Abstract
Increasing evidence has revealed a close interplay between the gut bacterial communities and host growth performance. However, until recently, studies generally ignored the contribution of eukaryotes, endobiotic organisms. To fill this gap, we used Illumina sequencing technology on eukaryotic 18S rRNA gene to compare the structures of gut eukaryotic communities among cohabitating retarded, overgrown, and normal shrimp obtained from identically managed ponds. Results showed that a significant difference between gut eukaryotic communities differed significantly between water and intestine and among three shrimp categories. Structural equation modeling revealed that changes in the gut eukaryotic community were positively related to digestive enzyme activities, which in turn influenced shrimp growth performance (λ = 0.97, P < 0.001). Overgrown shrimp exhibited a more complex and cooperative gut eukaryotic interspecies interaction than retarded and normal shrimp, which may facilitate their nutrient acquisition efficiency. Notably, the distribution of dominant eukaryotic genera and shifts in keystone species were closely concordant with shrimp growth performance. In summary, this study provides an integrated overview on direct roles of gut eukaryotic communities in shrimp growth performance instead of well-studied bacterial assembly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulating evidence has shown that gut microbiota plays significant roles in host physiology and metabolism; such roles include promoting nutrient digestibility, efficient harvest of energy, and acting as barrier against pathogen invasion (Flint et al. 2012; Krajmalnik-Brown et al. 2012; Xiong et al. 2016). Therefore, dysbiosis in the gut microbiota generally causes host metabolic syndrome and disease (Krajmalnik-Brown et al. 2012; Xiong et al. 2017a). In line with this condition, an imbalance of gut microbiota is closely associated with host obesity, which can be attributed to diet (De Lartigue et al. 2011) and host genetics (Ussar et al. 2015). However, recent studies that ruled out effects of these variables showed that cohabitating pigs (Ramayo-Caldas et al. 2016), sea cucumbers (Sha et al. 2016), and shrimps (Tacon et al. 2002; Zhu et al. 2016) exhibit significant differences in body weights, and these differences are consistent with distinct gut microbiota. This apparent discrepancy raises concern regarding factors contributing to variations in gut microbiota of cohabitating individuals. One possible explanation is that gut enterotype-like clusters contribute to differences in host growth traits (Ramayo-Caldas et al. 2016). A similar pattern was observed in human gut microbiota (Arumugam et al. 2011). However, this conception is questioned by the data that gut microbiome supports continuous gradients of dominant taxa rather than discrete enterotypes (Knights et al. 2014). Alternatively, microbial assembly, which affects host growth performance, can be attributed to biotic factors, such as interspecies interaction (Zhu et al. 2016). Indeed, interactions among taxa are essential drivers of ecological community structure and dynamics (Newell and Douglas 2013; Zhu et al. 2016). Recently, studies have shown a more complex gut bacterial interspecies interaction in overgrown shrimp (Zhu et al. 2016) and obese children (Riva et al. 2017) compared with corresponding normal subjects. However, whether this relationship also holds true for eukaryotes remains uncertain.
Currently, the relationship between host growth performance and its gut microbiota gained increasing attention (Pataky et al. 2009; Ussar et al. 2015; Zhu et al. 2016); however, existing literature focused mostly on bacteria. This gap in knowledge should be addressed, considering that members of gut microbiota harbor bacteria and eukaryotes (Parfrey et al. 2011). Biological and ecological performances also differ between eukaryotic and prokaryotic microbiota due to variations in their cell organization. Specifically, eukaryotes exhibit poorer metabolic versatility compared with their prokaryotic counterparts. However, eukaryotes can exploit diverse predatory life strategies. In addition, eukaryote assemblages are less resilient to environmental selection, including host filtering (Massana and Logares 2013). Microbial eukaryotes were traditionally referred to as parasites (Andersen et al. 2013). However, current studies have reported that the absence of Blastocystis and Dientamoeba in human gut microbiota is associated with active inflammatory bowel disease (Yakoob et al. 2010). Similarly, overgrowth of Candida spp. was found to inhibit Clostridium difficile infection (Manian and Bryant 2013). Given these reports, the role of host-associated eukaryotes can be pathogenic, commensal, or beneficial (Andersen et al. 2013; Lukeš et al. 2015). Hence, understanding how gut eukaryotic microbiota affects host growth performance is of significant interest.
Considering limited information on the relationship between gut eukaryotic microbiota and host growth performance, we characterized eukaryotic communities among three categories (retarded, overgrown, and normal subjects) of cohabitating shrimp. Shrimp samples were collected from identically managed ponds to control differences in abiotic factors, such as rearing conditions, diet, and host developmental stage, affecting assembly of gut microbiota. This design allowed us to (i) determine the relationship among gut eukaryotic community, digestive activities, and shrimp growth performance; (ii) screen dominant genera that contribute differences among retarded, normal, and overgrown shrimp categories; and (iii) evaluate to what extent that the interspecies interaction of gut eukaryotic microbiota was associated with shrimp growth performance.
