Abstract
The effect of biostimulation with ferric oxides, semiconductive ferric oxyhydroxide, and conductive magnetite on the anaerobic digestion of dairy wastewater was examined in a batch mode. The reactors supplemented with ferric oxyhydroxide (R2) and magnetite (R3) showed significantly enhanced biomethanation performance compared with the control (R1). The removal of chemical oxygen demand (COD) after 30 days was 31.9, 59.3, and 82.5 % in R1, R2, and R3, respectively. The consumed COD was almost fully recovered as biogas in R2 and R3, while only 79 % was recovered in R1. The total energy production as biogas was accordingly 32.2, 71.0, and 97.7 kJ in R1, R2, and R3, respectively. The reactors also differed in the acid formation profile with more propionate and butyrate found in R1 and more acetate found in R3. The enhanced biomethanation seems to be associated with variations in the bacterial community structure supposedly induced by the ferric oxides added. In contrast, no evident variation was observed in the archaeal community structure among the reactors. The potential electric syntrophy formed between Methanosaeta concilii-like methanogens and electroactive iron-reducing bacteria, particularly Trichococcus, was likely responsible for the enhanced performance. The stimulated growth of fermentative iron reducers may also have contributed by altering the metabolic characteristics of the bacterial communities to produce more favorable acidogenic products for methanogenesis. The overall results suggest the potential of biostimulation with (semi)conductive ferric oxides to enhance the rate and efficiency of the biomethanation of organic wastes. This seems to be potentially attractive, as increasing attention is being paid to the energy self-sufficiency of waste/wastewater treatment processes today.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anaerobic digestion (AD) is considered to be a promising technology to cope with today’s energy and environmental problems owing to its ability to convert organic pollutants into methane, a valuable energy carrier. AD has largely been applied to the treatment of high-strength organic waste and wastewater and is now anticipated to play a critical role in future energy security and sustainability. Despite such potentials, AD has some technical limitations due to its slow reaction rate. This is largely ascribed to the slow growth of anaerobic microorganisms involved in the methanogenic pathway. The limitations include significantly longer retention time and lower organic removal rate compared with aerobic treatment (Ahring 2003). Extensive efforts have been made to overcome these limitations and improve the feasibility of AD technology. Such efforts have conventionally relied on the empirical alteration of the process design and operation, but recently, the need for understanding at the microbial community level has been raised by many researchers. Given that a biological process basically depends on the concerted activity of the microorganisms involved, the microbial community composition will naturally have a significant influence on the process performance (Akarsubasi et al. 2005; Fernandez et al. 1999).
AD is a multi-stage process that is conducted by complex microbial consortia involving diverse metabolic groups, i.e., hydrolytic, fermentative, acetogenic, and methanogenic. Iron-reducing bacteria (IRBs), oxidizing a wide spectrum of organic substances using ferric iron as an electron acceptor, are commonly present in anaerobic environments and play a key role in iron cycling (Kim et al. 2014b). Although not directly engaged in the AD pathway, IRBs could reportedly produce either a positive or negative influence on the overall AD performance. Methanogenesis and ferric iron reduction are in direct competition for acetate and H2, the major precursors for methane, and the latter is thermodynamically more favorable due to its lower threshold levels for the electron donors (Lovely and Phillips 1987). Several previous studies actually showed a negative impact or no impact by adding different ferric iron compounds (in amorphous as well as crystalline forms) on AD in various settings (Mamais et al. 1994; Qu et al. 2004; Roden and Wetzel 2003). On the other hand, there are other conflicting observations on this matter. Different ferric oxides demonstrated a positive influence on methane production in several AD processes treating, for example, a fat-rich wastewater (Ivanov et al. 2002) and waste-activated sludge (Zhang et al. 2010). A recent study on a rice-paddy-soil microbial community reported clear increases in the methanogenesis rates from acetate and ethanol, with a stimulated growth of iron-reducing Geobacter, by the addition of ferric oxides (Kato et al. 2012). Although little is known about the underlying mechanism, these observations suggest that AD performance may be enhanced by stimulating the IRB activity using ferric iron. A recent study from our group tested this potential in a continuous digester treating waste-activated sludge and demonstrated improved methane production by biostimulation with ferric oxyhydroxide (Baek et al. 2014), suggesting that a stabilized AD microbial community could be altered by biostimulation with ferric iron, thus enhancing the AD efficiency. This observation is supported, at least in part, by the fact that iron respiration is less efficient with crystalline iron compounds, and thus, it inhibits methanogenesis only when the ferric iron is in an amorphous or poorly crystalline form (Ahring 2003; Lovely and Phillips 1987; Lovley 1991). In addition to substrate competition, a recent study demonstrated a direct inhibitory effect of amorphous Fe(OH)3 on methanogenesis in pure cultures (van Bodegom et al. 2004).
This study therefore aims to investigate the effect of crystalline, (semi)conductive ferric oxides on the development of the microbial community structure and the process performance during AD, particularly during startup. For this, three anaerobic batch reactors were operated in parallel using whey, a nutrient-rich cheese-processing wastewater, as a model waste stream for anaerobic treatment. Two reactors were supplemented with semiconductive ferric oxyhydroxide and conductive magnetite, while the other one, which served as a control for comparison, was not. The main focus of this study is on how the different biostimulation amendments influence the reaction course throughout the acidogenesis and methanogenesis steps. For more comprehensive insights into microbial as well as process responses, variations in the physicochemical parameters and microbial community structure were comparatively analyzed between the reactors during the operation period. This study helps improve our understanding of the role and potential of IRBs in AD environments.
