Abstract
Pathogen detection is a critical point for the identification and the prevention of problems related to food safety. Failures at detecting contaminations in food may cause outbreaks with drastic consequences to public health. In spite of the real need for obtaining analytical results in the shortest time possible, conventional methods may take several days to produce a diagnosis. Salmonella spp. is the major cause of foodborne diseases worldwide and its absence is a requirement of the health authorities. Biosensors are bioelectronic devices, comprising bioreceptor molecules and transducer elements, able to detect analytes (chemical and/or biological species) rapidly and quantitatively. Electrochemical immunosensors use antibody molecules as bioreceptors and an electrochemical transducer. These devices have been widely used for pathogen detection at low cost. There are four main techniques for electrochemical immunosensors: amperometric, impedimetric, conductometric, and potentiometric. Almost all types of immunosensors are applicable to Salmonella detection. This article reviews the developments and the applications of electrochemical immunosensors for Salmonella detection, particularly the advantages of each specific technique. Immunosensors serve as exciting alternatives to conventional methods, allowing “real-time” and multiple analyses that are essential characteristics for pathogen detection and much desired in health and safety control in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salmonella is a major foodborne pathogen in the world and can infect animals and humans resulting in morbidity and mortality (Centers for Disease Control and Prevention 2012; European Food Safety Agency 2014). Genus Salmonella is composed of two species, Salmonella enterica and Salmonella bongori, seven subspecies, and more than 2500 serovars (based on antigenic composition), all of which are believed to be capable of causing human illnesses, such as typhoid fever (serovar Typhi), paratyphoid fever (serovar Paratyphi), and gastroenteritis (all other serovars) (Food and Drug Administration 2012). Salmonella enterica serovars Typhimurium and Enteritidis are the most commonly identified in foodborne diseases worldwide (Centers for Disease Control and Prevention 2012; European Food Safety Agency 2014). Conventional methods of detecting Salmonella in food follow a complex sequence of steps. Typically, it entails a nonselective pre-enrichment step, followed by selective enrichment, isolation on selective agar media, bacterial identification by biochemical testing, and serotyping—the entire process taking at least 5 days to reach a diagnosis (Andrews et al. 2015).
Official food safety agencies, such as US Food and Drug Administration (FDA), US Department of Agriculture (USDA), Association of Official Analytical Chemist International (AOACI), and International Organization of Standardization (ISO), recommend conventional culture methods as the most reliable and accurate techniques for foodborne pathogen detection. Nevertheless, advances in technology and innovations have given microbiological laboratories a variety of kits and instruments based on different mechanisms of detection such as polymerase chain reaction (PCR) and enzyme-linked immuno sorbent assay (ELISA), which take less time than the conventional methods. Many of these tests have emerged as alternatives to conventional methods to reduce the analysis time (Velusamy et al. 2010). Although easy to perform, most of these alternative tests need 24 h for pre-enrichment in order to increase target bacteria population and reach the detection limits of the tests (Lee et al. 2015). In this context, there is an increased interest in having rapid new methodologies with the advantages of rapid response (without pre-enrichment step), high sensitivity, and ease of multiplexing (readings of many samples simultaneously) in order to address the current challenges in food hygiene inspection.
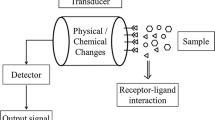
Biosensors are analytical devices, consisting of three associated elements: a bioreceptor or biological recognition element; a transducer (an electronic part which converts a biochemical signal from the interaction between analyte and bioreceptor into an electronic signal); and a processor, which amplifies and displays the analytical response signal. These innovative bioelectronic devices have a wide range of applications, such as diseases diagnosis, biomedicine, food processing, food safety, environmental monitoring, national defense, and security (Velusamy et al. 2010; Su et al. 2011; Holford et al. 2012; Saleem 2013). Currently, the most widespread application of this analytical tool is found in health care for the quantification of some substances produced by the human body, such as glucose, lactate, and cholesterol.
