Abstract
In natural and engineered environments, microorganisms often co-exist and interact with various minerals or mineral-containing solids. Microorganism-mineral interactions contribute significantly to environmental processes, including biogeochemical cycles in natural ecosystems and biodeterioration of materials in engineered environments. In this mini-review, we provide a summary of several key mechanisms involved in microorganism-mineral interactions, including the following: (i) solid minerals serve as substrata for biofilm development; (ii) solid minerals serve as an electron source or sink for microbial respiration; (iii) solid minerals provide microorganisms with macro or micronutrients for cell growth; and (iv) (semi)conductive solid minerals serve as extracellular electron conduits facilitating cell-to-cell interactions. We also highlight recent developments in harnessing microbe-mineral interactions for biotechnological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In natural and engineered environments, microorganisms often co-exist and, in many cases, interact with solid minerals (Fig. 1). The interaction of microorganisms with solid minerals in subsurface environments is generally considered a key factor in shaping and altering the physicochemical characteristics of the minerals (Gadd 2010). Indigenous microbial communities associated with rocks, such as granite and black shale, have been found to be involved in the bioweathering of the minerals present in these rocks (Frey et al. 2010; Gleeson et al. 2005, 2006; Matlakowska et al. 2010, 2012). Certain microorganisms interact with minerals to scavenge essential elements, which have low bioavailability (Ehrlich 1996). Consequently, microorganism-mineral interactions in natural ecosystems contribute significantly to environmental processes including biogeochemical cycling and metal/mineral transformations.
Ubiquitous presence of microorganism-mineral interactions in natural and built environments. Microbial biofilms can form at solid-air or solid-liquid interfaces. Biofilms on buildings, monuments and pipes cause discolouration and corrosion, which result in deterioration of these structures. In natural environments such as lakes, microorganism-mineral interactions on sediment, pebbles and rock are vital for biogeochemical cycling such as carbon and iron cycling
Microorganisms also play an active role in the deterioration of engineered materials, such as concrete in anthropogenic environments (Bertron 2014). The interaction of microorganisms with solid minerals is a major contributor to the deterioration of cultural heritage sites. Colonization of bacteria, fungi and cyanobacteria on stones may cause discolouration and mineral dissolution, resulting in the deterioration of monuments and frescoes (Cappitelli et al. 2007, 2009; Crispim and Gaylarde 2005; Fernandes 2006). Similarly, certain microorganisms promote corrosion and damage building materials, such as concrete (Bertron 2014), which compromises the integrity of important infrastructures, such as sewer pipes (Santo Domingo et al. 2011). On the other hand, microorganism-mineral interactions can be exploited in biotechnological applications, including anaerobic digestion, bioelectrochemical applications, metal recovery, repair of building materials and pH control. The interaction of microorganisms and minerals in natural and engineered environments has emerged as an exciting field of research that allows us to better understand environmental bioprocesses and to develop novel biotechnological applications.
In this mini-review, we provide a summary of the different mechanisms involved in microorganism-mineral interactions. In addition, we also highlight recent developments in biotechnological applications of microorganism-mineral interactions.
Microorganism-mineral interactions
Biofilm formation on solid minerals
Mineral surfaces provide a stable environment for the attachment and development of surface-associated microbial communities encased by an extracellular matrix comprising extracellular polymeric substances (EPS), known as biofilms (Costerton et al. 1995; Ding et al. 2014; Papida et al. 2000). Biofilms on solid minerals often form at the solid-air (subaerial biofilms) or solid-liquid (subaquatic biofilms) interfaces (Gorbushina 2007; Jenkinson and Lappin-Scott 2001; Spiers et al. 2003). Examples are subaerial biofilms on natural rocks and man-made stone monuments (Gorbushina 2007; Polo et al. 2012). Similarly, the majority of minerals, soil aggregates and sediment-water interfaces in aquatic environments are colonized by epilithic subaquatic biofilms (Gorbushina 2007; Lünsdorf et al. 2000). Subaquatic biofilms play important roles in carbon cycling and contribute significantly to primary production in oligotrophic lakes (Bartrons et al. 2012). The surface of solid minerals may provide microorganisms in biofilms with a favourable environment, facilitating extraction of inorganic nutrients directly from the mineral (Warren 2005).
Microbial biofilms on solid minerals often contain bacteria, fungi and algae. Table 1 shows various microbial communities that have been identified on minerals in natural and man-made environments. Airborne microorganisms may settle and colonize the exposed mineral surfaces to form biofilms at the solid-air interfaces (Abrusci et al. 2005; Borrego et al. 2010). Although chemolithotrophic, chemoorganotrophic and phototrophic microorganisms can be found in subaerial biofilms, chemoorganotrophs that trap minerals and organic substances from the air are often the first to colonize exposed surfaces (Viles and Gorbushina 2003). In addition, algae have been reported to be associated with green subaerial patinas on monuments, while black fungi have been shown to be the dominant microorganisms in black patinas (Polo et al. 2012). Subaquatic biofilms consist of prokaryotic and eukaryotic microorganisms with cyanobacteria and algae as being important taxa (Bartrons et al. 2012; Lyautey et al. 2005).
Surface adhesion is the initial step in biofilm formation (Hori and Matsumoto 2010). The attachment of microbial biofilms to mineral surfaces is mediated by an EPS matrix, which consists of extracellular polymers and high molecular weight cell surface compounds secreted by the microorganisms (Welch et al. 1999). The EPS protects the cells from mechanical damage and plays fundamental roles in the interactions of microorganisms with mineral surfaces (Tuson and Weibel 2013). High-molecular weight acidic polysaccharides in EPS increase mineral weathering by chelating metal ions derived from minerals as well as providing protons, which react with the mineral surface, resulting in proton-promoted dissolution (Welch et al. 1999). Batch reactor experiments demonstrated that Al and Si release to the bulk solution from feldspar dissolution under acidic conditions (pH 4.0) was enhanced up to 100-fold in the presence of commonly produced microbial acidic polysaccharides, such as gum xanthan, pectin, low-, medium- and high-molecular weight alginates (Welch et al. 1999). Similarly, biofilms of cyanobacterium Nostoc punctiforme and ascomycete Knufia petricola enhanced the leaching of Ca and Mg from carbonate and silicate minerals (Seiffert et al. 2014). Biofilms of bacteria isolated from black shale enhanced the release of K, P, Cu and As from shale into the aqueous phase, which resulted in partial dissolution and changes to the shale surface, such as pitting (Matlakowska et al. 2012). Matlakowska et al. (2012) proposed that the mobilization of elements was facilitated by the production of siderophores as well as metabolites.
