimb.2016.00024
Waters LS, Storz G (2009) Regulatory RNAs in bacteria. Cell 136:615–628. doi:10.1016/j.cell.2009.01.043
Waugh A, Gendron P, Altman R, Brown JW, Case D, Gautheret D, Harvey SC, Leontis N, Westbrook J, Westhof E, Zuker M, Major F (2002) RNAML: a standard syntax for exchanging RNA information. RNA 8:707–717
Wenner N, Maes A, Cotado-Sampayo M, Lapouge K (2014) NrsZ: a novel, processed, nitrogen-dependent, small non-coding RNA that regulates Pseudomonas aeruginosa PAO1 virulence. Environ Microbiol 16:1053–1068. doi:10.1111/1462-2920.12272
Wright PR, Georg J, Mann M, Sorescu DA, Richter AS, Lott S, Kleinkauf R, Hess WR, Backofen R (2014) CopraRNA and IntaRNA: predicting small RNA targets, networks and interaction domains. Nucleic Acids Res 42:W119–W123. doi:10.1093/nar/gku359
Xuanyuan Z, Wu Z, Li R, Jiang D, Su J, Xu H, Bai Y, Zhang X, Saris PE, Qiao M (2010) Loss of IrpT function in Lactococcus lactis subsp. lactis N8 results in increased nisin resistance. Curr Microbiol 61:329–334. doi:10.1007/s00284-010-9615-4
Zere TR, Vakulskas CA, Leng Y, Pannuri A, Potts AH, Dias R, Tang D, Kolaczkowski B, Georgellis D, Ahmer BM, Romeo T (2015) Genomic targets and features of BarA-UvrY (−SirA) signal transduction systems. PLoS One 10:e0145035. doi:10.1371/journal.pone.0145035
Zhang J, Caiyin Q, Feng W, Zhao X, Qiao B, Zhao G, Qiao J (2016) Enhance nisin yield via improving acid-tolerant capability of Lactococcus lactis F44. Sci Rep 6:27973. doi:10.1038/srep27973
Zhang YF, Liu SY, Du YH, Feng WJ, Liu JH, Qiao JJ (2014) Genome shuffling of Lactococcus lactis subspecies lactis YF11 for improving nisin Z production and comparative analysis. J Dairy Sci 97:2528–2541. doi:10.3168/jds.2013-7238
Zhu D, Zhao K, Xu H, Zhang X, Bai Y, Saris PEJ, Qiao M (2014) Construction of thyA deficient Lactococcus lactis using the Cre-loxP recombination system. Ann Microbiol 65:1659–1665. doi:10.1007/s13213-014-1005-x
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Zuker M, Jacobson AB (1998) Using reliability information to annotate RNA secondary structures. RNA 4:669–679
Acknowledgments
This project was financially supported by the National Key Technology Support Program (2015BAD16B04), the National Natural Science Foundation of China (31570049, 32570089), and the Funds for Creative Research Groups of China (21621004). J.Q. was supported by The New Century Outstanding Talent Support Program, Education Ministry of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing financial interests
The authors declare no competing financial interests.
Ethical approval
This work does not include any studies with human participants or animals.
Electronic supplementary material
ESM 1
(PDF 349 kb).
Rights and permissions
About this article
Cite this article
Qi, J., Caiyin, Q., Wu, H. et al. The novel sRNA s015 improves nisin yield by increasing acid tolerance of Lactococcus lactis F44. Appl Microbiol Biotechnol 101, 6483–6493 (2017). https://doi.org/10.1007/s00253-017-8399-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8399-x