Most portable commercial biosensors have electrochemical transducers, which are easier to use in automatic devices (Skládal et al. 2013). Many companies have fabricated this type of biosensors especially for glucose detection. Electrochemical techniques are very sensitive and when associated with biomolecules it is possible to enhance the specificity of analysis. In general, electrochemical signal involved in analytical response depends on electronic movements resulting from oxidation-reduction reactions captured by the transducer. Clark and Lyons (1962) reported the first electrochemical biosensor by immobilizing glucose oxidase on the surface of an oxygen electrode. Since then, numerous types of biosensors have been developed for various substances in different areas. At present, there are biosensors capable of determining molecules involved in food quality control (Arora et al. 2011; Niraj 2012; Mortari and Lorenzelli 2014), biomedical and drug sensing (Vilarino et al. 2009; Vidal et al. 2013) and toxicity analysis in the environment (Gil and Mello 2010; Qureshi et al. 2012; Singh et al. 2014; Burcu and Kemal 2015). Biosensors have improved as a result of improved molecular and biochemical understanding of analytical response and supporting technologies. For these reasons, it is possible to find today biosensors that are very small, cheap, and interface-friendly. Currently, different types of biosensors are classified by the type of biological molecules immobilized (genosensors, immunosensors), by interaction with analyte (catalytic or enzymatic), by analytical response (direct or indirect), or by transducer (electrochemical, optical, or acoustic wave).
Antibodies represent one group of biomolecules, which interact readily with different types of analytes, especially biological contaminants such as bacteria and viruses, via specific recognition of their antigens (Holford et al. 2012). An immunosensor is a biosensor having an antibody on the surface as a bioreceptor, and it functions similarly as ELISA, except that it is faster, cheaper, and easier to handle as detailed in the next sections. The ELISA has been applied as a “gold-standard” for the validation of all recently developed immunoassays and immunosensors. One of the first papers on the use of immunosensors was written by Vo-Dihn et al. (1987); they demonstrated that antibodies could be engaged in situ for chemical carcinogen detection.
Analytical methods must overcome different challenges to detect bacteria efficiently. First, these detection methods have to be rapid to permit adoption as an emergency measure when necessary. Secondly, a high sensitivity is required, since the presence of even a single strain of pathogenic bacterium is able to develop an infection depending on the health status of the infected body and the virulence of the microorganism. Thirdly, detection must be extremely selective, especially in food, because a low number of pathogenic bacteria are often present in a complex matrix with proteins, fat, carbohydrates, hormones, and other nutrients. These kinds of molecules can hide the presence of bacteria. All the above challenges can be met by an electrochemical immunosensor, which proves to be a powerful tool in bacteria detection and prevention of bacterial outbreaks. The purpose of this paper is to review the developments and the applications of electrochemical immunosensors for Salmonella detection in food and to document the procedures for evaluation and characterization of the performance of immunosensors.
Alternative methods for Salmonella detection
A wide variety of alternative methods for Salmonella detection has been developed, and they can be grouped into several categories. Based on their operational principles, we can distinguish three main groups of techniques, immunology-based assays, nucleic acid-based assays, and biosensors. Among these methods, ELISA and PCR procedures have the specificity and the sensitivity that are almost similar to conventional methods. ELISA assays are able to detect Salmonella concentration at the level of 105 UFC mL−1 while PCR-based assays provide a level of sensitivity at 104 UFC mL−1 after pre-enrichment step (Lee et al. 2015). The sensitivity and specificity of these methods can be strongly altered by the intrinsic characteristics of the food involved, such as background microbiota, sample matrix, presence of non-culturable cells, and inhibitory substances (e.g., fats, proteins, carbohydrates, heavy metals, antibiotics, and organic compounds) (Mortari and Lorenzelli 2014). Thus, comparative studies are necessary to ensure that a particular assay is effective in analyzing a specific type of food. Some alternative tests have distinctive performance characteristics and are applicable to a restricted array of food.
Several papers reported comparisons between alternative and conventional methods. For example, Margot et al. (2013) compared methods for the detection of Salmonella species using pure cultures of Salmonella and others bacterial species commonly found in food products and concluded that rapid methods were as sensitive and specific as the conventional methods. Sometimes difficult matrices such as black tea can pose a problem with false negative results due to atypical colony colors. Therefore, rapid methods have some limitations in its application as will be shown in the following discussions on the three major categories.
Immunology-based assays
Immunology-based assays have been often used for the detection of Salmonella spp.; they generally employ specific antibodies that bind with antigens. This type of assays includes ELISA tests, latex agglutination tests, immunodiffusion, and immunochromatography. As in other rapid tests, these methods have some potential drawbacks for Salmonella detection such as the need of a prior pre-enrichment step to recover stressed cells, cross-reactions with closely related antigens, antigen variation, limits in sensitivity for some sample matrices, and high cost for automation and application to industrial scale.