Biofilms also contribute to the biomechanical deterioration of solid minerals. The EPS matrix is hydrophilic and absorbs water to prevent microbial communities from drying out (Papida et al. 2000). The presence of moisture in the EPS maintains diffusion pathways and supplies microbial cells with readily available nutrients at low water potential, enabling cells to survive periods of desiccation (Chenu and Roberson 1996; Papida et al. 2000). It was shown that the diffusion of glucose in clay was significantly facilitated by EPS at low water potentials (Chenu and Roberson 1996). Thus, trapping of moisture by the EPS may help to preserve the physiological activity of the microorganisms even during desiccation events, increasing the time available for mineral hydrolysis by microbial action and contributing to the acceleration of weathering (Chenu and Roberson 1996; Ercole et al. 2007). During repeated wetting and drying cycles, water absorption by the EPS also causes expansion of the biofilm matrix, while water evaporation causes its contraction, resulting in mechanical stress, which widens fissures in minerals (Papida et al. 2000; Warscheid and Braams 2000).
Solid minerals as electron acceptors and donors for respiration
Metals in solid minerals may have important biological functions in microorganisms as terminal electron acceptors and donors for respiration (Ehrlich 1996; Gadd 2010; Karlsson et al. 2012) (Fig. 2). Insoluble minerals like oxides of iron and manganese are amongst the most abundant terminal anaerobic electron acceptors in the Earth’s subsurface environments (Richardson et al. 2013) (Fig. 2a). Mineral respiration constitutes one of the most widespread respiratory processes in anoxic zones and has significant influence on the balance of several biogeochemical cycles, such as the nitrogen, sulphur and carbon cycles (Fredrickson and Gorby 1996; Lovley et al. 2004; Nealson and Myers 1992). The reduction of metals for energy generation is coupled to the oxidation of carbon substrates, such as acetate or inorganic electron donors such as hydrogen (Fredrickson and Gorby 1996; Nealson and Myers 1992), and is the main contributor to degradation of organic matter in aquatic sediments (Lovley 1991; Thamdrup 2000). Bacteria from diverse taxonomic groups, such as Shewanella, Pseudomonas, Bacillus, Arcobacter and Colwellia capable of reducing manganese and oxidizing organic carbon substrates, have been isolated from the Black Sea and manganese oxide-rich marine sediments, suggesting that these bacteria may play a pivotal role in carbon-linked Mn(IV) reduction in these anoxic environments (Nealson et al. 1991; Vandieken et al. 2012). Similarly, Fe(III) reduction is responsible for the anaerobic oxidation of organic carbon in freshwater and marine sediments (Lovley et al. 2004). In addition to manganese and iron, bacteria of the Shewanella genus have been found to be able to reduce an array of electron acceptors, such as uranium, chromium, technetium, neptunium, plutonium, selenite, tellurite and vanadate (Fredrickson et al. 2008). Microbial respiration on solid minerals increases the solubility of these minerals in the environment and accelerates mineral weathering (Gadd 2010).
Minerals function as electron acceptors and donors for respiration in microorganisms. a Metal ions (e.g. Mn4+ and Fe3+) in metal oxides are reduced during anaerobic mineral respiration. b In chemolithotrophic and photolithotrophic respiration, microorganisms obtain electrons from elements and ions (e.g. S, S2−, S2O3 2−, Fe2+)
Minerals can also function as electron donors for lithotrophic bacteria, which have been reported to oxidize a variety of inorganic substrates (e.g. sulphide, metal, ammonium, nitrite) in the generation of ATP during respiration (Tolli and King 2005). Acidophilic S and Fe oxidizing bacteria, such as Thiobacillus sp. and Acidithiobacillus sp., have been isolated from mine tailings (Baker and Banfield 2003; Wakelin et al. 2012). The oxidation of sulphide and iron by chemolithotrophic bacteria accelerates the dissolution of sulphide minerals, such as pyrite, arsenopyrite, chalcopyrite, marcasite and sphalerite, and significantly impacts rates of weathering (Baker and Banfield 2003). Chemolithotrophs obtain their energy from the oxidation of certain inorganic compounds, which can occur in the presence or absence of light. In contrast, photolithotrophs, such as green and purple sulphur bacteria, which oxidize elemental sulphur as well as reduced sulphur compounds (e.g. sulphides and thiosulphates), obtain their energy from sunlight (Frigaard and Dahl 2008; Imhoff 1995; Madigan and Jung 2009). Sulphur oxidizing bacteria are widely distributed in anoxic aquatic environments and are important for the cycling of sulphur as well as sulphide detoxification (Madigan and Jung 2009).
Solid minerals as reservoirs for macro and/or micronutrients
Solid minerals are also sources of macro and/or micronutrients, such as cobalt, nickel and iron (Fig. 3). Cobalt is an important co-factor of methyltransferases, while nickel is essential for the activity of several hydrogenases in methanogenic microorganisms (Karlsson et al. 2012). Iron is a macronutrient that is a key component of metalloenzymes, such as cytochromes, nitrogenases and monooxygenases (Konhauser et al. 2011). Hence, microorganisms interact with minerals to acquire these essential macro and micronutrients. Microbial metabolites including organic acids, siderophores and cyanide cause the leaching of metal ions and contribute to mineral solubilization. Carboxylic acids provide protons for protonolysis as well as metal chelating anions, while oxalic acids form oxalate complexes with metal ions (Gadd 2010; Uroz et al. 2009). Siderophores are organic ligands, which chelate and sequester iron. They are produced by a wide range of environmental microorganisms, especially under conditions with low soluble iron levels (Cabaj and Kosakowska 2009; Neilands 1995). Cyanide solubilizes metals by forming complexes with them (Frey et al. 2010). The production of these metal chelating metabolites, which facilitate metal ion acquisition, has been frequently reported in microorganisms isolated from rocks. Microorganisms isolated from black shale at the Lubin copper mine produced siderophores during growth on medium-lacking soluble iron so as to facilitate the mobilization of Fe3+ ions from added insoluble iron-containing compounds (Matlakowska and Sklodowska 2009). Likewise, a positive correlation was observed between the production of oxalate and cyanide and the iron release from granite, when bacterial isolates from a glacier forefield were cultivated in the presence of granite powder (Frey et al. 2010).