Materials and methods
Experimental design and sample collection
In this study, investigated shrimp ponds are located in Xiangshan, Ningbo in Eastern China (29° 29′ 11.37″ N 121° 48′ 27.30″ E). There are six cement bottom ponds which are uniform in size (3000 m2) and depth (1.2 m). To maintain a suitable dissolved oxygen level, these greenhouse ponds were aerated at the bottom (Supplementary Fig. S1). Ponds were identically managed with disinfectant seawater inputs (salinity 25‰), 5% daily water exchange, feed type, and feed schedule. For subsequent standardization management, farmers adjusted water parameters, such as pH and salinity, before shrimp inoculation. On 8 April 2016, congeneric postlarvae of Litopenaeus vannamei were inoculated to each pond with stocking density of 480,000 shrimp. After 70 days of cultivation, adult shrimp exhibited distinct body size and weight. Shrimp samples were collected from each pond and were separately stored and aerated in tanks with water from their original pond during transportation. In addition, to evaluate the relationship of microbial between the gut and rearing water, we also collected water samples from the corresponding ponds. The samples were transported to the laboratory within 2 h.
On sampling day, shrimp from each pond were sorted into three groups according to body size: retarded, normal, and overgrown shrimp. Specifically, the size that accounted for the most proportion was artificially categorized into normal shrimp (Supplementary Table S1). Expected size at this growth stage was confirmed by farmers. Subsequently, we categorized sizes that were markedly smaller or larger than normal shrimp as retarded or overgrown individuals, respectively (Supplementary Table S1). To minimize effects of detecting surface-associated microbes on gut microbiota, shrimp were wiped with alcohol-soaked cotton and washed twice with cold distilled water before isolation of intestines. Intestines from every three shrimp from each size category and pond were dissected on ice and pooled to compose one biological sample.
Measurement of digestive enzyme activity
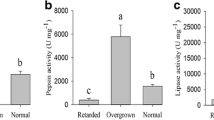
Stomach and hepatopancreas from every three shrimp (the same shrimp for intestine collection) were immediately extracted and pooled. Tissues were homogenized with four volumes (weight/volume) of ice-cold distilled water and centrifuged at 6000 rpm for 10 min at 4 °C. Then, the supernatant was collected to measure digestive enzyme activities, and protein content of the supernatant was determined using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL, USA). Digestive enzyme activities, including amylase, pepsin, and lipase enzymes, were analyzed according to commercial kit protocols (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). In brief, amylase and pepsin were determined using iodine-starch colorimetric method and casein hydrolysis, respectively. Assay of lipase represented colorimetric estimation of p-nitrophenol released due to enzymatic hydrolysis of p-nitrophenyl palmitate. Enzyme activities were expressed as a relative unit per milligram of soluble protein (U mg−1).
DNA extraction, eukaryotic 18S rRNA gene amplification, and sequencing
Approximately 1 L of rearing water sample for each pond was sequentially prefiltered through nylon mesh (100 μm pore size) and then filtered on 0.22 μm polycarbonate membrane (Type GTTP 2500, Millipore) (Fisher Scientific, Fair Lawn, NJ, USA) to collect the planktonic microbial biomass. Genomic DNA (gDNA) of water and shrimp intestine was extracted using the FAST DNA Spin kit (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s protocols. The extracts were quantified using a NanoDrop ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, USA) and then immediately frozen at −80 °C prior to amplification.
The eukaryotic V2–V3 regions of 18S rRNA gene were amplified using a primer set: 18S–F82: GAAACTGCGAATGGCTC and Euk-R516: ACCAGACTTGCCC-TCC (Voudanta et al. 2016). An aliquot of 100 ng purified DNA was used as the template from each sample for amplification. To minimize polymerase chain reaction (PCR)-induced biases, each sample was amplified in triplicate (50 μL reaction system) under the following conditions: predenaturation at 95 °C for 3 min, 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s, with a final extension at 72 °C for 5 min and hold at 4 °C. A second PCR (8 cycles) was performed to link sample with specific tag sequences with the same reaction conditions as described above. PCR products for each sample were pooled and purified using a QIAquick PCR purification kit (Qiagen, GmbH, Hilden, Germany). The amplicon products were sequenced using an Illumina MiSeq platform (Illumina, San Diego, CA, USA) after multiple sample libraries for quantitation and mixture.
Processing of Illumina sequencing data
The paired-end reads were joined with FLASH (Magoč and Salzberg 2011), then were processed using the Quantitative Insights Into Microbial Ecology (QIIME v1.9.0) pipeline (Caporaso et al. 2010a). In brief, the sequences were quality filtered on the basis of quality score, sequence length, ambiguous sequence, and primer mismatch thresholds in QIIME. Briefly, chimeras were detected using UCHIME (Edgar et al. 2011). The eukaryotic phylotypes were identified using UCLUST (Edgar 2010) and binned into operational taxonomic units (OTUs, 97% similarity level) for diversity estimators. The most abundant sequence in a given OTU was selected as the representative sequence and was aligned using PyNAST (Caporaso et al. 2010b). The taxonomic identity of each phylotype was determined using the Silva database (release 119) taxonomy (Quast et al. 2013). After taxonomies have been assigned, OTUs unaffiliated with the domain of eukaryotes and singletons were removed from the dataset prior to subsequent analysis. To allow comparison of diversity without bias from unequal sequencing effort, we used a randomly selected subset of 19,350 sequences (corresponding to integral number of the smallest sequencing effort for any of the samples) from each sample in the QIIME pipeline to calculate the diversities and dissimilarities among samples. The raw sequences data generated in this study were deposited in the DDBJ (http://www.ddbj.nig.ac.jp/) Sequence Read Archive under the accession number DRA005322.