Materials and methods
Anaerobic reactors
Three completely mixed tank reactors with a working volume of 2 L, designated as R1 to R3, were run to anaerobically treat whey, a byproduct from cheese production, in batch mode for a period of 30 days. Whey has widely been treated by AD due to its high organic strength and biodegradability (Hwang and Hansen 1998; Lee et al. 2010). The basic characteristics of the substrate and inoculum are presented in Table 1. Most organic matter in the substrate existed in soluble form and thus was more easily available for microorganisms. Whey used as the substrate in this study was obtained from a domestic dairy company (Samik Dairy and Food Co., Ltd., South Korea). Each reactor was filled with dilute whey (5 g/L as chemical oxygen demand (COD)) and inoculated with anaerobic sludge from a municipal sewage treatment plant in Busan, South Korea (seeding ratio, 1 % (v/v)). Reactors R2 and R3 were supplemented with ferric oxyhydroxide (FeOOH) and magnetite (Fe3O4) to give a final Fe concentration of 20 mM, respectively. The reactor-operating temperature was maintained at 35 ± 2 °C using an automatic controller combined with a heating tape, and the pH was kept above 7.0 by dosing with a 3-N NaOH solution with a pH controller. Each reactor was routinely sampled and monitored for a set of physicochemical parameters.
DNA extraction
Community DNA was purified from the reactor samples using an automated nucleic acid extractor (ExiProgen, Bioneer, Daejeon, South Korea) according to the manufacturer’s instructions. A 1-mL aliquot of a sample was centrifuged in a 1.5-mL microtube at 13,000×g for 3 min, and the pelleted biomass was then washed with distilled water by repeated centrifugations (13,000×g, 1 min), decanting (900 μL), and resuspending (up to 1 mL in distilled water) to remove cell debris and other impurities. A 200-μL portion of the resuspension was loaded on the extractor with the ExiProgen Bacteria Genomic DNA Kit (Bioneer). The purified DNA was eluted in a DNase-free elution buffer solution (200 μL) and stored at −20 °C until use.
Molecular fingerprinting and sequencing analysis
Denaturing gradient gel electrophoresis (DGGE), a widely used molecular fingerprinting method to analyze the microbial community structure, was employed to monitor the variations in the archaeal and bacterial community structures in the reactors. Archaeal and bacterial 16S ribosomal RNA (rRNA) gene fragments for DGGE analysis were prepared by polymerase chain reaction (PCR) using the ARC787F/1059R and BAC338F/805R primer sets (Yu et al. 2005), respectively. A 40-base gas chromatograph (GC) clamp was added to the 5′-end of each forward primer (i.e., ARC787F and BAC338F) to obtain a stable melting behavior and better separation of the amplicons on a gel (Muyzer et al. 1993). A touch-down thermal cycling protocol was applied for PCR: initial denaturation at 94 °C for 10 min; 20 cycles of denaturation at 94 °C for 30 s, annealing at 65 to 55 °C for 30 s (decreasing the temperature by 0.5 °C/cycle), and extension at 72 °C for 30 s; an additional 25 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and further extension at 72 °C for 7 min. The PCR fragments (20 μL) were loaded on 8 % (w/v) polyacrylamide gels with denaturant gradients of 35–65 and 20–60 % for the archaeal and bacterial DGGE runs (100 % denaturant contains 7 M urea and 40 % (v/v) formamide), respectively. Electrophoresis was run in a D-code system (Bio-Rad, Hercules, CA, USA) for 16 h at 80 V. The DGGE gels were stained with SYBR Safe dye (Molecular Probe, Eugene, OR) and scanned at 470 nm using a Safe Imager Blue-Light Transilluminator (Invitrogen, USA) to visualize the band patterns. Bands of interest were cut out of the gels and eluted in 40 μL of sterile water. A 2-μL aliquot of each elution was reamplified with the same primer sets as used for the DGGE analysis but without the GC clamp. The resulting PCR products were gel-purified (Gel/PCR purification mini kit, Favorgen, Taiwan), cloned (pGEM-T Easy, Progema, USA), and sequenced (Macrogen Sequencing Service, South Korea). The recovered sequence information was compared against the GenBank and Ribosomal Database Project (RDP) databases. The RDP classifier was used for taxonomic classification at a bootstrap confidence threshold of 80 %. All nucleotide sequences retrieved in this study have been deposited in the GenBank database: KP241962–KP241975.
Cluster analysis and ordination of DGGE fingerprints
A binary matrix was constructed from each of the archaeal and bacterial DGGE fingerprints by recording the presence or absence of individual DGGE bands as 1 or 0, respectively, in all lanes. Cluster analysis on the resulting matrices was performed using the unweighted pair group method with arithmetic means (UPGMA) algorithm to measure the relatedness between the analyzed community structures. A cluster dendrogram construction was conducted using PAST 3.03 software (Hammer et al. 2001). Non-metric multidimensional scaling (NMS), one of the most effective ordination methods for ecological community data (McCune and Grace 2002), was employed to visualize the shifts in archaeal and bacterial community structures over time in each reactor. NMS compresses a complex data set (e.g., the band profile of a DGGE lane) into a point in a low-dimensional space so that the distance between two points in a NMS plot reflects the dissimilarity between the corresponding data sets. A NMS calculation was performed using PC-ORD software 5.0 (MjM software, Gleneden Beach, OR, USA). The UPGMA clustering and NMS were both performed using the Sorensen distance measure generally recommended for community data (McCune and Grace 2002).
Real-time polymerase chain reaction
The Methanosaeta and Trichococcus 16S rRNA gene concentrations in the reactors were measured by real-time PCR using the Mst primers/probe set (Yu et al. 2005) and the Trich primer set (VandeWalle et al. 2012), respectively, which are among the most widely used for detecting the target microbes. Real-time PCR amplification was performed in a Quantstudio 12K Flex system (Life Technologies, Singapore). A reaction mixture for Methanosaeta detection (20 μL) was prepared using the Thunderbird Probe qPCR mix (Toyobo, Osaka, Japan): 10 μL of the premix, 2 μL of the TaqMan probe (final concentration, 200 nM), 1 μL of each primer (final concentration, 500 nM), 4 μL of PCR-grade water, and 2 μL of template DNA. On the other hand, for Trichococcus detection based on SYBR Green I chemistry, a reaction mixture was prepared using the Thunderbird SYBR qPCR mix (Toyobo): 10 μL of the premix, 1 μL of each primer (final concentration, 500 nM), 7 μL of PCR-grade water, and 1 μL of template DNA. Both target sequences were amplified in a two-step thermal procedure: pre-denaturation for 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. All Trichococcus runs were checked for amplification specificity by melting curve analysis (60 to 95 °C with a ramping rate of 0.05 °C/s) after the amplification cycles.