Minerals function as reservoirs for macro and/or micronutrients. Macro and micronutrients function as co-factors for enzymes and are essential for microbial function. To acquire these essential nutrients, microorganisms in biofilms produce metabolites such as organic acids, siderophores and cyanide, which cause leaching of metal ions and contribute to mineral solubilization. Some of the leached metal ions will be used by the microorganisms, while the remainder will be released into the environment
Solid minerals as electron transfer conduits facilitating intercellular interactions
Conductive or semiconductive iron oxide minerals, such as pyrite, magnetite and hematite, are abundant in soil and sediments and can function as natural electron conduits (Kato et al. 2012b). These conductors connect spatially separated biogeochemical redox processes by transferring electrons between microorganisms. Unlike large crystals, these iron oxide particles are often small enough to fit into the intercellular spaces of microbial communities in soil and sediments and form electron conduits between cells (Kato et al. 2010) (Fig. 4). Interspecies electron transfer (IET) was observed in a magnetite and hematite supplemented anaerobic co-culture of the soil bacteria Geobacter sulfurreducens and Thiobacillus denitrificans, containing acetate and nitrate as the electron donor and acceptor, respectively. IET in the hematite-supplemented culture was mostly mediated by iron redox cycles, whereas magnetite served as an electron conduit between the two bacteria (Kato et al. 2012b). IET via (semi)conductive iron oxide minerals in microbial communities can also accelerate methanogenesis (Kato et al. 2012a).
(Semi)conductive solid minerals such as hematite and magnetite can function as electron transfer conduits facilitating intercellular interactions. These conduits connect spatially separated biogeochemical redox processes by transferring electrons between microorganisms. Interspecies electron transfer (IET) via hematite and magnetite nanoparticles couple acetate oxidation to nitrate reduction and accelerate thermodynamically unfavourable reactions such as methanogenesis from acetate and ethanol
Biotechnological applications of microbe-mineral interactions
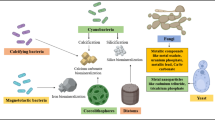
Microbe-mineral interactions have been exploited in several recent biotechnological applications, including the improvement of anaerobic digestion (AD) performance, bioelectrochemical performance, metal recovery, repair of concrete and carbonate stone and pH control (Fig. 5).
Improvement of AD performance
The addition of minerals, such as metal oxides, has been shown to improve the performance of anaerobic digesters. During the AD of dairy wastewater, methane generation and organic degradation were significantly enhanced in reactors supplemented with semiconductive ferric oxyhydroxide and conductive magnetite, with the greatest improvement observed in a reactor treated with magnetite. Similarly, the degradation efficiency and methane yields of plant biomass were increased in the presence of the iron oxides goethite, hematite and magnetite. In both cases, it is likely that these iron oxides facilitated direct IET (electric syntrophy) between methanogens and electroactive iron-reducing bacteria, thus resulting in more acidogenic substrates (e.g. volatile fatty acids) for methanogenesis (Baek et al. 2015; Ma et al. 2015).
Other minerals have also been shown to improve AD. The sulphate-containing mineral gypsum promotes the growth of sulphate-reducing bacteria (SRB) by enabling their decomposition of organic compounds. Mere addition of hematite or gypsum accelerated the removal of total organic carbon (TOC) from beef extract and peptone (Chen et al. 2014). Simultaneous addition of gypsum and hematite promoted both TOC degradation and formation of FeS and CaCO3. In other studies, anaerobic digestion processes of swine manure were improved by the addition of zeolite, which decreased the inhibitory effect of ammonia on methanogenesis by ammonium adsorption to the mineral particles, thus resulting in an improvement in methane production (Lin et al. 2013; Montalvo et al. 2005).
Bioelectrochemical applications
Electrochemically active bacteria are capable of exchanging electrons with extracellular (semi)conductive minerals. The bioelectrochemical interaction between bacteria and (semi)conductive minerals can be harnessed to enhance the performance of bioelectrochemical devices, such as microbial fuel cells. The performance of microbial fuel cells can be improved by the addition of (semi)conductive solid minerals to establish electrically conductive networks. For example, supplementation of an electrochemical cell containing rice paddy soil bacteria with hematite, magnetite and ferrihydrite enhanced the current density and coulombic efficiency of the culture (Kato et al. 2010). In another study, coating ruthenium oxide on the anode of a microbial fuel cell enhanced bioelectrochemical performance by 17 times, in which the mechanism remains unknown (Lv et al. 2012). For a detailed summary of conductive mineral (e.g. metal oxides) enabled high-performance bioelectrochemical devices, the audience may refer to several recently published reviews on the fundamentals and applications of bioelectrochemical systems (Ge et al. 2014; Lovley and Nevin 2013; Sun et al. 2015; Wang and Ren 2013; Yong et al. 2015).
Metal recovery
High value, critical and scarce metals as well as rare earth elements (REE) can be recovered by bioleaching from ores or electronic wastes for subsequent industrial reuse. Such metals are usually locked in sulphide and iron minerals. Several bacterial species with potential metal bioleaching capacities, such as acid and siderophore production, have been isolated from mineral ores and mining environments (Curutchet et al. 2001; Matlakowska and Sklodowska 2009). Immobilized biofilms of the acidophilic bacterium Thiobacillus ferrooxidans, which produce sulphuric acid (promoter of mineral dissolution) and Fe(III) (oxidant), have been developed for metal recovery from the dissolution of sulphide minerals. Treatment of a low-grade sulphur ore with sulphuric acid and Fe(III) produced by the bacteria resulted in the recovery of 69.7 % of copper, 39.5 % of zinc and 99.5 % of manganese after 75 days (Curutchet et al. 2001). Another acidophilic bacterium, Acidithiobacillus ferrooxidans, has been shown to be able to leach copper from printed circuit boards, as well as cobalt and lithium from spent lithium ion batteries (Mishra et al. 2008; Yang et al. 2009).