Among immunology-based assays, ELISA has been the most commonly used for Salmonella detection with several commercial kits available on the market. ELISA method is a biochemical technique used to detect the presence of an antibody or an antigen in a sample. Briefly, it involves immobilization of a biomolecule (an antibody or antigen) onto a solid surface—with enzymes being used as markers for the presence of a specific antibody-antigen coupling. As examples, the most used commercial kits for Salmonella detection are Assurance GDS™ for Salmonella (BioControl Systems, Inc., Bellevue, WA), TECRA Salmonella (Tecra International Pty Ltd., French Forest, New South Wales, Australia), Salmonella ELISA Test SELECTA/OPTIMA (Bioline APS, Denmark), and Vitek Immuno Diagnostic Assay System (VIDAS) (BioMerieus, Hazelwood, MO) (Lee et al. 2015).
Nucleic acid-based assays
Also known as molecular methods, the nucleic acid-based assays are tests that utilize a specific nucleic acid target sequence within the organism’s genome (in this specific case, bacterial genome). The most widespread technique in this category of tests is the PCR method, a procedure based on the specific amplification of a short target DNA sequence. In recent years, molecular methods have attracted attention by providing enough specificity and sensitivity for detecting only one molecule of the target DNA in a defined sample. Because of the capability to detect a low concentration of Salmonella, enrichment times are considerably shorter to reach the Salmonella concentration needed for reliable detection by PCR when compared to other assays. However, tests based on nucleic acids (DNA and RNA) have some limitations, because they are specific in identifying genes and cannot pick out viable bacteria or detect the presence of toxins (Feng 2010). Examples of commercial rapid tests based on PCR for Salmonella detection include ABI Prism 7500 (Applied Biosystems, Warrington, UK), Probelia (Sanofi-Diagnostics Pasteur, Marnes-la-Coquette, France), BAX system (DuPont Qualicon, Wilmington, DE), TagMan (PE-Applied Biosystems, Foster City, CA), Gene-Trak (Neogen Corporation, Lansing, MI), iQ-Check™ PCR (BioRad Laboratories, Hercules, CA), LightCycler (Roche Diagnostics, Manheim, Germany), and SmartCycler (Cepheid Inc., Sunnyvale, CA) (Lee et al. 2015).
Biosensors
Microbial biosensor represents a rapidly developing research area, and there are numerous publications in this area. Biosensors have the potential to shorten the time between sampling and results, but they need improved selectivity and sensitivities and reduced cost, when compared to other methods. The use of biosensors permits both miniaturization and automation. It is possible to work with sample volumes in the range of nanoliters or less, which implies a lower cost of reagents. Also, multi-analyte analysis can be done in the same device, which shortens the analysis time. Biological recognition elements used in the biosensor application include enzymes, antibodies, nucleic acids, whole cells, tissue/whole organisms, and biomimetic materials. The signal recognition of biosensors is achieved through different types of transducers: electrochemical, optical, thermometric, and piezoelectric. There are many papers related to the development of biosensors for Salmonella detection (e.g., Afonso et al. 2013; Dong et al. 2013; Chumyim et al. 2014; Freitas et al. 2014; Hu et al. 2014; Ma et al. 2014). Table 1 lists some immunosensors including their detection limits and detection times.
Assembly and evaluation of electrochemical immunosensors
The basic composition of an immunosensor consists of antibodies immobilized on an electrode surface. The surface must have appropriate electrochemical characteristics, in addition to being compatible with the immobilization method. A wide variety of materials can be used for the surface; among them gold has been applied most frequently (Ricci et al. 2012), because it is an inert metal and compatible with cell structures and biomolecules. However, gold and other metals normally do not allow adhesion of biomolecules; therefore, they require some form of surface modification. Self-assembled monolayers (SAMs) are highly ordered molecular assemblies formed spontaneously by chemisorption and self-organization of molecules on the surface (Prashar 2012). This technique has been recently applied to modify the electrode surface and found to be useful in immunosensor assembly because it allows a high degree of control of the composition and thickness of the transducer surface. Surface functional groups (−CN, −NH2, or –SH) on SAMs form covalent bond with biomolecules and metal surface and serve as bridges among them. Short-chain molecules such as cysteamine can be self-assembled on the electrode and confer a lower degree of blockage for electron transfer than long-chain molecules (Anandan et al. 2009). Thus, the biomolecule which is used in SAM formation must be chosen carefully to match the type of transducers used.