Statistical analysis
A one-way analysis of variance (ANOVA) was used to test the significances in body weight, digestive enzyme activity, and eukaryotic diversity among the three shrimp categories (Churchill 2004). Nonmetric multidimensional scaling (NMDS) and analysis of similarity (ANOSIM) were performed to evaluate the overall differences in water and shrimp gut eukaryotic communities based on the Bray–Curtis distance metrics (Clarke 1993). SIMPER (similarity percentage) was performed to determine which OTU drives the differences in eukaryotic microbiota among the three shrimp categories (Clarke 1993). A Mantel test was employed to evaluate the association between gut eukaryotic community and growth traits, including body sizes and weight, and digestive enzyme activities. Subsequently, a structural equation modeling (SEM) was used to illustrate the interplay among the gut eukaryotic community, enzyme activity, and shrimp body weight/size in Amos 21.0 (IBM, Chicago, IL, USA). We established the SEM to validate: (i) whether the gut eukaryotic community directly influenced shrimp growth performance and (ii) whether the gut eukaryotic community led to changes in enzyme activity, which in turn affects shrimp growth performance. The data matrix was fitted to the model using the maximum-likelihood estimation method. A favorable model fit was suggested by nonsignificant χ 2 test (P > 0.05), high goodness-of-fit index (GFI > 0.90), root of mean square residual (RMR < 0.1), and Tucker-Lewis index (TLI > 0.90). A ternary plot was applied to reveal the distribution of the dominant genera (>1% at least in one sample) among the normal, retarded, and overgrown shrimp categories using the package “ggtern” in R V3.0.2 (R Core Team 2013). Further, the correlations between digestive enzyme activities (including amylase, lipase, and pepsin), body size/weight, and the dominant eukaryotic genera among three categories were evaluated by Pearson correlation analysis. These Pearson correlations were visualized in a heatmap. Interspecies interaction was evaluated using an open-accessible pipeline (http://ieg2.ou.edu/MENA) (Deng et al. 2012). To quantitatively compare the differences in gut interspecies interaction among the three shrimp categories, a set of topology properties were calculated, including the average path length, clustering coefficient, modularity, and positive co-occurrences (Deng et al. 2012; Mej 2003). The network was visualized in Cytoscape 3.1.1 (Shannon et al. 2003).
Results
Shrimp categories and digestive enzyme activity
Average body sizes of retarded, normal, and overgrown shrimp were 4.50 ± 0.19 (mean ± standard deviation), 6.47 ± 0.09, and 8.27 ± 0.39 cm, and corresponding weights totaled 3.62 ± 0.48, 12.15 ± 1.29, and 24.07 ± 3.07 g, respectively. Shrimp body size, body weight, and average daily growth significantly differed among the three shrimp categories (P < 0.001 in all cases) (Supplementary Table S1). Digestion activities of amylase, pepsin, and lipase in stomach and hepatopancreas were the highest in overgrown shrimp and were the lowest in retarded shrimp (Supplementary Table S2). Remarkably, activities of amylase, pepsin, and lipase of stomach and hepatopancreas were positively related to shrimp body size and weight (Pearson correlations r > 0.81, P < 0.001 in all cases) (Table 1).
Distribution of taxa and phylotypes
After quality control, we obtained a total of 930,378 high-quality sequences with an average of 38,766 ± 2942 (mean ± standard deviation) reads per sample. The rarefaction curve for each group was not saturated (Supplementary Fig. S2), suggesting high gut eukaryotic diversity. Thus, increased sequencing depth is needed to explore eukaryotic diversity in future works. After rarefaction to equal sequencing depth, 4400 OTUs were observed across all samples. Water eukaryotic diversity was higher at each taxonomical level than in shrimp gut (Table 2). Dominant classes (relative abundance > 1% at least in one sample) were Chloroplastida (mean ± standard deviation, 83.9 ± 6.2%), followed by Stramenopiles (6.5 ± 4.1%), Rhodophyceae (5.2 ± 2.6%), Alveolata (2.9 ± 1.9%), and Holozoa (1.0 ± 0.4%). High standard deviations indicate profound alterations in eukaryotic assemblages among retarded, normal, and overgrown shrimp categories. Significant differences were found in relative abundances of dominant classes between water and intestines and among the three shrimp categories (Supplementary Fig. S3A). For example, relative abundance of Chloroplastida was significantly lower in water than in shrimp intestines, whereas that of Rhodophyceae exhibited the opposite pattern. In general, the relative abundances of these dominant classes were substantially varied among retarded, overgrown, and normal shrimp, e.g., Stramenopiles was significantly lower in retarded shrimp (3.0 ± 0.37%) but was dramatically enriched in normal shrimp (8.8 ± 0.63%) (Supplementary Fig. S3A). By contrast, relative abundance of the predominant Chloroplastida class was comparable among the three shrimp categories (Supplementary Fig. S3A). However, at the finer level, its family members were significantly changed. For example, the relative abundance of Chlorophyceae significantly decreased in retarded and overgrown shrimp as compared with normal subjects, whereas that of Trebouxiophyceae exhibited an opposite trend (Supplementary Fig. S3B).
Eukaryotic community diversity between water samples and among shrimp categories
The α-diversity of eukaryotic communities was determined in retarded, normal, and overgrown shrimp; this variable included observed species richness and Shannon diversity index. Both eukaryotic richness and diversity in water were significantly higher compared with those found in shrimp intestines. Diversities of the gut eukaryotic communities in retarded and overgrown shrimp were comparable, which were significantly lower (P < 0.05 in both cases) than those in normal shrimp (Fig. 1).