Organic acids produced by filamentous fungi have been demonstrated to be able to leach REEs from ores, such as bauxite reside and monazite sand (Brisson et al. 2016; Qu and Lian 2013). Citric acid produced by Penicillium tricolor was shown to have the potential for recovering REEs and radionuclides from bauxite residue by the formation of soluble complexes. Leaching efficiencies ranging from 20 to 40 % were achieved (Qu and Lian 2013). Aspergillus niger and two newly isolated strains, Aspergillus terreus strain ML3-1 and Paecilomyces spp. strain WE3-F, were found to be able to use monazite as their sole phosphate source and, hence, were able to solubilize phosphate to release REEs (Brisson et al. 2016).
Microorganisms also produce metal chelating compounds, which can be used in metal recovery. Cyanogenic bacteria produce cyanide, which forms complexes with metal ions and can also be employed to recover high-value metals, such as gold from electronic wastes. Chromobacterium violaceum has been investigated in the recovery of gold from electronic waste. Metabolically engineered C. violaceum strains, which produced 1.5 times more cyanide than the wild type, made possible three times more gold biorecovery from electronic scrap metal (Tay et al. 2013). Likewise, mutant strains of C. violaceum that have been generated by random mutagenesis were shown to cause twofold improvement of gold recovery from electronic scrap metal (Natarajan and Ting 2014).
In addition to bioleaching, microorganisms can also be employed to mobilize critical and scarce metals and remove them from contaminated sites during bioremediation. These scarce elements are essential in many industries ranging from electronics to healthcare. However, they may also accumulate in the environment and cause pollution (Nancharaiah et al. 2016). Bacterial recovery and recycling of these elements by immobilization are potentially safer and more efficient than the current practice makes possible. Tellurium, one of the rarest elements in the Earth’s crust (0.00001 %), has multiple applications in antimicrobials, photovoltaic (PV) modules and thermoelectric generators. A hydrothermal vent bacterium Pseudoalteromonas sp. strain EPR3 has been shown to be able to reduce and methylate tellurium into insoluble metallic tellurium particles and gaseous tellurium species, respectively, from various tellurium-containing compounds, such as cadmium telluride, bismuth telluride, autoclave slime and tellurium dioxide (Bonificio and Clarke 2014). This concentrates the scarce element for downstream recovery processes.
Selenium, chromium and uranium from anthropogenic sources, such as wastewater, can contaminate the environment. These metals are also widely used in the industry and by the military. Hence, they should be recovered to meet industrial needs besides being removed in bioremediation. Microbial conversion of soluble selenium, chromium and uranium into the insoluble elements may be exploited in the biological treatment of wastewater. Soluble oxyanions of selenium (SeO4 2−, SeO3 2−) can be reduced by microorganisms to insoluble selenium, which lowers its bioavailability in the environment (Nancharaiah and Lens 2015). The dissimilatory metal-reducing bacterium Shewanella oneidensis MR-1 can reduce soluble chromium(VI) to insoluble chromium(III) precipitates, which accumulate in the extracellular matrix (Belchik et al. 2011). Soluble uranium(VI) species in the environment may be extracted by microbial processes, such as biosorption onto cells, intracellular bioaccumulation, bioprecipitation to hydrogen uranyl phosphate (HUO2PO4) and bioreduction as uranite (UO2) (Nancharaiah et al. 2016). Enzymatic bioprecipitation of U(VI) to uranium phosphate precipitates is a recent attractive approach for bioremediation of uranium, as the precipitates are not susceptible to changes in oxidation state, thus functioning as a stable long-term sink for uranium (Kulkarni et al. 2013). The insoluble particles of these elements may then be recovered and used in industrial applications.
Repair of concrete and carbonate stone
Bacteria capable of biomineralization can be used for the biological repair of small cracks in concrete as well as the development of self-healing concrete (DeJong et al. 2006; Seifan et al. 2016). Alkali resistant Bacillus sp., which precipitates calcium carbonate as calcite, aragonite and vaterite minerals, have been investigated as potential healing agents (Jonkers and Schlangen 2008; Van Tittelboom et al. 2010; Wiktor and Jonkers 2011). In addition, Bacillus sp. form thick-walled spores, which can be viable for more than 200 years in dry conditions (Schlegel 1993). Bacillus sp. have been reported to be able to survive when incorporated in cement and, hence, are potential candidates in the development of self-healing concrete (Jonkers 2007). The EPS produced by the biofilms of calcifying bacteria trap calcium ions, thus facilitating calcium carbonate mineralization and healing of the cracks (Ercole et al. 2012). Bacteria can be externally applied or incorporated into concrete as a healing agent for the filling of cracks and the production of self-healing concrete respectively (Jonkers and Schlangen 2008; Van Tittelboom et al. 2010). As compared to conventional methods of concrete repair, which pose environmental and health hazards, bacterial-induced calcium carbonate precipitation is a pollution free alternative (Van Tittelboom et al. 2010). It has been shown that precipitation of calcium carbonate in the presence of calcium by externally applied Bacillus sphaericus resulted in complete filling of 10-mm deep cracks, similar to the results observed when the cracks were traditionally treated with epoxy. The calcium carbonate crystals formed may also contribute to a further decrease in water permeability observed in cracks treated with active bacteria, as compared to autoclaved bacteria. In another study, it was shown that the incorporation of high numbers of Bacillus pseudofirmus (5.8 × 108 cm−3 cement stone) did not significantly decrease the compressive strength of concrete (Jonkers and Schlangen 2008). Calcium carbonate precipitation by bacteria may also be a method to protect monuments and sculptures made of carbonate stone (Mapelli et al. 2012). Hence, the use of calcifying bacteria such as Bacillus sp. is a promising tool for concrete and carbonate stone repair.