The quantitative standard curve for Methanosaeta was generated as previously described using an equimolar mixture of the standard plasmids as the standard template (Kim et al. 2013). A 10-fold serial dilution series (102–108 copies/μL) of the standard template was prepared and analyzed by real-time PCR with Mst-set. The crossing point (CP) values determined from the standard dilutions were plotted against the logarithm of their corresponding initial copy concentrations of template. The target sequence concentration in an unknown sample was quantified against the linear regression line of the resulting plot. The Trichococcus standard curve was prepared in the same manner using a standard plasmid made in this study as the standard template. The standard plasmid was constructed by cloning the target sequence PCR-amplified from the day 12 sample of R3 using the Trich-set into pGEM-T Easy vector (Promega, Madison, WI, USA). A wide dynamic range (r 2 > 0.999) over six or more log units was observed for both standard curves: 8.6 × 101–8.6 × 107 copies/μL for Mst-set and 8.7 × 101–8.7 × 108 copies/μL for Trich-set. Each DNA sample was analyzed in duplicate, and real-time PCR data were acquired and processed using Quantstudio 12K Flex Software ver. 1.2 (Life Technologies).
Analytical methods
COD was measured spectrophotometrically using HS-COD-MR kit (HUMAS, Daejeon, Korea). Solids were measured following the protocols in Standard Methods (APHA-AWWA-WEF. 2005). Volatile fatty acids (VFAs; C2–C7) were analyzed using a gas chromatograph (7820A, Agilent, Palo Alto, CA, USA) equipped with a flame ionization detector and an Innowax column (Agilent). The same gas chromatograph equipped with a thermal conductivity detector and a ShinCarbon ST column (Restek, Bellefonte, PA) was used to analyze biogas composition. Soluble COD and VFA analyses were performed with samples prepared by filtering through a membrane filter (pore size, 0.45 μm). All analyses were performed at least in duplicate. Biogas volume was measured by water displacement and converted to standard temperature and pressure (STP; 0 °C and 1 atm).
Results
Anaerobic digestion performance
The three reactors showed visibly different reaction profiles during the 30 days of batch operation. The residual-soluble COD (sCOD) concentration decreased rapidly along with an immediate production of hydrogen during the first 2 days of incubation and remained fairly constant at approximately 3.7 g/L until day 12 in all reactors (Fig. 1). Afterwards, however, the sCOD removal rates in the reactors diverged substantially, and its removal efficiency on day 30 was 82.5, 59.3, and 31.9 % in R3, R2, and R1, respectively. Correspondingly, the cumulative methane production during the operation period was highest in R3 (2529 mL), followed by R2 (1730 mL) and R1 (577 mL). Such differences in the organic removal rates are also reflected in the VFA profiles (Fig. 2). The total VFA (tVFA) level accumulated up to approximately 4 g COD/L during the initial few days in all reactors. Similar to the sCOD removal patterns, the lowest residual tVFA level (i.e., indicating largest tVFA consumption) after 30 days of operation was observed in R3 (0.8 g COD/L), followed by R2 (1.8 g COD/L) and R3 (3.7 g COD/L). These clearly indicate that the organic removal and methanogenesis rates were significantly higher in R2 and R3 supplemented with ferric oxides than in the control reactor R1. Acetate, propionate, and butyrate were the dominant acidogenic products in all reactors. The complete degradation of both acetate and butyrate was achieved within 30 days in R3. On the other hand, a significant level of acetate remained undegraded in R1 (2.2 g COD/L) and R2 (1.2 g COD/L) on day 30, while butyrate was almost completely removed. Especially in R1, the residual acetate concentration increased gradually with the degradation of butyrate till the end of the reactor operation. One mole of butyrate is broken down syntrophically to 2 mol of acetate and further converted to CH4 and CO2 via methanogenesis (Ahring 2003). The delayed acetate consumption, particularly in R1, may indicate that aceticlastic methanogenic activity was not high enough to efficiently handle the released acetate in the system. The faster and more effective stabilization of acetate in R2 and R3, on the other hand, may reflect a positive effect of the ferric oxides (FeOOH and Fe3O4 in R2 and R3, respectively) on aceticlastic methanogenesis. Meanwhile, no apparent degradation of propionate was shown in any reactor. This suggests that a 30-day operation period was not sufficient for effective syntrophic propionate oxidation, a highly endergonic (ΔG 0’ = +76.1 kJ/mol) process, in the reactors (Ahring 2003). Because of the thermodynamic hindrance, propionate oxidation occurs after the other easily utilizable VFAs are fully consumed in AD processes (Kobayashi et al. 2009). The residual propionate concentration on day 30 was considerably higher in R1 (821 mg COD/L) than in R2 (412 mg COD/L) and R3 (549 mg COD/L).