Antibody immobilization on sensor surface is considered a critical point to the sensitivity and specificity determination of immunosensor. There is no perfect immobilization method that provides high sensitivity and superior stability for these devices. Optimization studies for each kind of device must be carried out to produce the best responses. In the literature, some methods have been reported that orient the antibodies, leaving the antigen-recognizing region (paratope region) free while the fragment crystallizable (Fc) region of the antibody is surface-bound. Oriented immobilization of antibodies through protein A and protein G has been successfully achieved in the process of immunosensor development (Liu et al. 2012; Ferreira and Sales 2014; Derkus et al. 2014; Cao et al. 2015).
The basic structure of an immunosensor with antibody immobilization oriented by a protein is illustrated in Fig. 1. In this figure, we schematically show the immunosensor (work electrode) enclosing an electrochemical cell containing the counter and the reference electrodes, thus forming an electrochemical system. In the next scheme, the immunosensor structure is shown sequentially. First, a self-assembled monolayer (SAM) was formed on the electrode surface, and it acted as a bridge between the metal (electrode surface) and protein A linked by a covalent bond. This arrangement provides for protein A to bind to the primary antibody in order to recognize the antigen. A secondary antibody labeled with enzymes is used to generate an analytical response as a sandwich system. The enzymes function as markers because when conjugated to the secondary antibody they catalyze the reaction with their substrates. As products of this reaction, electroactive species emit analytical response signal for the immunosensor (Fig. 1). In indirect immunosensors, different kinds of markers can be utilized beyond the enzymes (Zhao et al. 2016), like biotin (Martín-Yerga et al. 2013), avidin (Kim and Choi 2014), and nanoparticles (Özel et al. 2014).
Mediators are commonly used in this type of biosensors even in commercial ones. They are called biosensors of second generation (Murugaiyan et al. 2014). Mediators are low molecular weight molecules that participate in redox processes with a high rate of electron transfer. During the catalytic reaction, the mediator reacts with the prosthetic group of enzyme and diffuses to the electrode surface in order to receive or transfer electrons (Dominguez-Benetton et al. 2013). Additionally, the work potential is determined by the oxidation/reduction potential. This application is important because in the presence of a mediator, the electrochemical reaction becomes less dependent on the oxygen concentration in the solution. The use of mediators in redox processes is advantageous also because they reduce the operating potential of the device, thus avoiding interference from unwanted redox species.
During immunosensor development, two factors need to be considered. The first factor is characterization, and it is necessary after each stage of assembly to confirm the efficiency of the immobilization set-up. The second factor is the evaluation of the device performance in real food samples, since this is the main goal of device development. These two factors are described below.
Characterization of immunosensors
The immunosensors may be characterized by using the same techniques employed for the analytical response of the device. In this case, each immunosensor is characterized by morphology, topography, electrochemical behavior, or by the presence of functional groups on the surface as determined by attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR). In amperometric immunosensors, it is common for the electrochemical characterization to be done by cyclic voltammetry in potassium ferricyanide K3[Fe(CN)6] solution. In this case, it is possible to observe the decrease in current electric amplitude of the cathode and anodic peaks due to isolation in the electron flow after each layer is formed on the electrode surface. K3[Fe(CN)6] is a redox couple used in the studies of electrochemical characterization (Mantzila et al. 2008). Pimenta-Martins et al. (2012) characterized the changes on the surface during the immunosensor assembly using the redox probe K3[Fe(CN)6/K4[Fe(CN)6]. After pretreatment, the gold electrode was covered with cysteamine and the penetration of the redox probe close to the surface electrode was found to be slightly reduced. Subsequently, the immobilization of protein A on the modified gold electrode left the penetration of the redox probe further reduced. This fact was also validated from the binding of the (antibody-antigen-antibody) sandwich assembly.