pH control
Calcite and silicate minerals can also be used for pH control in biological systems. Both calcite and silicate minerals are easily obtainable from natural environments. Calcium carbonate may potentially be used as a neutralizing agent in microbial biotechnological applications such as anaerobic fermentation to produce acetic and lactic acid or in wastewater treatment plants (Salek et al. 2015). Calcium carbonate is 60–80 % cheaper than the current neutralizing agent sodium carbonate, translating to a reduction in operational costs (Halmann and Steinfeld 2006). In addition, active pH control is not required as the use of calcium carbonate forms a self-regulating pH system in which the pH will not exceed 8.5 due to the nature of calcium carbonate (Salek et al. 2015). The pH control is required in lactic acid fermentation processes, as the decrease in pH with increasing lactic acid production will result in feedback inhibition, inhibiting further production of lactic acid by the microorganisms. Although ammonia, sodium hydroxide and potassium hydroxide are efficient neutralizers in lactic acid fermentation, ammonia toxicity can result in lowering of the final acid yield and cell concentration. The use of calcium hydroxide not only results in high lactic acid yields but also generates calcium sulphate, as a solid waste in the extraction process. Hence, a method for the production of lactic acid was developed using recycled calcium carbonate as a neutralizing agent without the generation of calcium sulphate (Yang et al. 2015). It was reported that recycled calcium carbonate can maintain a higher pH at higher lactate concentrations, as compared to that of original calcium carbonate in a fermentation culture of Lactobacillus lactis, leading to improved lactic acid yields. This was due to the small particle size and increased surface area of the recycled calcium carbonate, which resulted in higher dissolution rates. In addition, L. lactis was adsorbed to the rough surface of the recycled calcium carbonate and formed holes on the particles, which enable them to function as favourable microenvironments for microbial growth and production of lactic acid (Yang et al. 2015). Other studies have demonstrated the buffering potential of nepheline, an aluminosilicate mineral, in acid mine water (Kleiv and Sandvick 2000).
Recent studies examined the use of silicates as long-term pH buffering agents to maintain pH in acidified groundwater during bioremediation. Chlorinated ethenes, such as tetrachloroethene (PCE) and trichloroethene (TCE), are common groundwater contaminants in industrialized countries (Lacroix et al. 2014a). A cost-effective method of bioremediation of chlorinated ethenes is the dechlorination of these compounds by organohalide-respiring bacteria (OHRB) in organohalide respiration (OHR) to form non-toxic ethene (Lee et al. 1998). However, substrate fermentation and OHR generate acids, which acidify the groundwater. This drastically inhibits OHR, as OHRB are inactivated at decreased pH (Lacroix et al. 2014b). Circulation of sodium bicarbonate or sodium carbonate solutions is commonly used to adjust the pH of groundwater during OHR (Robinson et al. 2009). However, this only has a short-term effect and requires constant monitoring with frequent injections. In comparison, the dissolution of silicate minerals is relatively slow, which enables them to function as long-term sources of alkalinity. Formate dehydrogenation, an alternative method of pH control that generates only bicarbonate without acid production, may be insufficient in cases of high dechlorination rates (Philips et al. 2013). Additionally, the dissolution of silicate minerals is stimulated by acidity, thus avoiding pH overshooting and wastage of buffering material (Marini 2006). Silicate minerals forsterite, diopside and fayalite maintained pH in the neutral range in batch cultures of OHRB, facilitating complete dechlorination of chlorinated ethenes to ethene (Lacroix et al. 2014a, b). Hence, silicates may be potential good-buffering agents in the bioremediation of acidified groundwater.
Conclusion and future perspectives
Microorganisms interact with minerals in various ways, and these interactions play vital roles in natural and engineered environments by mediating biological and geochemical processes. Solid minerals provide a surface for the stable attachment of biofilms, function as electron acceptors and donors and as reservoirs for macro and/or micronutrients, as well as electron transfer conduits facilitating intercellular interactions. Microbe-mineral interactions are major contributors to the biodeterioration of engineered materials, resulting in degradation of cultural heritage sites and infrastructures. On the other hand, the interactions between microorganisms and solid minerals have been exploited for various biotechnological applications, such as improvement of anaerobic digestion performance, enhancement of bioelectrochemical performance, metal recovery, repair of concrete and carbonate stone, and pH control in microbial fermentation.
Although extensive studies have been conducted in elucidating microorganism-mineral interactions in natural and engineered environments, our understanding of biofilm formation on mineral surfaces is limited. While most studies have focussed on the effects of biofilm formation on minerals and their significance, the molecular mechanisms involved in biofilm development on mineral surfaces are not well understood. Whether and how interactions with mineral surfaces influence the genetic regulation networks of the biofilm lifestyle are interesting research topics to explore. Further understanding of the genetic regulation of biofilm formation on solid minerals may be exploited for other novel biotechnological applications in the future.