An interesting thing to note is that the cumulative production of hydrogen was highest in R1 (1068 mL), followed by R2 (835 mL) and R3 (661 mL), which is contrary to the methane production profiles (Fig. 1b). This, together with the VFA composition data on day 9 (Fig. 3), implies that the reactors varied in their acidogenic fermentation pathways likely due to the effect of the ferric oxides added. There is more than one pathway for the mixed-culture fermentation of organic matter, particularly when the substrate includes diverse organic compounds, such as the whey used in this study (Venetsaneas et al. 2009). Figures 2 and 3 show the evident differences in the acid production profiles between the ferric oxide-supplemented reactors and the control reactors. Although acetate accounted for the largest portion in all reactors, its proportion to the tVFA concentration on day 9 (i.e., the point of maximum tVFA accumulation) differed significantly among the reactors: 37.7 % in R1, 50.3 % in R2, and 55.1 % in R3. Correspondingly, the reactors showed high variations in the propionate/acetate ratio (0.53 in R1, 0.19 in R2, and 0.23 in R3) and the butyrate/acetate ratio (0.91 in R1, 0.64 in R2, and 0.45 in R3). It is generally known that butyric-type fermentation benefits biohydrogen production and thus the butyrate/acetate ratio correlates positively with hydrogen yield (Seifert et al. 2009; Wicher et al. 2013). This was in agreement with the hydrogen production profiles observed in the reactors tested. The changes in the butyrate/acetate ratio reportedly represent alterations in the metabolic characteristics of the reactor microbial communities (Khanal et al. 2004). The metabolic shifts towards acetic-type fermentation observed in R2 and R3 were supposedly induced by the ferric oxides added. The lesser production of hydrogen in the ferric oxide-supplemented reactors may also be in part attributed to the iron reduction consuming hydrogen as an electron donor (Caccavo et al. 1992; Lovley et al. 1989).
Energy recovery as biogas
Hydrogen production ceased on day 2, before which the biogas was composed mostly of hydrogen (≥90 % (v/v)) in all reactors and methane evolved after different lengths of delay in the reactors (Fig. 1b). The greatest 30-day cumulative methane production was in the order of R3, R2, and R1. This could be attributed to the higher production of acetate, along with the lower formation of longer-chain VFAs, during the acidogenesis in the ferric oxide-supplemented reactors (Figs. 2 and 3). It is well known that acetate is a more favorable substrate than other longer-chain VFAs for methanogenesis, and thus, a higher acetate/other VFA ratio generally leads to a higher methanogenesis rate (Ahring 2003). The methanogenesis profile was further described by fitting the cumulative methane production data to a modified Gompertz equation (Eq. 1) for each reactor as previously described (Lay et al. 1998).
where M(t) is the cumulative methane production (mL) at time t, P is the methane production potential (mL), R m is the maximum methane production rate (mL/d), λ is the lag phase length (day), and t is the incubation time (day). The estimated P and R m values were much greater in R2 (>2-fold) and R3 (>3-fold) than in R1, while the lag time was estimated to be longer in R1 than in the other reactors (Table 2). These, which were in agreement with the observed reaction profiles, suggest that the ferric oxide addition had a positive effect on the reactor performance in both terms of methanogenesis rate and yield. The total energy production as hydrogen plus methane during the experimental period, calculated based on the net heating value, was 32.2, 71.0, and 97.7 kJ (at STP) in R1, R2, and R3, respectively. This is due to the greater methane production in the ferric oxide-supplemented reactors and the greater heating value of methane (35.8 kJ/L) than of hydrogen (10.8 kJ/L). The recovery of COD as biogas was also examined in each reactor by balancing the sum of COD equivalents of hydrogen and methane produced against the substrate COD removed. Interestingly, COD recovery was nearly 100 % in both R2 and R3, while a significantly lower value of 79 % was obtained in R1. This implies that the production of hydrogen and methane served as the absolutely dominant electron sink in R2 and R3, while a substantial fraction was used for other metabolic functions in R1. This is another indication of changes in the reactor metabolic functions likely induced by the addition of ferric oxides.
DGGE analysis and phylogenetic affiliation
DGGE and further phylogenetic analysis were performed to characterize the archaeal and bacterial community structures in the reactors tested. The seed sludge and the reactor samples on days 1, 12, and 30 were examined to monitor the shifts in community structures during the 30-day operation period (Fig. 4). Two archaeal (WA1 to WA2) and twelve bacterial (WB1 to WB12) bands were excised from the DGGE gels and sequenced for phylogenetic affiliation (Table 3). Both the archaeal sequences were closely related (≥97 % sequence similarity) to a well-known methanogen species, while two out of twelve bacterial sequences were not. WA1 and WA2 were both closely related to Methanosaeta concilii and detected in all lanes as the prevailing methanogen bands, indicating that the aceticlastic pathway was probably the main route for methanogenesis in all reactors. These bands appeared with a significantly higher band intensity, although not robustly quantitative, in the 30-day samples of R2 and R3. This corresponds to the general knowledge that Methanosaeta species often dominate methanogen communities in stabilized AD systems with low levels of residual VFAs (Ahring 2003).
Ten out of twelve bacterial sequences were assigned to known bacterial species across three phyla, Firmicutes (WB1, WB2, WB4, WB8, WB10, and WB12), Proteobacteria (WB6 and WB9), and Bacteroidetes (WB3). The remaining two were classifiable only at the order (WB5) and the genus (WB11) level, respectively, but both were affiliated with the phylum Firmicutes. Six Firmicutes sequences (WB1, WB2, WB4, WB8, WB10, and WB11) were assigned to the obligatory anaerobic genus Clostridium of which members are frequently found in abundance in anaerobic fermentation processes. Clostridium species are metabolically very versatile and capable of utilizing a wide range of organic substances to produce different organic acids and hydrogen (Quéméneur et al. 2012). The Clostridium-related bands were observed from the early period, where acidogenesis with hydrogen production actively occurred, in all reactors. Most of the Clostridium sequences were affiliated with saccharolytic species, and the Clostridium populations in the reactors were likely responsible for the rapid fermentation of carbohydrate (mainly lactose) during the initial operation period. WB3 showed a high sequence similarity of 99.1 % to Macellibacteroides fermentans and Parabacteroides chartae, both of which can ferment carbohydrates, including lactose, into diverse organic acids (Jabari et al. 2012; Tan et al. 2012). Differentiating between M. fermentans and P. chartae strains based on the 16S rRNA gene sequence information is often difficult due to their high sequence similarity. For example, their type of strains shares a 16S rRNA gene sequence similarity of 99.7 % (Kitahara et al. 2013). WB3 was assigned to the genus Parabacteroides by the RDP classifier at a confidence threshold of 80 %. WB6, WB7, and WB9 were all affiliated with the genus Acinetobacter, which is known to be aerobic. The presence of Acinetobacter has, however, been extensively reported in anaerobic or oxygen-limited environments (Baek et al. 2014; Bertin et al. 2010; Supaphol et al. 2011; Ueno et al. 2006), and the Acinetobacter-related populations in the reactors supposedly played a functional role in the AD reactions. WB12 was affiliated with a fermentative genus, Trichococcus, which utilizes various sugars and polysaccharides (De Vos et al. 2009). The metal-reducing capability of Trichococcus pasteurii under anoxic/anaerobic conditions has recently been suggested. The abundant growth of T. pasteurii was reported with the microbial U(VI) and Fe(III) reduction in trench slurry (Fox et al. 2006) and the Cr(VI) reduction in the cathode biofilm of a microbial fuel cell (Tandukar et al. 2009). Accordingly, given the prominent occurrence of WB12 in R2 and R3, the corresponding Trichococcus-related population was suggested to be electroactive and directly involved in the functioning of the ferric oxides. WB5, the only band not affiliated to a known genus, was classified to the order Clostridiales, indicating that the population corresponding to this band probably took part in the fermentation of substrate organics.