Microscopy is often used for morphological and topographic studies (Kaur et al. 2004). This is important because each assembly step for an immunosensor causes changes on the surface that can be monitored. Canbaz and Sezgintürk (2014) characterized the surface morphologies of the proposed biosensor layer. The modified surface with anti-HER-3 had an almost uniform granular morphology attributed to the dispersion of protein onto the surface. After application of bovine serum albumin (BSA) used for blocking active ends of the surface, the granular morphology of anti-HER-3 changed into an even more granular form due to the three-dimensional structure of BSA. Topographic characterization using atomic force microscopy (AFM) in the contact mode did not rupture the protein surface; thus, images of the protein-coated surfaces could be obtained (Hayes et al. 1998; Coen et al. 2001). Lee et al. (2003) verified that the surface topography was increased by depositing modified protein A, and there were antibodies immobilized onto the self-assembled protein A layers as an aggregate. Moreover, FTIR spectra show spectral features corresponding to the amide II bands of IgGs (β-sheet, main secondary structure element of IgG) indicating the presence of IgGs immobilized onto the film.
FTIR is a non-destructive spectroscopic technique recommended for the characterization of immunosensors in order to obtain information concerning the interfaces and the nature of the bonds in the material and at the interface. Many papers have reported the use of this technique to characterize immunosensor assembly. Sibai et al. (1996) verified that the FTIR spectrum of the antibody obtained was characteristic of a protein, for amide I was present around 1660 cm−1, amide II around 1550 cm−1, as well as the N-H band around 3300 cm−1 that was overlapped with O-H band at 3430 cm−1.
Quartz crystal microbalance (QCM) technique can be very useful for characterizing the assembly of immunosensors and providing relevant information about interfaces and surfaces involved in the electrochemical response. This technique consists of mass sensitive detectors which operate on oscillating crystals resonating at the fundamental frequency of the quartz crystal (Babacan et al. 2000). Each change in the surface causes perturbations in the frequency of the crystal, which is associated with binding or desorption of molecules. QCM technique has been used to compare different immobilization methods and determine the best conditions for the biosensor response. Many papers have reported QCM characterization of immunosensors for Salmonella detection based on the layer-by-layer or the self-assembly technique for immobilization of the biomolecules (Pathirana et al. 2000; Si et al. 2001; Wong et al. 2002; Olsen et al. 2003). The results provided new perspectives on the development of amperometric sensors using a similar assembly system. Furthermore, the QCM technique has been used to confirm the specificity and applicability of immunosensors during repetitive use after the regeneration step (Prusak-Sochaczewski et al. 1990; Park et al. 2000).
Evaluation of immunosensors in real samples
After it is assembled, the immunosensor should be studied in order to optimize the analytical responses and to improve sensitivity and other operational parameters. Operational parameters include linearity, quantification limit, detection limit, accuracy, precision (reproducibility and repeatability), and specificity. These parameters can also be influenced by temperature, pH, and other environmental conditions. Parallel to these studies, the conditions that can reduce interfering substances present in solution should be verified. Sample preparation should be studied in order to understand the best form to submit to the biosensor. Undoubtedly, the preferred mode is to analyze the sample in nature, i.e., without any type of pretreatment, but for the most part, the sample needs to go through some pretreatments. Like other rapid tests such as immunological tests, the sample can preferably be centrifuged with a solvent in order to remove interfering elements such as fats in foods. For Salmonella detection, rapid testing kits usually also require a pre-enrichment step. Biosensors are advantageous in comparison to other methods because in general they do not need any sample treatment. Yet, in the specific case of immunosensors with a marker (i.e., not label-free), the final measurement depends on the addition of a substrate to the marker in a standard solution after the incubation step with the sample. For label-free immunosensors, the response is direct and depends exclusively on the nature of sample (not on fat or pH), if a response is obtained after contact with sample. In both cases, it is necessary to wash the sample after incubation time to eliminate all free substances that are not bound to the antibody.
Electrochemical immunosensors for Salmonella detection
Electrochemical immunosensors can be based on potentiometric, amperometric, impedimetric, or conductometric transduction principles. A working electrode, a counter electrode, and a reference electrode usually compose the electrochemical sensor (Fig. 1). In the specific case of immunosensors, antibodies are immobilized on the working electrode and the signal is generated as a function of electronic transfer which occurs between working electrode and counter electrode. This signal is proportional to analyte concentration present in the sample.