References
Abrusci C, Martín-González A, Del Amo A, Catalina F, Collado J, Platas G (2005) Isolation and identification of bacteria and fungi from cinematographic films. Int Biodeterior Biodegrad 56(1):58–68
Baek G, Kim J, Cho K, Bae H, Lee C (2015) The biostimulation of anaerobic digestion with (semi)conductive ferric oxides: their potential for enhanced biomethanation. Appl Microbiol Biotechnol 99(23):10355–10366
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44(2):139–152
Bartrons M, Catalan J, Casamayor EO (2012) High bacterial diversity in epilithic biofilms of oligotrophic mountain lakes. Microb Ecol 64(4):860–869
Belchik SM, Kennedy DW, Dohnalkova AC, Wang Y, Sevinc PC, Wu H, Lin Y, Lu HP, Fredrickson JK, Shi L (2011) Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77(12):4035–4041
Bertron A (2014) Understanding interactions between cementitious materials and microorganisms: a key to sustainable and safe concrete structures in various contexts. Mater Struct 47(11):1787–1806
Bonificio WD, Clarke DR (2014) Bacterial recovery and recycling of tellurium from tellurium-containing compounds by Pseudoalteromonas sp. EPR3. J Appl Microbiol 117(5):1293–1304
Borrego S, Guiamet P, Gómez de Saravia S, Batistini P, Garcia M, Lavin P, Perdomo I (2010) The quality of air at archives and the biodeterioration of photographs. Int Biodeterior Biodegrad 64(2):139–145
Brisson VL, Zhuang WQ, Alvarez-Cohen L (2016) Bioleaching of rare earth elements from monazite sand. Biotechnol Bioeng 113(2):339–348
Cabaj A, Kosakowska A (2009) Iron-dependent growth of and siderophore production by two heterotrophic bacteria isolated from brackish water of the southern Baltic Sea. Microbiol Res 164(5):570–577
Cappitelli F, Principi P, Pedrazzani R, Toniolo L, Sorlini C (2007) Bacterial and fungal deterioration of the Milan Cathedral marble treated with protective synthetic resins. Sci Total Environ 385(1–3):172–181
Cappitelli F, Abbruscato P, Foladori P, Zanardini E, Ranalli G, Principi P, Villa F, Polo A, Sorlini C (2009) Detection and elimination of cyanobacteria from frescoes: the case of the St. Brizio Chapel (Orvieto Cathedral, Italy). Microb Ecol 57(4):633–639
Chen T-H, Wang J, Zhou Y-F, Yue Z-B, Xie Q-Q, Pan M (2014) Synthetic effect between iron oxide and sulfate mineral on the anaerobic transformation of organic substance. Bioresour Technol 151:1–5
Chenu C, Roberson EB (1996) Diffusion of glucose in microbial extracellular polysaccharide as affected by water potential. Soil Biol Biochem 28(7):877–884
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 49(1):711–745
Crispim CA, Gaylarde CC (2005) Cyanobacteria and biodeterioration of cultural heritage: a review. Microb Ecol 49(1):1–9
Curutchet G, Donati E, Oliver C, Pogliani C, Viera MR (2001) [11] Development of Thiobacillus biofilms for metal recovery. In: Ron JD (ed) Methods in enzymology, vol 337. Academic, pp. 171–186
DeJong JT, Fritzges MB, Nüsslein K (2006) Microbially induced cementation to control sand response to undrained shear. J Geotech Geoenviron Eng 132(11):1381–1392
Ding Y, Peng N, Du Y, Ji L, Cao B (2014) Disruption of putrescine biosynthesis in Shewanella oneidensis enhances biofilm cohesiveness and performance in Cr(VI) immobilization. Appl Environ Microbiol 80(4):1498–1506
Ehrlich HL (1996) How microbes influence mineral growth and dissolution. Chem Geol 132(1–4):5–9
Ercole C, Cacchio P, Botta AL, Centi V, Lepidi A (2007) Bacterially induced mineralization of calcium carbonate: the role of exopolysaccharides and capsular polysaccharides. Microsc Microanal 13(01):42–50
Ercole C, Bozzelli P, Altieri F, Cacchio P, Del Gallo M (2012) Calcium carbonate mineralization: involvement of extracellular polymeric materials isolated from calcifying bacteria. Microsc Microanal 18(04):829–839
Fernandes P (2006) Applied microbiology and biotechnology in the conservation of stone cultural heritage materials. Appl Microbiol Biotechnol 73(2):291–296
Fredrickson JK, Gorby YA (1996) Environmental processes mediated by iron-reducing bacteria. Curr Opin Biotechnol 7(3):287–294
Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JLM, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM (2008) Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6(8):592–603
Frey B, Rieder SR, Brunner I, Plötze M, Koetzsch S, Lapanje A, Brandl H, Furrer G (2010) Weathering-associated bacteria from the Damma glacier forefield: physiological capabilities and impact on granite dissolution. Appl Environ Microbiol 76(14):4788–4796
Frigaard N-U, Dahl C (2008) Sulfur metabolism in phototrophic sulfur bacteria. Adv Microb Physiol 54:103–200
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156(3):609–643
Ge Z, Li J, Xiao L, Tong Y, He Z (2014) Recovery of electrical energy in microbial fuel cells. Environ Technol Lett 1(2):137–141
Gleeson D, Clipson N, Melville K, Gadd G, McDermott F (2005) Characterization of fungal community structure on a weathered pegmatitic granite. Microb Ecol 50(3):360–368
Gleeson D, Kennedy N, Clipson N, Melville K, Gadd G, McDermott F (2006) Characterization of bacterial community structure on a weathered pegmatitic granite. Microb Ecol 51(4):526–534
Gorbushina AA (2007) Life on the rocks. Environ Microbiol 9(7):1613–1631
Halmann M, Steinfeld A (2006) Production of lime, hydrogen, and methanol by the thermo-neutral combined calcination of limestone with partial oxidation of natural gas or coal. Energy 31(10):1533–1541
Hori K, Matsumoto S (2010) Bacterial adhesion: from mechanism to control. Biochem Eng J 48(3):424–434
Imhoff JF (1995) Taxonomy and physiology of phototrophic purple bacteria and green sulfur bacteria. In: Anoxygenic photosynthetic bacteria. Springer, pp. 1–15
Jenkinson HF, Lappin-Scott HM (2001) Biofilms adhere to stay. Trends Microbiol 9(1):9–10