Microbial community structure
Although the intensity of Methanosaeta-related bands (WA1 and WA2) varied with time and among the reactors, no marked differences or changes in the archaeal band patterns (Sorensen distance (D S ) < 0.15) were observed (Fig. 4). In contrast, the bacterial DGGE patterns showed clear variations over time and among the reactors (D S < 0.55). These results mean that the magnitude of changes in the community structure was significantly greater for bacteria (i.e., acidogens) than for archaea (i.e., methanogens) and that the changes in the bacterial community structure had little effect on the evolution of the methanogen community structure in the reactors. This could be ascribed to the much less diverse and dynamic characteristics of methanogens than of acidogens in AD environments, which are largely due to the limited substrate spectrum of methanogens (Kim et al. 2014a; Zumstein et al. 2000). The cluster dendrogram shown in Fig. 5a illustrates the relationships between the bacterial community structures analyzed. The bacterial community structures of three reactors grew apart with the progress of batch digestion, with the R1 community being remotely located from those of R2 and R3 on day 30. This, together with the acid production profiles (Figs. 2 and 3), further supports the metabolic pathways of the reactor microbial communities being changed by the influence of the ferric oxides added in R2 (FeOOH) and R3 (Fe3O4). Changes in the microbial community structure can lead to changes in metabolic functioning in a bioreactor as the functional attributes of a biological process depend on the activity of the microbes working in the system (Akarsubasi et al. 2005).
A cluster dendrogram (a) and NMS plot (b) illustrating the relationships between the bacterial community structures analyzed over the experimental period. Each data point is labeled with the reactor name and time of sampling in days. Arrows indicate the time-course community structure shifts in each reactor
The direction and magnitude of the temporal variations in the bacterial community structure in each reactor were visualized by NMS analysis (Fig. 5b). The first two ordination axes accounted for 71.0 % (r 2 = 0.710) and 18.1 % (r 2 = 0.181) of the total variance in the analyzed community structure data, respectively, and thus, 89.1 % (cumulative r 2 = 0.891) of the variability in the data can be explained by the two-dimensional plot. The final stress (<5) and instability (<10−4) of the solution were sufficiently low to provide a reliable NMS solution (McCune and Grace 2002). As in the cluster analysis, the community profiles of the three reactors were closely clustered initially, but gradually became more distantly related as the reaction proceeded. The seed bacterial community structure was located in the lower-right corner of the NMS plot and shifted with time in all reactors towards the upper-left region of the plot. The bacterial communities in the control and the ferric oxide-supplemented reactors evolved in visibly different directions after day 12, and the community profile of the control reactor was located distant from those of R2 and R3 at the conclusion of the batch test. Correspondingly, WB12 retrieved from a putative metal-reducing bacterium likely engaged in the utilization of the ferric oxides added was detected in R2 and R3 only. The NMS results linked with the reactor performance data (Figs. 1, 2, and 3) suggest that the ferric oxides induced the bacterial communities in R2 and R3 to develop and function differently from those in R1.
Microbial quantification
Real-time PCR analysis revealed that both Methanosaeta and Trichococcus were present in all reactors with a 16S rRNA concentration of >106 copies/mL during the operation period (Fig. 6). All reactors showed a continuous increase in the Methanosaeta level over time. This increase was much more pronounced in the ferric oxide-supplemented reactors, particularly in R3 with magnetite. On day 30, the Methanosaeta 16S rRNA gene concentrations in R2 and R3 were 5.1- and 16.4-fold higher than that in R1, which seems to reflect the more stabilized AD environment in R3 (Ahring 2003). The Trichococcus 16S rRNA gene concentration was also visibly higher in the ferric oxide-supplemented reactors compared with that in R1. Its abundance after 30 days of batch incubation was 2.9- and 10.4-fold higher in R2 and R3, respectively, than in R1, while it decreased with the stabilization of organic matter with time (Figs. 1 and 2). These observations suggest that the supplementation with (semi)conductive ferric oxides likely formed a favorable environment for Methanosaeta and Trichococcus to flourish in the AD systems.