Bacteria present in food can promote reactions, and they can be detected by applying appropriate electrochemical methods. For example, when microorganisms metabolize uncharged substrates to a charged product, such as the conversion of carbohydrates to lactic acid, a change in the conductivity of the medium occurs. Microbial growth can be shown in the same way by an increase in both conductance and capacitance, causing a decrease in impedance. Another evidence of microbial metabolism can be verified by the hydrolysis of specific substances due to enzymatic activity at the microbial layer, and it can be accompanied by the production of protons near the pH electrode. The response comes from the change of electric potential difference between working electrode and reference electrode, which are separated by a selective membrane. Furthermore, the specific interaction between biomolecules, like an enzyme and its substrate or an antibody and its antigen, can produce an electronic transfer capable of generating an electric current in an applied potential, which is related to the concentration of the species in solution. These characteristics can be observed during bacterial presence in food and may be used for the development of different electrochemical immunosensors for Salmonella detection. In the following section, we explore each of these features. Some examples of the techniques for immunosensor are summarized in Table 1.
Amperometric immunosensors
Amperometric measurements are based on electrical current between working and counter electrodes as a function of analyte concentration after applying a constant potential. This technique is preferred in the development of many biosensors. Most of the current commercial biosensors utilize this technique. In the literature, there are many papers with different modes of immobilization and detection using amperometric technique. It is necessary because each biological molecule has a preferred immobilization method and specific steps for analyte detection for any given application. In amperometric immunosensors, the direct sandwich ELISA format is very common in the device assembly, whereas it is possible to find a variety of immobilization methods for the primary antibody and labeling of the secondary antibody (Fig. 1).
Amperometric immunosensors represent a modern version of ELISA, which often incurs false negative results due to extremely low amounts of contaminants in sample. Conventional ELISA for Salmonella spp. detection provides a limit of detection (LOD) of 104–105 CFU mL−1 (Lee et al. 2015). Some immunosensors reported have a detection limit much lower than the ELISA methods (Salam and Tothill 2009; Freitas et al. 2014; Zhu et al. 2014). As we can see in Table 1, there are amperometric immunosensors with LOD as low as 6 CFU mL−1 (Zhu et al. 2014). Another aspect observed is the detection time that varies from 50 min to 3 h in amperometric devices. Moreover, the main advantage of immunosensors using a sandwich system in a solution is the response that is free of interferences, thereby reducing the risks of false positives. This happens because bacteria bind to the primary antibody immobilized on the surface, and the response occurs as a function of the labeled secondary antibody that links to bacteria and remains in immunosensor surface after successive washes of surface. The washes are important in order to remove nonbonding molecules, and an electric current is produced by electron transfer from the substrate reaction (Fig. 1). In this kind of immunosensor, it is very common to use mediators, which generally reduce the electrical potential of immunosensors. The decrease in electrical potential is important in reducing the chances of biomolecular denaturation and the interference with other substances in the sample. The main mediator used in biosensors is ferrocene (Morales et al. 2007), but there are other mediators such as ferricyanide and osmium that can be used (Bally and Voros 2009; Alonso-Lomillo et al. 2010; Vashist et al. 2011; Kirsch et al. 2013).
In our laboratory, we have worked with thiol and protein A in order to orientate the primary anti-Salmonella antibody and have achieved an excellent detection limit of 10 CFU mL−1. Salam and Tothill (2009) immobilized monoclonal antibody against Salmonella Typhimurium using physical and covalent immobilization via amine coupling of carboxymethyldextran on gold surface. A sandwich ELISA format was developed using a polyclonal anti-Salmonella antibody conjugated to horseradish peroxidase (HRP) as an enzyme label. An electron transfer mediator, 3,3,5,5-tetramethylbenzidine dihydrochloride (TMB) with H2O2 as substrate system, was utilized. Detection levels of 5 × 103 cells mL−1 and 20 cells mL−1 were achieved respectively for physical and covalent immobilization. Delibato et al. (2006) developed a multichannel electrochemical immunosensor for the detection of Salmonella. It consisted of a disposable screen-printed array, coupled with a multichannel pulse monitor, which was assembled in a sandwich system. Croci et al. (2001) preferred to seed the contaminated samples in pre-enrichment broth (buffer peptone water), and samples were taken at different times and analyzed by immunosensor to determine the minimum incubation time needed to detect Salmonella. The results showed that this method was efficient and sensitive only after 5 h of incubation in pre-enrichment broth. It was possible to detect Salmonella in meat artificially contaminated with low concentrations of Salmonella (1–10 cells 25 g−1).