Jonkers H (2007) Self healing concrete: a biological approach. In: van der Zwaag S (ed) Self healing materials. Springer series in materials science, vol 100. Springer, Dordrecht, pp 195–204
Jonkers HM, Schlangen E (2008) A two component bacteria-based self-healing concrete. In: Concrete repair, rehabilitation and retrofitting II. CRC Press, pp. 119–120
Karlsson A, Einarsson P, Schnürer A, Sundberg C, Ejlertsson J, Svensson BH (2012) Impact of trace element addition on degradation efficiency of volatile fatty acids, oleic acid and phenyl acetate and on microbial populations in a biogas digester. J Biosci Bioeng 114(4):446–452
Kato S, Nakamura R, Kai F, Watanabe K, Hashimoto K (2010) Respiratory interactions of soil bacteria with (semi)conductive iron-oxide minerals. Environ Microbiol 12(12):3114–3123
Kato S, Hashimoto K, Watanabe K (2012a) Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol 14(7):1646–1654
Kato S, Hashimoto K, Watanabe K (2012b) Microbial interspecies electron transfer via electric currents through conductive minerals. Proc Natl Acad Sci U S A 109(25):10042–10046
Kerfahi D, Hall-Spencer JM, Tripathi BM, Milazzo M, Lee J, Adams JM (2014) Shallow water marine sediment bacterial community shifts along a natural CO2 gradient in the Mediterranean Sea off Vulcano, Italy. Microb Ecol 67(4):819–828
Kleiv R, Sandvick K (2000) Using tailings as heavy metal adsorbents—the effect of buffering capacity. Miner Eng 13(7):719–728
Konhauser KO, Kappler A, Roden EE (2011) Iron in microbial metabolisms. Elements 7(2):89–93
Kulkarni S, Ballal A, Apte SK (2013) Bioprecipitation of uranium from alkaline waste solutions using recombinant Deinococcus radiodurans. J Hazard Mater 262:853–861
Lacroix E, Brovelli A, Barry DA, Holliger C (2014a) Use of silicate minerals for pH control during reductive dechlorination of chloroethenes in batch cultures of different microbial consortia. Appl Environ Microbiol 80(13):3858–3867
Lacroix E, Brovelli A, Maillard J, Rohrbach-Brandt E, Barry DA, Holliger C (2014b) Use of silicate minerals for long-term pH control during reductive dechlorination of high tetrachloroethene concentrations in continuous flow-through columns. Sci Total Environ 482:23–35
Lee M, Odom J, Buchanan R Jr (1998) New perspectives on microbial dehalogenation of chlorinated solvents: insights from the field. Annu Rev Microbiol 52(1):423–452
Lin L, Wan C, Liu X, Lei Z, Lee D-J, Zhang Y, Tay JH, Zhang Z (2013) Anaerobic digestion of swine manure under natural zeolite addition: VFA evolution, cation variation, and related microbial diversity. Appl Microbiol Biotechnol 97(24):10575–10583
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55(2):259
Lovley DR, Nevin KP (2013) Electrobiocommodities: powering microbial production of fuels and commodity chemicals from carbon dioxide with electricity. Curr Opin Biotechnol 24(3):385–390
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. In: Advances in microbial physiology, vol 49. Academic, pp. 219–286
Lünsdorf H, Erb R, Abraham W, Timmis K (2000) ‘Clay hutches’: a novel interaction between bacteria and clay minerals. Environ Microbiol 2(2):161–168
Lv Z, Xie D, Yue X, Feng C, Wei C (2012) Ruthenium oxide-coated carbon felt electrode: a highly active anode for microbial fuel cell applications. J Power Sources 210:26–31
Lyautey E, Lacoste B, Ten-Hage L, Rols J-L, Garabetian F (2005) Analysis of bacterial diversity in river biofilms using 16S rDNA PCR-DGGE: methodological settings and fingerprints interpretation. Water Res 39(2):380–388
Ma D, Wang J, Chen T, Shi C, Peng S, Yue Z (2015) Iron-oxide-promoted anaerobic process of the aquatic plant of curly leaf pondweed. Energy Fuel 29(7):4356–4360
Madigan MT, Jung DO (2009) An overview of purple bacteria: systematics, physiology, and habitats. In: The purple phototrophic bacteria. Springer, pp. 1–15
Magniont C, Coutand M, Bertron A, Cameleyre X, Lafforgue C, Beaufort S, Escadeillas G (2011) A new test method to assess the bacterial deterioration of cementitious materials. Cem Concr Res 41(4):429–438
Mapelli F, Marasco R, Balloi A, Rolli E, Cappitelli F, Daffonchio D, Borin S (2012) Mineral–microbe interactions: biotechnological potential of bioweathering. J Biotechnol 157(4):473–481
Marini L (2006) Geological sequestration of carbon dioxide: thermodynamics, kinetics, and reaction path modeling, vol 11. Elsevier
Matlakowska R, Sklodowska A (2009) The culturable bacteria isolated from organic-rich black shale potentially useful in biometallurgical procedures. J Appl Microbiol 107(3):858–866
Matlakowska R, Narkiewicz W, Sklodowska A (2010) Biotransformation of organic-rich copper-bearing black shale by indigenous microorganisms isolated from Lubin copper mine (Poland). Environ Sci Technol 44(7):2433–2440
Matlakowska R, Skłodowska A, Nejbert K (2012) Bioweathering of Kupferschiefer black shale (Fore-Sudetic Monocline, SW Poland) by indigenous bacteria: implication for dissolution and precipitation of minerals in deep underground mine. FEMS Microbiol Ecol 81(1):99–110
Mishra D, Kim D-J, Ralph DE, Ahn J-G, Rhee Y-H (2008) Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag 28(2):333–338
Montalvo S, Díaz F, Guerrero L, Sánchez E, Borja R (2005) Effect of particle size and doses of zeolite addition on anaerobic digestion processes of synthetic and piggery wastes. Process Biochem 40(3):1475–1481
Nancharaiah YV, Lens PN (2015) Selenium biomineralization for biotechnological applications. Trends Biotechnol 33(6):323–330
Nancharaiah Y, Mohan SV, Lens P (2016) Biological and bioelectrochemical recovery of critical and scarce metals. Trends Biotechnol 34(2):137–155
Natarajan G, Ting Y-P (2014) Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour Technol 152(0):80–85
Nealson KH, Myers CR (1992) Microbial reduction of manganese and iron: new approaches to carbon cycling. Appl Environ Microbiol 58(2):439–443