Discussion
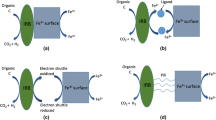
Our overall experimental data demonstrated that the addition of ferric oxyhydroxide (R2) and magnetite (R3) significantly enhanced the AD performance in terms of energy recovery as well as organic removal over the control reactor (R1). This implies that biostimulation with the (semi)conductive ferric oxides was beneficial for high-rate AD. An interesting point is that the performance enhancement was more pronounced in R3 than in R2, suggesting that the conductivity of the ferric oxides potentially influenced the performance variations among the reactors. This may, in part, explain the conflicting observations on the influence of ferric iron on AD in the previous studies mentioned in the “Introduction.” It seems that such differences are related to the crystallinity and conductivity of the ferric compounds rather than other factors, such as different inoculum sources and substrates. Kato et al. (2012) recently reported that the methanogenesis rate was improved by adding (semi)conductive ferric oxides (i.e., hematite and magnetite) in cultures enriched from a rice-paddy soil microcosm using synthetic media with acetate or ethanol as the sole substrate. On the other hand, no positive effect was recorded with insulative ferrihydrite for either carbon source. Limited solubilization of Fe(III) to Fe(II) was observed in the cultures supplemented with ferric oxides, and therefore, the authors attributed the accelerated methanogenesis mainly to the electric syntrophy rather than the nutritional effects or electron shuttling of soluble iron. They also revealed that H2-mediated syntrophy did not work in the ferric oxide-supplemented cultures and suggested that the syntrophy between the electroactive bacteria (i.e., Geobacter) and methanogens (i.e., Methanosarcina) mediated by direct electron flow through the ferric oxide particles supposedly led to enhanced methanogenesis. Similarly, more recent studies reported an accelerated methanogenesis from propionate by magnetite addition under mesophilic (Cruz Viggi et al. 2014) and thermophilic (Yamada et al. 2015) conditions. Such observations made with synthetic media correspond well with the results from our reactors fed with whey, a real wastewater containing various organic compounds, except that neither a Geobacter- nor a Methanosarcina-related sequence was found in our DGGE analysis.
Methanosaeta strains, the dominant methanogens in our reactors, have been thought to be able to grow exclusively on acetate and not to act as electron donors by directly utilizing electrons for methane generation, whereas Methanosarcina and several hydrogenotrophic methanogens have been suggested to have the ability to directly accept electrons from conductive materials (Cheng et al. 2009; Dinh et al. 2004; Uchiyama et al. 2010). However, a recent study on the genome sequence of Methanosaeta revealed the existence of a complete set of genes that are required for the reduction of carbon dioxide to methane (Smith and Ingram-Smith 2007). Additionally, more recent metagenomic and metatranscriptomic investigations on the anaerobic granules in an upflow anaerobic sludge blanket reactor treating synthetic brewery wastewater showed the dominance of M. concilii, with a high expression of the genes that are necessary for CO2 reduction to CH4 (Morita et al. 2011; Rotaru et al. 2014). These imply the potential of Methanosaeta to directly accept electrons and participate in the electric syntrophy, given that they cannot utilize H2 or formate as an electron donor to reduce CO2 (Kato et al. 2012; Rotaru et al. 2014). In our experiment, accordingly, the formation of an electric syntrophy between M. concilii-related methanogens (WA1 and WA2) and the electroactive bacteria, particularly a Trichococcus-related population corresponding to WB12, was suggested in the ferric oxide-supplemented reactors (Fig. 4 and Table 3). Supportively, real-time PCR analysis showed that both Methanosaeta and Trichococcus occurred in markedly higher abundance in R2 and R3 than in R1 (Fig. 6). This implies that the growth of the putative electric-syntrophy partners was stimulated by the (semi)conductive ferric oxides added. Interestingly, the Trichococcus 16S rRNA gene concentration was much higher in R3 (3.6–10.4-fold) than the other reactors on day 30 despite the fact that the residual level of utilizable organics was lowest in R3 (Figs. 1 and 2). The high abundance of Trichococcus in R3 may have been formed by the enhanced electric syntrophy owing to the conductive nature of magnetite. The different rates of methanogenesis in R2 and R3 may be attributed to the difference in conductivity between semiconductive ferric oxyhydroxide and conductive magnetite. Such differences in chemical and microbial properties were well reflected by the gradual divergence between the bacterial community structures of R2 and R3 with the progress of the batch culture (Fig. 5).
IRBs are capable of oxidizing poorly biodegradable organic compounds, such as aromatic hydrocarbons (Kim et al. 2014b). The enhanced degradation of refractory organics through ferric reduction may therefore also lead to an increase in methanogenesis rate. A recent work on the AD of waste-activated sludge reported an increase in methane production by ferric oxyhydroxide addition concurrent with a significant increase (2.3-fold) in the soluble Fe(II) concentration (Baek et al. 2014). This indicates that at least some of the ferric oxide particles added were reduced and consumed as the final electron acceptor (i.e., iron reduction) rather than being used as conduits for electron transfer (i.e., electric syntrophy). Unlike sewage sludge, however, the whey used as the substrate for our batch reactors is readily biodegradable and contains little refractory organic material. In such a case, active iron reduction can negatively affect methanogenesis because IRBs and methanogens compete for acetate (Lovely and Phillips 1987) and a system dominated by the slow-growing Methanosaeta, such as our experimental reactors, is known to be more sensitive to this competition. However, the acetate consumption was much faster and more effective in R2 and R3 than in R1, implying that the ferric oxides benefited the aceticlastic methanogens (Figs. 1 and 2). Therefore, the accelerated methanogenesis in the ferric oxide-supplemented reactors was more likely attributed to the electric syntrophy between the methanogens and electroactive bacteria mediated by the (semi)conductive ferric oxides. In support of this, a recent study revealed that magnetite facilitates extracellular electron transfer in a similar manner to c-type cytochromes and further demonstrated the compensation of the deficiency of the functions of a c-type cytochrome (OmcS) by magnetite (Liu et al. 2015).