In addition to the adoption of enzyme labels in this technique, magnetic particles are also widely used. Superparamagnetic particles are highly attractive for use in biosensors for their capability to magnetize under an applied magnetic field. Analytes can be labeled with magnetic beads as an immobilization platform and as a tool to separate measurable molecules found in immunosensors (Wang 2005). The particles can be separated easily from a liquid phase with a small magnet but can be redispersed immediately once this magnet is removed. When coated with recognition molecules, magnetic spheres are ideal for efficient capture and separation of target. Unwanted sample constituents may be washed away, following a simple magnetic separation step. Several procedures other than amperometric technique may be used for subsequent final measurements, such as conventional impendance and wave accoustic assays (Liu et al. 2001; Kim et al. 2003). More recently, magnetic beads have been used not only for labeling and separation of an analyte but also for direct quantification of antibodies with this particular label (Brzeska et al. 2004; Meyer et al. 2007). Gehring et al. (1996) used antibody-coated superparamagnet beads in a format termed enzyme-linked immunomagnetic electrochemistry. Salmonella Typhimurium was sandwiched between antibody-coated magnetic beads and an enzyme-conjugated antibody. With the aid of a magnet, beads (with or without bound bacteria) were localized onto the surface of disposable graphite ink electrodes in a multi-well plate format. With this technique, a minimum detectable level of 8 × 103 cells mL−1 of Salmonella Typhimurium in a buffer was achieved in about 80 min.
Other nanoparticles such as carbon nanotubes and gold nanotubes have been used on the surface of electrodes in order to increase the active surface area for immobilization of biomolecules and consequently the sensitivity for the biosensor. Chumyim et al. (2014) developed an immunosensor with a detection limit of 103 CFU mL−1 Salmonella based on tyrosinase-amplified labeling platform and the recycling system of catechol/o-quinone redox couple with multiwall carbon nanotubes as an amplified labeling electrochemical sensor.
Impedimetric immunosensors for Salmonella
Impedimetric biosensors are less frequently compared to potentiometric and amperometric biosensors; nevertheless, there have been some interesting publications on these immunosensors. Impedimetric immunosensors function by antigen-antibody interaction causing a change in capacitance and electron transfer resistance across a working electrode (Pohanka and Skládal 2008). For these cases, electrochemical impedance spectroscopy (EIS) is often engaged to characterize the surfaces after the immobilization of biomolecules and binding of antigen. The most popular format for evaluating EIS data are the Nyquist and Bode plots (Wang et al. 2012). In the Nyquist plot, the imaginary impedance component (z″) is plotted against real impedance component (z′). In the Bode plot, both the logarithm of the absolute impedance (ǀZǀ) and the phase shift (φ) are plotted against the logarithm of the excitation frequency. Impedance immunosensors can be classified into two main categories according to Prodromidis (2010): (a) capacitive—where the surface of the electrode is completely covered by a dieletric layer and the whole electrode assembly behaves as an insulator. In this type of sensor, no redox probe is present in the measuring solution and the capacitive current is measured under a small-amplitude sinusoidal voltage signal at low excitation frequencies (typically 10–1000 Hz). The antibody-antigen interactions are expected to cause a decrease in the measured capacitance; and (b) faradic or faradaic impedimetric—where the surface of the electrode, which is covered by an insulating layer, is able to catalyze a redox probe placed in the measuring solution. In this case, the measured parameter is the charge transfer resistance (the real component of impedance at low frequency values, typically 0.1–1.0 Hz) and antibody-antigen interactions are expected to cause an increase in its value as the faradic reaction becomes increasingly hindered. In general, faradic impedimetric immunosensors exhibit a higher sensitivity due to antibody-antigen interaction. However, the redox probes may have an effect on both the stability and the activity of the electrode.