Nealson KH, Myers CR, Wimpee BB (1991) Isolation and identification of manganese-reducing bacteria and estimates of microbial Mn(IV)-reducing potential in the Black Sea. Deep Sea Res Part A Oceanogr Res Pap 38. Suppl 2(0):S907–S920
Neilands J (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
Papida S, Murphy W, May E (2000) Enhancement of physical weathering of building stones by microbial populations. Int Biodeterior Biodegrad 46(4):305–317
Philips J, Maes N, Springael D, Smolders E (2013) Acidification due to microbial dechlorination near a trichloroethene DNAPL is overcome with pH buffer or formate as electron donor: experimental demonstration in diffusion-cells. J Contam Hydrol 147:25–33
Polo A, Gulotta D, Santo N, Di Benedetto C, Fascio U, Toniolo L, Villa F, Cappitelli F (2012) Importance of subaerial biofilms and airborne microflora in the deterioration of stonework: a molecular study. Biofouling 28(10):1093–1106
Qu Y, Lian B (2013) Bioleaching of rare earth and radioactive elements from red mud using Penicillium tricolor RM-10. Bioresour Technol 136:16–23
Richardson DJ, Butt JN, Clarke TA (2013) Controlling electron transfer at the microbe–mineral interface. Proc Natl Acad Sci U S A 110(19):7537–7538
Robinson C, Barry D, McCarty PL, Gerhard JI, Kouznetsova I (2009) pH control for enhanced reductive bioremediation of chlorinated solvent source zones. Sci Total Environ 407(16):4560–4573
Salek S, van Turnhout A, Kleerebezem R, van Loosdrecht M (2015) pH control in biological systems using calcium carbonate. Biotechnol Bioeng 112(5):905–913
Santo Domingo JW, Revetta RP, Iker B, Gomez-Alvarez V, Garcia J, Sullivan J, Weast J (2011) Molecular survey of concrete sewer biofilm microbial communities. Biofouling 27(9):993–1001
Schlegel HG (1993) General microbiology, 7th edn. Cambridge University Press
Seifan M, Samani AK, Berenjian A (2016) Bioconcrete: next generation of self-healing concrete. Appl Microbiol Biotechnol 100(6):2591–2602. doi:10.1007/s00253-016-7316-z
Seiffert F, Bandow N, Bouchez J, von Blanckenburg F, Gorbushina AA (2014) Microbial colonization of bare rocks: laboratory biofilm enhances mineral weathering. Proc Earth Planet Sci 10(0):123–129
Spiers AJ, Bohannon J, Gehrig SM, Rainey PB (2003) Biofilm formation at the air–liquid interface by the Pseudomonas fluorescens SBW25 wrinkly spreader requires an acetylated form of cellulose. Mol Microbiol 50(1):15–27
Sun J-Z, Kingori GP, Si R-W, Zhai D-D, Liao Z-H, Sun D-Z, Zheng T, Yong Y-C (2015) Microbial fuel cell-based biosensors for environmental monitoring: a review. Water Sci Technol 71(6):801–809
Tay SB, Natarajan G, Rahim MNBA, Tan HT, Chung MCM, Ting YP, Yew WS (2013) Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci Report 3:2236
Thamdrup B (2000) Bacterial manganese and iron reduction in aquatic sediments. In: Advances in microbial ecology. Springer, pp. 41–84
Tolli J, King GM (2005) Diversity and structure of bacterial chemolithotrophic communities in pine forest and agroecosystem soils. Appl Environ Microbiol 71(12):8411–8418
Tuson HH, Weibel DB (2013) Bacteria-surface interactions. Soft Matter 9(17):4368–4380
Uroz S, Calvaruso C, Turpault M-P, Frey-Klett P (2009) Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol 17(8):378–387
Van Tittelboom K, De Belie N, De Muynck W, Verstraete W (2010) Use of bacteria to repair cracks in concrete. Cem Concr Res 40(1):157–166
Vandieken V, Pester M, Finke N, Hyun J-H, Friedrich MW, Loy A, Thamdrup B (2012) Three manganese oxide-rich marine sediments harbor similar communities of acetate-oxidizing manganese-reducing bacteria. ISME J 6(11):2078–2090
Viles HA, Gorbushina AA (2003) Soiling and microbial colonisation on urban roadside limestone: a three year study in Oxford, England. Build Environ 38(9–10):1217–1224
Vollertsen J, Nielsen AH, Jensen HS, Wium-Andersen T, Hvitved-Jacobsen T (2008) Corrosion of concrete sewers—the kinetics of hydrogen sulfide oxidation. Sci Total Environ 394(1):162–170
Wakelin SA, Anand RR, Reith F, Gregg AL, Noble RR, Goldfarb KC, Andersen GL, DeSantis TZ, Piceno YM, Brodie EL (2012) Bacterial communities associated with a mineral weathering profile at a sulphidic mine tailings dump in arid Western Australia. FEMS Microbiol Ecol 79(2):298–311
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31(8):1796–1807
Warren LA (2005) Biofilms and metal geochemistry: the relevance of micro-organism-induced geochemical transformations. In: Micro-organisms and earth systems—advances in geomicrobiology. Cambridge University Press
Warscheid T, Braams J (2000) Biodeterioration of stone: a review. Int Biodeterior Biodegrad 46(4):343–368
Welch SA, Barker WW, Banfield JF (1999) Microbial extracellular polysaccharides and plagioclase dissolution. Geochim Cosmochim Acta 63(9):1405–1419
Wiktor V, Jonkers HM (2011) Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem Concr Compos 33(7):763–770
Yang T, Xu Z, Wen J, Yang L (2009) Factors influencing bioleaching copper from waste printed circuit boards by Acidithiobacillus ferrooxidans. Hydrometallurgy 97(1–2):29–32
Yang P-B, Tian Y, Wang Q, Cong W (2015) Effect of different types of calcium carbonate on the lactic acid fermentation performance of Lactobacillus lactis. Biochem Eng J 98:38–46
Yong Y-C, Wu X-Y, Sun J-Z, Cao Y-X, Song H (2015) Engineering quorum sensing signaling of Pseudomonas for enhanced wastewater treatment and electricity harvest: a review. Chemosphere 140:18–25
Acknowledgments
This material is based on research/work supported by the Singapore Ministry of National Development and National Research Foundation under L2 NIC Award No. L2NICCFP1-2013-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ng, D.H.P., Kumar, A. & Cao, B. Microorganisms meet solid minerals: interactions and biotechnological applications. Appl Microbiol Biotechnol 100, 6935–6946 (2016). https://doi.org/10.1007/s00253-016-7678-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7678-2