Another point to consider is the fact that several Clostridium species are capable of using Fe(III) as a minor electron acceptor (<5 % of the reducing equivalent) for fermentative growth (Lovley 2006). Although these bacteria do not conserve energy from ferric reduction, thermodynamic estimations proved that fermentation coupled with ferric reduction is energetically more favorable than without it (Lovley and Phillips 1989). This implies that the growth of the fermentative iron reducers can potentially be stimulated by providing ferric iron. It is therefore interesting to observe that WB10, a DGGE band assigned to Clostridium, was observed to have a substantially greater intensity in R2 and R3 than in R1 during the active acidogenesis period (Figs. 2 and 4b). In addition, a previous study actually reported the increase in cellular activity of Clostridium chauvoei, to which WB10 was most closely related, by the addition of ferric salts (Scott 1926). These suggest that the Clostridium-related population corresponding to WB10 was likely a fermentative iron reducer and was stimulated to active growth in R2 and R3. Given that both C. chauvoei and Clostridium tertium (Table 3) are able to ferment lactose and produce acetate followed by butyrate as the major fermentation products (De Vos et al. 2009), this stimulated growth may have contributed to the alteration in the composition of the VFAs produced (i.e., higher proportion of acetate) in the reactors (Figs. 2 and 3). Such a shift in the metabolic characteristics towards more acetic-type fermentation seems to be beneficial to methanogenesis (Fig. 1). The biostimulation of fermentative iron reducers and its influence on methanogenesis have been little explored, and this interesting potential deserves further study.
References
Ahring BK (2003) Biomethanation I, vol. 1. Springer, New York
Akarsubasi AT, Ince O, Kirdar B, Oz NA, Orhon D, Curtis TP, Head IM, Ince BK (2005) Effect of wastewater composition on archaeal population diversity. Water Res 39:1576–1584
APHA-AWWA-WEF (2005) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association, Washington, DC
Baek G, Kim J, Lee C (2014) Influence of ferric oxyhydroxide addition on biomethanation of waste activated sludge in a continuous reactor. Bioresour Technol 166:596–601
Bertin L, Lampis S, Todaro D, Scoma A, Vallini G, Marchetti L, Majone M, Fava F (2010) Anaerobic acidogenic digestion of olive mill wastewaters in biofilm reactors packed with ceramic filters or granular activated carbon. Water res 44:4537–4549
Caccavo F, Blakemore RP, Lovley DR (1992) A hydrogen-oxidizing, Fe (III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl Environ Microbiol 58:3211–3216
Cheng S, Xing D, Call DF, Logan BE (2009) Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Technol 43:3953–3958
Cruz Viggi C, Rossetti S, Fazi S, Paiano P, Majone M, Aulenta F (2014) Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ Sci Technol 48:7536–7543
De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB (2009) In: Bergey’s Manual of Systematic Bacteriology, vol Vol. 3: The Firmicutes, 2nd edn. Springer, New York
Dinh HT, Kuever J, Muszmann M, Hassel AW, Stratmann M, Widdel F (2004) Iron corrosion by novel anaerobic microorganisms. Nature 427:829–832
Fernandez A, Huang S, Seston S, Xing J, Hickey R, Criddle C, Tiedje J (1999) How stable is stable? Function versus community composition. Appl Environ Microbiol 65:3697–3704
Fox JR, Mortimer RJ, Lear G, Lloyd JR, Beadle I, Morris K (2006) The biogeochemical behavior of U (VI) in the simulated near-field of a low-level radioactive waste repository. Appl Geochem 21:1539–1550
Hammer O, Harper DAT, Ryan PD (2001) PAST: Palaeontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hwang S, Hansen C (1998) Characterization of and bioproduction of short-chain organic acids from mixed dairy-processing wastewater. Trans ASAE 41:795–802
Ivanov VN, Stabnikova EV, Stabnikov VP, Kim IS, Zubair A (2002) Effects of iron compounds on the treatment of fat-containing wastewaters. Appl Biochem Microbiol 38:255–258
Jabari L, Gannoun H, Cayol J-L, Hedi A, Sakamoto M, Falsen E, Ohkuma M, Hamdi M, Fauque G, Ollivier B, Fardeau M-L (2012) Macellibacteroides fermentans gen. Nov., sp. nov., a member of the family Porphyromonadaceae isolated from an upflow anaerobic filter treating abattoir wastewaters. Int J Syst Evol Microbiol 62:2522–2527
Kato S, Hashimoto K, Watanabe K (2012) Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ Microbiol 14:1646–1654
Khanal SK, Chen W-H, Li L, Sung S (2004) Biological hydrogen production: effects of pH and intermediate products. Int J Hydrogen Energ 29:1123–1131
Kim J, Jung H, Lee C (2014a) Shifts in bacterial and archaeal community structures during the batch biomethanation of Ulva biomass under mesophilic conditions. Bioresour Technol 169:502–509
Kim J, Lee S, Lee C (2013) Comparative study of changes in reaction profile and microbial community structure in two anaerobic repeated-batch reactors started up with different seed sludges. Bioresour technol 129:495–505
Kim S-J, Park S-J, Cha I-T, Min D, Kim J-S, Chung W-H, Chae J-C, Jeon CO, Rhee S-K (2014b) Metabolic versatility of toluene-degrading, iron-reducing bacteria in tidal flat sediment, characterized by stable isotope probing-based metagenomic analysis. Environl Microbiol 16:189–204
Kitahara M, Sakamoto M, Tsuchida S, Kawasumi K, Amao H, Benno Y, Ohkuma M (2013) Parabacteroides chinchillae sp. nov., isolated from chinchilla (Chincilla lanigera) feces. Int J Syst Evol Microbiol 63:3470–3474
Kobayashi T, Yasuda D, Li Y-Y, Kubota K, Harada H, Yu H-Q (2009) Characterization of start-up performance and archaeal community shifts during anaerobic self-degradation of waste-activated sludge. Bioresour Technol 100:4981–4988
Lay J-J, Li Y-Y, Noike T (1998) Developments of bacterial population and methanogenic activity in a laboratory-scale landfill bioreactor. Water Res 32:3673–3679
Lee C, Kim J, Shin SG, O’Flaherty V, Hwang S (2010) Quantitative and qualitative transitions of methanogen community structure during the batch anaerobic digestion of cheese-processing wastewater. Appl Microbiol Biotechnol 87:1963–1973
Liu F, Rotaru A-E, Shrestha PM, Malvankar NS, Nevin KP, Lovley DR (2015) Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ Microbiol 17:648–655
Lovely D, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 53:1536–1540
Lovley D (2006) Dissimilatory Fe(III)- and Mn(IV)-Reducing Prokaryotes. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The Prokaryotes. Springer, New York, pp. 635–658
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 55:259–287
Lovley DR, Phillips EJ, Lonergan DJ (1989) Hydrogen and formate oxidation coupled to dissimilatory reduction of iron or manganese by Alteromonas putrefaciens. Appl Environ Microbiol 55:700–706
Lovley DR, Phillips EJP (1989) Requirement for a microbial consortium to completely oxidize glucose in Fe(III)-reducing sediments. Appl Environ Microbiol 55:3234–3236
Mamais D, Pitt PA, Cheng YW, Loiacono J, Jenkins D (1994) Determination of ferric chloride dose to control struvite precipitation in anaerobic sludge digesters. Water Environ Res 66:912–918
McCune B, Grace JB (2002) Analysis of Ecological Communities. Vol. 2. Glenden Beach, OR: MjM Software Design, USA.