Comparison of species richness (a) and Shannon diversity (b) among retarded, normal, and overgrown shrimp gut and water eukaryotic communities. Means ± standard deviations were compared using one-way analysis of variance (ANOVA). Different lowercase letters indicate significant differences (P < 0.05) among groups
Variation in eukaryotic communities across habitats and shrimp categories
NMDS ordination biplot revealed clear differences in the structures of eukaryotic communities among water, retarded, normal, and overgrown shrimp (Fig. 2). This result was confirmed by analysis of similarities, which revealed that eukaryotic community structures were significantly distinct (P < 0.05) between each pair of groups, with an exception between retarded and overgrown shrimp (Table 3). No significant differences were observed between samples from the gut and corresponding water and those between gut and unrelated water eukaryotic communities for each shrimp category (Supplementary Fig. S4). Therefore, variations in gut eukaryotic communities were not affected by their ponds of origin. Similarity percentage analysis showed that the top 14 dominant OTUs cumulatively contributed 78.6% of the dissimilarities in eukaryotic community among the three shrimp categories; these OTUs primarily belong to Chloroplastida, Alveolata, and Holozoa lineages (Supplementary Table S3).
The gut eukaryotic communities were significantly correlated with shrimp weight/size and enzyme activities (Mantel test, P < 0.05 in all cases) (Supplementary Table S4). In this regard, we further applied SEM to explore direct and indirect effects of the gut eukaryotic communities on shrimp enzyme activities and body weight/sizes. The model fit data well: χ 2 = 1.067, P = 0.302, GFI = 0.961, RMR = 0.001, TLI = 0.995. SEM accounted for 97% of variances in shrimp body weight/size. The gut eukaryotic community contributed significantly positive (λ = 0.23, P < 0.05) effects on enzyme activities, which in turn promoted shrimp body weight/size (λ = 0.97, P < 0.001). By contrast, direct effects of the gut eukaryotic communities on shrimp body weight/size were relatively weak (λ = 0.05, P = 0.148) (Fig. 3). To conclude, shrimp growth performance was directly influenced by digestive enzyme activities that were mediated by the gut eukaryotic microbiota.
Key gut eukaryotic genera associated with shrimp growth traits
Ternary plots illustrated the distribution patterns of dominant eukaryotic genera among retarded, normal, and overgrown shrimp (Fig. 4a). Gymnodiniphycidae was the solely dominant genera found across all three shrimp categories. Each shrimp category harbored unique assemblages; for example, Rotifera was predominant in normal shrimp, whereas Arthropoda and Mucoromycotina were exclusively abundant in retarded and overgrown shrimp, respectively. Notably, a few potential pathogens, such as Ascomycota and Mucoromycotina, dominated in retarded and overgrown shrimps, respectively, but were rare or absent in normal shrimp (Fig. 4a).
Ternary plots showing percentage of mean relative abundances of dominant eukaryotic genera (>1% at least in one sample) among normal, retarded, and overgrown shrimp (a). Sizes of circles are proportional to relative abundances of genera. Genus in orange and purple frame is shared among the three shrimp categories. Genera in blue ellipse frames are primarily distributed in one shrimp category. Genera in green ellipse frames are primarily distributed in two shrimp categories. The position in triangle indicates relative abundance of each taxon among three shrimp categories. The heatmap shows Pearson correlations between enzyme activities (including amylase, lipase, and pepsin) and body size/weight and these dominant eukaryotic genera (>1% at least in one sample) among three shrimp categories (b)
To further explore potential roles of gut eukaryotic genera in shrimp growth performance, we calculated Pearson correlation between relative abundances of these dominant genera and enzyme activities or body size/weight, respectively. The results showed that variations in relative abundances of these dominant genera were significantly correlated to enzyme activities and shrimp body size and weight, as depicted by constructed heatmap (Fig. 4b). For example, Craniata and Nannochloris strongly and positively correlated to the three measured enzyme activities, whereas the presence of Desmodesmus correlated with shrimp body size and weight (Fig. 4b).
Difference in complexity of the gut eukaryotic interspecies interaction among shrimp categories
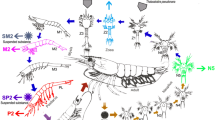
It is now recognized that the interspecies interaction in a community play critical roles in determining the functions of microbial community (Mej 2003). Thus, we evaluated to what extent that the interspecies interaction was associated with shrimp growth performance. The OTU table was split into three datasets: retarded, overgrown, and normal shrimp gut eukaryotic communities, respectively. The co-occurrence patterns of microbial taxa depended on Pearson correlation among taxa (Deng et al. 2012; Faust and Raes 2012). For this reason, OTUs detected in five or more replicates were selected for correlation calculation to improve statistical confidence, resulting in 110, 203, and 218 OTUs obtained from retarded, overgrown, and normal shrimps, respectively (Table 4). Network connectivity distribution curves fitted well with the power law model (R 2 > 0.90 in all cases, Table 4), suggesting that the constructed networks were scale-free. Interspecies interaction was more complex and better connected in retarded and overgrown shrimp than in normal ones (Fig. 5). This pattern was confirmed by key topological properties, that is, average path of retarded and overgrown shrimp was lower than that of normal shrimp, whereas average clustering coefficient and average degree exhibited the opposite trend (Table 4). Notably, percentage of positive co-occurrence was the highest in overgrown shrimp (Table 4), suggesting more cooperative activities in gut eukaryotic taxa in this group (Montoya et al. 2006). The keystone species were dominated by Nannochloris species (affiliated with Trebouxiophyceae family) in overgrown shrimp, while these in normal shrimp were Desmodesmus (affiliated with Chlorophyceae family) and Rotifera species. Furthermore, some potential pathogens belonging to Ascomycota and Basidiomycota (affiliated with fungi) were detected in retarded and overgrown shrimp (Fig. 5).
Interspecies interactions of the gut eukaryotic communities for retarded (a), overgrown (b), and normal (c) shrimp, respectively. Each node represents a eukaryotic OTU. Colors of nodes indicate OTUs affiliated to different major genus. A blue edge indicates positive interaction between two individual nodes, whereas a red edge indicates negative interaction
Discussion
Given the inadequate control for differences in diet, rearing conditions, and other factors that contribute determinant effects on the gut microbiome (Bennett et al. 2016; Filippo et al. 2010), identifying the causal relationship between the gut microbiota and growth performance remains a challenge. Available studies have focused on roles of the gut bacterial communities on mammalian growth traits (Parfrey et al. 2011). By contrast, little is known about the functional importance of the gut eukaryotes in invertebrate. In this study, we provide the first attempt to explore the direct roles of gut eukaryotic communities on shrimp growth performance using cohabitating subjects.
It is now recognized that host exerts a strong selective pressure on colonization of alien species (Bailey 2012; Ley et al. 2006; Zhu et al. 2016), thus resulting in successful establishment of only some planktonic taxa in intestines (Zhu et al. 2016). Consistent with previous studies, we observed that eukaryotic diversity was significantly higher in water than in shrimp intestines (Fig. 1). It is worthy to note that the gut eukaryotic diversities were comparable between retarded and overgrown shrimp but were substantially lower than that in normal shrimp (Fig. 1). Ample evidence has shown that a positive association of high gut eukaryotic diversity with healthy individuals, thereby conferring lower incidence of disease (Lozupone et al. 2012; Rook et al. 2014), although the shrimp gut assemblages differ from those of vertebrates. Therefore, we assume that a high gut eukaryotic diversity, just as it has been shown for bacteria, could be essential in maintaining host health. Increasing evidence has shown that the gut microbiota contributes significant roles in regulating nutrient digestion and disease resistance (Richards et al. 2005; Sukantak 2010; Xiong et al. 2015). Thus, dysbiosis of gut microbiota generally concurs with changes in microbial-mediated functions and host phenotypes (Tremaroli and Bäckhed 2012). Consistent with this notion, the gut eukaryotic microbiotas significantly differed among the three shrimp categories (Fig. 2 and Table 3); this result was also evident at the coarse phylum/class level (Supplementary Fig. S3A). In this study, application of cohabitating subjects adequately ruled out differences in some abiotic and biotic factors, which markedly affect gut microbiota. This raises the question on which factors drive the variations in gut eukaryotic community among the shrimp categories. A plausible explanation is that the stochastic processes, such as priority effect, determine the gut eukaryotic assembly in individuals (Fukami 2015; Vannette and Fukami 2014). This explanation was affirmed by previous reports, in which indicated that pioneer colonizers of larval gut exert long-term effects on host health later in life (Rodríguez et al. 2015). However, priority effects do not completely overshadow host selection on the gut microbiota, as evidenced by marked differences in relative abundances of dominant eukaryotic assemblages between water and intestine (Supplementary Fig. S3). For example, Rhodophyceae lineage was abundant in water, whereas it was selected against by shrimp, with a significantly lower abundance in intestines. By contrast, it is likely that Chloroplastida lineage is adaptive to shrimp gut habitats. Substantial differences were also observed in dominant lineages among the three shrimp categories. Specifically, relative abundances of Stramenopiles significantly decreased in retarded and overgrown shrimp in comparison with normal ones (Supplementary Fig. S3A), coinciding with the notion that Blastocystis species (which belong to Stramenopiles) species are dominant and common gut commensals in healthy individuals (Scanlan et al. 2014). Notably, some Chytridiomycota and Zygomycota species were exclusively detected in the intestines of retarded and overgrown shrimp (data not shown). It has been reported that these species can infect vertebrates by virulence and cause diseases in human and other animals (Heitman 2011). As such, retarded and overgrown shrimp are at a high risk for developing shrimp diseases. Similarly, in our previous study, we found that relative abundances of opportunistic bacterial pathogens, Vibrionaceae and Pseudoalteromonadaceae species, were enriched in retarded and overgrown shrimp as compared with these in normal ones (Xiong et al. 2017b). Indeed, there was an outbreak of disease after our sampling. Therefore, additional work is needed to explore the role of these taxa on host health and growth.