Morita M, Malvankar NS, Franks AE, Summers ZM, Giloteaux L, Rotaru AE, Rotaru C, Lovley DR (2011) Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2:e00159–e00111
Muyzer G, De Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ microbiol 59:695–700
Qu D, Ratering S, Schnell S (2004) Microbial reduction of weakly crystalline iron (III) oxides and suppression of methanogenesis in paddy soil. Bull Environ Contam Toxicol 72:1172–1181
Quéméneur M, Hamelin J, Barakat A, Steyer J-P, Carrère H, Trably E (2012) Inhibition of fermentative hydrogen production by lignocellulose-derived compounds in mixed cultures. Int J Hydrogen Energ 37:3150–3159
Roden EE, Wetzel RG (2003) Competition between Fe(III)-reducing and methanogenic bacteria for acetate in iron-rich freshwater sediments. Microb Ecol 45:252–258
Rotaru A-E, Shrestha PM, Liu F, Shrestha M, Shrestha D, Embree M, Zengler K, Wardman C, Nevin KP, Lovley DR (2014) A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energ Environ Sci 7:408–415
Scott JP (1926) A method of increasing the virulence of Clostridium chauvoei by the use of ferric salts. J Infect Dis 38:511–513
Seifert K, Waligorska M, Wojtowski M, Laniecki M (2009) Hydrogen generation from glycerol in batch fermentation process. Int J Hydrogen Energ 34:3671–3678
Smith KS, Ingram-Smith C (2007) Methanosaeta, the forgotten methanogen? Trends Microbiol 15:150–155
Supaphol S, Jenkins SN, Intomo P, Waite IS, O’Donnell AG (2011) Microbial community dynamics in mesophilic anaerobic co-digestion of mixed waste. Bioresour Technol 102:4021–4027
Tan H-Q, Li T-T, Zhu C, Zhang X-Q, Wu M, Zhu X-F (2012) Parabacteroides chartae sp. nov., an obligately anaerobic species from wastewater of a paper mill. Int J Syst Evol Microbiol 62:2613–2617
Tandukar M, Huber SJ, Onodera T, Pavlostathis SG (2009) Biological chromium (VI) reduction in the cathode of a microbial fuel cell. Environ Sci Technol 43:8159–8165
Uchiyama T, Ito K, Mori K, Tsurumaru H, Harayama S (2010) Iron-corroding methanogen isolated from a crude-oil storage tank. Appl Environ Microbiol 76:1783–1788
Ueno Y, Sasaki D, Fukui H, Haruta S, Ishii M, Igarashi Y (2006) Changes in bacterial community during fermentative hydrogen and acid production from organic waste by thermophilic anaerobic microflora. J Appl Microbiol 101:331–343
Van Bodegom PM, Scholten JCM, Stams AJM (2004) Direct inhibition of methanogenesis by ferric iron. FEMS Microbiol Ecol 49:261–268
VandeWalle JL, Goetz GW, Huse SM, Morrison HG, Sogin ML, Hoffmann RG, Yan K, McLellan SL (2012) Acinetobacter, Aeromonas and Trichococcus populations dominate the microbial community within urban sewer infrastructure. Environ Microbiol 14:2538–2552
Venetsaneas N, Antonopoulou G, Stamatelatou K, Kornaros M, Lyberatos G (2009) Using cheese whey for hydrogen and methane generation in a two-stage continuous process with alternative pH controlling approaches. Bioresour Technol 100:3713–3717
Wicher E, Seifert K, Zagrodnik R, Pietrzyk B, Laniecki M (2013) Hydrogen gas production from distillery wastewater by dark fermentation. Intl J Hydrogen Energ 38:7767–7773
Yamada C, Kato S, Ueno Y, Ishii M, Igarashi Y (2015) Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J Biosci Bioeng 119:678–682
Yu Y, Lee C, Kim J, Hwang S (2005) Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol Bioeng 89:670–679
Zhang D, Chen Y, Zhao Y, Zhu X (2010) New sludge pretreatment method to improve methane production in waste activated sludge digestion. Environ Sci Technol 44:4802–4808
Zumstein E, Moletta R, Godon J (2000) Examination of two years of community dynamics in an anaerobic bioreactor using fluorescence polymerase chain reaction (PCR) single-strand conformation polymorphism analysis. Environ Microbiol 2:69–78
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) through Basic Science Research Program (2014R1A1A1002329) granted by the Ministry of Science, ICT, and Future Planning and also through International Cooperation Program managed by NRF (2013K2A1A2054369). The authors are also grateful for the support of the Korea Ministry of Environment (MOE) through a Waste-to-Energy Human Resource Development Project.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baek, G., Kim, J., Cho, K. et al. The biostimulation of anaerobic digestion with (semi)conductive ferric oxides: their potential for enhanced biomethanation. Appl Microbiol Biotechnol 99, 10355–10366 (2015). https://doi.org/10.1007/s00253-015-6900-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6900-y