Apparent differences in shrimp growth performance can be attributed to several factors, such as genetic background, diet, and rearing conditions (Forberg et al. 2016), whereas these variables were ruled out with cohabitating subjects. However, it is still difficult to untangle the effects of feeding behavior. To improve host fitness, gut microbiota can manipulate host feeding behavior and vice versa (Alcock et al. 2014). Specifically, overgrown shrimp can be prioritized during feeding, placed in a more habitable location, or they may show aggressiveness than smaller ones; all these factors affect host growth performance. Notably, variations in gut eukaryotic community directly translated into changes in digestive enzyme activities, which further affected shrimp growth performance, though the direct effects of gut eukaryotic microbiota on shrimp growth performance were relatively weak (Fig. 3). This observation agrees with previous reports, which showed that digestive enzyme activities can directly influence shrimp growth traits (Hernández-Acosta et al. 2016). In this regard, it is likely that the gut microbiota participates in intermediary metabolism by the effect on the enzyme activities (Pataky et al. 2009), thereby affecting shrimp growth performance.
Subsequently, we examined which assemblages contributed to variations in eukaryotic communities for the three shrimp categories. Ternary plots showed that each shrimp category harbored unique eukaryotic genera, that is, specific symbionts (Fig. 4a). Notably, the distribution pattern for a given genus is generally concordant with its known biological functions. For example, Ascomycota species were specific symbionts in retarded shrimp (Fig. 4a). It has been reported that some members of Ascomycota are opportunist pathogens; as such, its enrichment leads to increased incidence of disease (Luan et al. 2015). Consistently, the relative abundance of Ascomycota assemblage was negatively correlated with shrimp body weight and size and measured digestive enzyme activities (Fig. 4b). Desmodesmus, Mucoromycotina, and Coscinodiscophytina were predominant in overgrown shrimp (Fig. 4a). Nannochloris species exhibit the ability to utilize inorganic carbon (Huertas and Lubián 1998), and thus, its enrichment will facilitate host digestion and absorption. Consistently, two Nannochloris OTUs (OTU4293 and OTU7606) were detected as top contributors to variations in gut eukaryotic communities among the three shrimp categories (Supplementary Table S3). By contrast, saprobiotic Mucoromycotina can compete nutrient with host (Mélida et al. 2015; Benny et al. 2014), which may pose adverse effects on the host. As a result, relative abundances of this genera negatively correlated with enzyme activities and body sizes/weight (Fig. 4b). However, it has been reported that Mucoromycotina species are producer of lipids (Takeda et al. 2014), thereby providing additional nutrients to hosts. Divergent effects of a given genus on host traits coincide with the notion that the same eukaryotes may be parasitic in some cases and commensal in others (Lukeš et al. 2015), which raises the question what differentiates pathogenic eukaryotes (true parasites) from mutualists in future work. Desmodesmus species are capable of utilizing complex carbon sources, thereby facilitating acquisition of additional nutrients in hosts (Samorì et al. 2013). Consistently, relative abundance of Desmodesmus was positively correlated (Pearson correlation r > 0.30, P < 0.05 in all cases) to measured enzyme activities (Fig. 4b), suggesting that this lineage is beneficial to shrimp growth. Thus, overgrowth can be partially explained by enrichment of this genus in the shrimp intestine. Gymnodiniphycidae was the only dominant and shared genus among the three shrimp categories, indicating that this lineage is a core member in shrimp intestine. However, owing to limited information on biological roles of this group, extrapolating function of these organisms in determining shrimp growth traits can be difficult at the moment. Collectively, differences in growth performance among cohabiting shrimp can be attributed to their distinct specific symbionts.
Interspecies interactions reflect niche processes, which are used to infer ecological traits and stability in assembling communities (Shade et al. 2012; Williams et al. 2014). Previous reports showed that interspecies interaction can substantially affect microbial assembly and microbial-mediated functions (Riva et al. 2017; Zhu et al. 2016). In this study, we noted that gut eukaryotic interspecies interaction was more complex and better connected in overgrown shrimp (Fig. 5), as evidenced by increased average clustering coefficient and average degree of network connectivity in overgrown shrimp (Table 4). In addition, percentage of the positive co-occurrence of eukaryotic taxa in overgrown shrimp was higher than that in normal shrimp, suggesting that eukaryotic assemblages are more cooperative in overgrown shrimp. Similarly, more complex and synergic interspecies interactions were observed in gut bacteria from the same overgrown shrimp (Xiong et al. 2017b). Thus, we propose to integrate data on common eukaryotic, endobiotic organisms to interpret roles of gut microbiota. Further work is also necessary to determine the relative significance of gut bacterial and eukaryotic microbiota in host health status. Notably, the keystone species affiliated with Desmodesmus in normal shrimp gut eukaryotic microbiota shifted to Arthropoda species in retarded shrimp and to Nannochloris species in overgrown shrimp (Fig. 5). It has been shown that the gut microbial interspecies interaction contributes important roles in host digestion and feed efficiency (Combes et al. 2013), which subsequently affects the interplay between the gut microbiota and host metabolism (Tremaroli and Bäckhed 2012). In this regard, marked shifts in keystone species may partially contribute to differences in growth traits of cohabitating shrimp. For example, Nannochloris species were keystone taxa in overgrown shrimp, and its relative abundance was positively associated with body weight and sizes (Fig. 4b). Indeed, Nannochloris strains have been applied as probiotics to promote growth performance in marine aquaculture (Witt et al. 1981). For this reason, future work should explore the interplay between shifts in gut keystone species and host growth performance.
To our knowledge, no study has explored the correlation between the gut eukaryotic microbiota and shrimp growth performance. Our results demonstrate that marked changes in the gut eukaryotic community contribute to variations in digestive activities, which in turn influence shrimp growth performance. Differences in growth performance can be attributed to altered complexity and cooperation of interspecies interaction. Additionally, unique and keystone eukaryotic assemblages are detected, and their ecological functions are concordant with corresponding growth performance. Collectively, these findings considerably expand our understanding of the role of gut eukaryotic microbiota in shrimp growth performance instead of the well-studied bacterial assembly.
References
Alcock J, Maley CC, Aktipis C (2014) Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 36:940–949
Andersen LOB, Nielsen HV, Stensvold CR (2013) Waiting for the human intestinal Eukaryotome. ISME J 7:1253–1255
Arumugam M, Raes J, Pelletier E, Paslier DL, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, Vos WMD, Brunak S, Doré J, Consortium M, Weissenbach J, Ehrlich SD, Bork P (2011) Enterotypes of the human gut microbiome. Nature 473:174–180
Bailey MT (2012) The contributing role of the intestinal microbiota in stressor-induced increases in susceptibility to enteric infection and systemic immunomodulation. Horm Behav 62:286–294
Bennett G, Malone M, Sauther ML, Cuozzo FP, White B, Nelson KE, Stumpf RM, Knight R, Leigh SR, Amato KR (2016) Host age, social group, and habitat type influence the gut microbiota of wild ring-tailed lemurs (Lemur catta). Am J Primatol 78:883–892
Benny GL, Humber RA, Voigt K (2014) Zygomycetous fungi: phylum Entomophthoromycota and subphyla Kickxellomycotina, Mortierellomycotina, Mucoromycotina, and Zoopagomycotina. In: Mclaughlin DJ, Spatafora JW (eds) Systematics and evolution. Springer, Berlin, Part A, pp 209–250
Caporaso JG, Kuczynski J, Stombaugh J (2010a) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Caporaso JG, Bittinger K, Bushman FD, Desantis TZ, Andersen GL, Knight R (2010b) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Churchill GA (2004) Using ANOVA to analyze microarray data. Biotechniques 37:173–177
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143
Combes S, Fortun-Lamothe L, Cauquil L, Gidenne T (2013) Engineering the rabbit digestive ecosystem to improve digestive health and efficacy. Animal 7:1429–1439
Core Team R (2013) R: a language and environment for statistical computing. The R Foundation for Statistical Computing, Vienna http://www.R-project.org/
De Lartigue G, de La Serre CB, Raybould HE (2011) Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 105:100–105
Deng Y, Jiang YH, Yang Y, He Z, Luo F, Zhou J (2012) Molecular ecological network analyses. BMC Bioinformatics 13:113
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550
Filippo CD, Cavalieri D, Paola MD, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696
Flint HJ, Scott KP, Louis P, Duncan SH (2012) The role of the gut microbiota in nutrition and health. Nat Rev Gastro Hepat 9:577–589
Forberg T, Sjulstad EB, Bakke I, Olsen Y, Hagiwara A, Sakakura Y, Vadstein O (2016) Correlation between microbiota and growth in mangrove killifish (Kryptolebias marmoratus) and Atlantic cod (Gadus morhua). Sci Rep 6:21192
Fukami T (2015) Historical contingency in community assembly: integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst 46:1–23
Heitman J (2011) Microbial pathogens in the fungal kingdom. Fungal Biology Reviews 25:48–60
Hernández-Acosta M, Gutiérrez-Salazar GJ, Guzmán-Sáenz FM, Aguirre-Guzmán G, Alvarez-González CA, Lópezace-Vedo EA, Fitzsimmons K (2016) The effects of Yucca schidigera and Quillaja saponaria on growth performance and enzymes activities of juvenile shrimp Litopenaeus vannamei cultured in low-salinity water. Lat Am J Aquat Res 44:121–128
Huertas IE, Lubián LM (1998) Comparative study of dissolved inorganic carbon utilization and photosynthetic responses in Nannochloris (Chlorophyceae) and Nannochloropsis (Eustigmatophyceae) species. Can J Bot 76:1104–1108
Knights D, Ward TL, McKinlay CE, Miller H, Gonzalez A, McDonald D (2014) Rethinking “Enterotypes”. Cell Host Microbe 16:433–437
Krajmalnik-Brown R, Ilhan ZE, Kang DW, DiBaise JK (2012) Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract 27:201–214
Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837–848
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability, and resilience of the human gut microbiota. Nature 489:220–230
Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, Xiao X, Hu Y, Liu Y, Wu N (2015) Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep 5:7980
Lukeš J, Stensvold CR, Jirkůpomajbíková K, Wegener PL (2015) Are human intestinal eukaryotes beneficial or commensals? PLoS Pathog 11:e1005039
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Manian FA, Bryant A (2013) Does Candida species overgrowth protect against Clostridium difficile infection? Clin Infect Dis 56:464–465
Massana R, Logares R (2013) Eukaryotic versus prokaryotic marine picoplankton ecology. Environ Microbiol 15:1254–1261
Mej N (2003) The structure and function of complex networks. SIAM Rev 45:40–45
Mélida H, Sain D, Stajich JE, Bulone V (2015) Deciphering the uniqueness of Mucoromycotina cell walls by combining biochemical and phylogenomic approaches. Environ Microbiol 17:1649–1662
Montoya JM, Pimm SL, Solé RV (2006) Ecological networks and their fragility. Nature 442:259–264
Newell PD, Douglas AE (2013) Interspecies interactions determine the impact of the gut microbiota on nutrient allocation in Drosophila melanogaster. Appl Environ Microb 80:788–796
Parfrey LW, Walters WA, Knight R (2011) Microbial eukaryotes in the human microbiome: ecology, evolution, and future directions. Front Microbiol 2:153
Pataky Z, Bobbioniharsch E, Hadengue A, Carpentier A, Golay A (2009) Gut microbiota, responsible for our body weight? Rev Med Suisse 5:662–666
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596
Ramayo-Caldas Y, Mach N, Lepage P, Levenez F, Denis C, Lemonnier G, Leplat JJ, Billon Y, Berri M, Doré J (2016) Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J 10:2973–2977
Richards JD, Gong J, De Lange CFM (2005) The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: current understanding, possible modulations, and new technologies for ecological studies. Can J Anim Sci 85:421–435
Riva A, Borgo F, Lassandro C, Verduci E, Morace G, Borghi E, Berry D (2017) Pediatric obesity is associated with an altered gut microbiota and discordant shifts in Firmicutes populations. Environ Microbiol 19:95–105
Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC (2015) The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 26:24–29
Rook GAW, Raison CL, Lowry CA (2014) Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol 177:1–12
Samorì G, Samorì C, Guerrini F, Pistocchi R (2013) Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: part I. Water Res 47:791–801
Scanlan PD, Stensvold CR, Heilig HGHJ, Vos WMD, O’Toole PW, Cotter PD (2014) The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol 90:326–330
Sha Y, Liu M, Wang B, Jiang K, Sun G, Wang L (2016) Gut bacterial diversity of farmed sea cucumbers Apostichopus japonicus with different growth rates. Microbiology 85:109–115
Shade A, Peter H, Allison SD, Baho DL, Berga M, Bürgmann H, Huber DH, Langenheder S, Lennon JT, Martiny JB (2012) Fundamentals of microbial community resistance and resilience. Front Microbiol 3:417
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T (2003) Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13:2498–2504
Sukantak N (2010) Role of gastrointestinal microbiota in fish. Aquacult Rse 41:1553–1573
Tacon AGJ, Cody JJ, Conquest LD, Divakaran S, Forster IP, Decamp OE (2002) Effect of culture system on the nutrition and growth performance of Pacific white shrimp Litopenaeus vannamei (Boone) fed different diets. Aquac Nutr 8:121–137
Takeda I, Tamano K, Yamane N, Ishii T, Ai M, Umemura M, Terai G, Baker SE, Koike H, Machida M (2014) Genome sequence of the Mucoromycotina fungus Umbelopsis isabellina, an effective producer of lipids. Genome Announ 2:e00071–e00014
Tremaroli V, Bäckhed F (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489:242–249
Ussar S, Griffin NW, Bezy O, Fujisaka S, Vienberg S, Softic S, Deng L, Bry L, Gordon JI, Kahn CR (2015) Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab 22:516–530
Vannette RL, Fukami T (2014) Historical contingency in species interactions: towards niche-based predictions. Ecol Lett 17:115–124
Voudanta E, Kormas KA, Monchy S, Delegrange A, Vincent D, Genitsaris S, Christaki U (2016) Mussel biofiltration effects on attached bacteria and unicellular eukaryotes in fish-rearing seawater. PeerJ 4:e1829
Williams RJ, Howe A, Hofmockel KS (2014) Demonstrating microbial co-occurrence pattern analyses within and between ecosystems. Front Microbiol 5:358
Witt U, Koske PH, Kuhlmann D, Lenz J, Nellen W (1981) Production of Nannochloris spec. (Chlorophyceae) in large-scale outdoor tanks and its use as a food organism in marine aquaculture. Aquaculture 23:171–181
Xiong J, Wang K, Wu J, Qiuqian L, Yang K, Qian Y, Zhang D (2015) Changes in intestinal bacterial communities are closely associated with shrimp disease severity. Appl Microbiol Biotechnol 99:6911–6919
Xiong J, Dai W, Li C (2016) Advances, challenges, and directions in shrimp disease control: the guidelines from an ecological perspective. Appl Microbiol Biotechnol 100:6947–6954
Xiong J, Zhu J, Dai W, Dong C, Qiu Q, Li C (2017a) Integrating gut microbiota immaturity and disease-discriminatory taxa to diagnose the initiation and severity of shrimp disease. Environ Microbiol 19:1490–1501
Xiong J, Dai W, Zhu J, Liu K, Dong C, Qiu Q (2017b) The underlying ecological processes of gut microbiota among cohabitating retarded, overgrown and normal shrimp. Microb Ecol 73:988–999
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107:679–684
Zhu J, Dai W, Qiu Q, Dong C, Zhang J, Xiong J (2016) Contrasting ecological processes and functional compositions between intestinal bacterial community in healthy and diseased shrimp. Microb Ecol 72:975–985
Acknowledgements
We appreciate Editor Ursula Kües and the two anonymous reviewers for their constructive comments. This work was supported by the Zhejiang Province Public Welfare Technology Application Research Project (2016C32063), and Education Department (Y201327177), the Project of Science and Technology Department of Ningbo (2017C10044), and the K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 694 kb)
Rights and permissions
About this article
Cite this article
Dai, W., Yu, W., Zhang, J. et al. The gut eukaryotic microbiota influences the growth performance among cohabitating shrimp. Appl Microbiol Biotechnol 101, 6447–6457 (2017). https://doi.org/10.1007/s00253-017-8388-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8388-0