Various types of impedimetric immunosensors for Salmonella based on the different types of formation of sensitive layer such as electropolymerization and self-assembly have been proposed. Pournaras et al. (2008) employed functional impedimetric immunosensors based on polytyramine electropolymerized films for the detection of Salmonella Typhimurium in real samples. Interestingly, since the detection was performed directly on cultures, it eliminated various centrifugation and washing steps, which were used for the isolation of bacteria cells from culture, thus making the proposed immunosensors promising candidates for on-site applications. Yang et al. (2009) described a capacitive immunosensor for Salmonella spp. detection based on grafted ethylene diamine and self-assembled gold nanoparticle on glassy carbon electrode. The antibodies were immobilized on gold nanoparticle and the limit of detection was found to be 1.0 × 102 CFU mL−1. Dev Das et al. (2009) studied self-assembled array for trapping channels on oxidized macroporous silicon substrate for detection of Salmonella Typhimurium. It was found that oxidized macroporous silicon substrate with its regular network of pores at 1–2 μm diameter is capable of detecting concentrations from 103 CFU mL−1 to 107 CFU mL−1 in pure culture of the bacteria.
The development of faradic impedimetric immunosensors for the detection of Salmonella Typhimurium in milk also were reported (Rickert et al. 1996; Mantzila et al. 2008). The alteration of the interfacial features of the electrodes due to different modification or recognition steps was measured in the presence of hexacyanoferrate (II)/(III) redox couple. A substantial amplification of the measured signal was achieved by performing the immunoreaction directly in culture samples. The efficiency of the immunosensors was evaluated in a series of standard culture samples over the final concentration range of 102–106 CFU mL−1 for Salmonella Typhimurium (Mantzila et al. 2008). However, the hexacyanoferrate (II)/(III) system was found to damage SAMs or to reduce the activity of the immobilized protein according to Rickert et al. (1996) who developed a mixed self-assembled monolayer of a synthetic peptide and 11-hydroxyundecanethiol. Faradic impedance spectroscopy is usually considered more sensitive as compared to capacitance measurements at electrically blocked electrodes.
Although promising results have been achieved with impedimetric immunosensors for Salmonella detection, some papers reported in the literature have highlighted the complexity of the analytical procedures (Nandakumar et al. 2008; Nguyen et al. 2014).
Conductometric immunosensors
This kind of immunosensor is based on the consumption or production of charged species, thus leading to conductance changes resulting from the antibody-antigen interaction. This immunosensor can be labeled free or not free. In the former case, the use of enzymes is often adopted because a large number of enzymes are known to produce changes in the conductivity of the sensor surface (Table 2). Some enzymes produce ionic products that increase the conductivity, but there are also those, for example, glucose oxidase, whose products induce a decrease in conductivity (Soto et al. 2001). Immunosensors based on conductometric principle present some advantages: they do not require any reference electrode; driving voltage can be sufficiently low to decrease significantly the power consumption; and transducers are not light sensitive (Jaffrezic-Renault and Dzyadevych 2008). Nevertheless, in the literature, there are relatively few papers on this subject, especially involving Salmonella detection. Muhammad-Tahir and Alocilja (2003) proposed an immunosensor for Escherichia coli O157:H7 and Salmonella spp. based on sandwich assay and using polyaniline as the antibody label. The signal (change in conductance) was due to the presence of polyaniline, a conductive polymer, which increased its signal intensity when Salmonella was present. This immunosensor provided a specific, sensitive, low-volume, and near real-time detection mechanism for the lower limit of detection of 7.9 × 101 UFC mL−1 within a 10-min process. However, it was limited for use at high concentrations of Salmonella because binding sites may be over-occupied with the antigen, thus obstructing the charge transfer within the conductive polymer structure. In general, the wide application of conductometric immunosensors has been hindered by specific difficulties, such as low specificity of the technique and the need to employ certain experimental conditions (e.g., buffer concentrations and dissolution of ingredients in solution). The latter conditions are prompted by the need to avoid reduction in signal/noise ratio (which should be greater than 2 %).
Potentiometric immunosensors
Potentiometric transducers measure the potential difference between working and reference electrodes. Potential changes may be caused by electrochemical, chemical, or biological interactions. Thus, changes in pH, ionic, or redox at the surface influence the response of potentiometric sensors. Examples of potentiometric sensors are the solid state ion selective field effect transistors (ISFETs) and pH electrode-based ion selective electrodes (ISEs). These are used for pH, ion, chemical, or gas sensing and are marketed by iStat Corp and others. There are few examples of potentiometric biosensors that are generally applicable to enzymes or immunosensing system. An example is light-addressable potentiometric sensor (LAPS) used in the Molecular Diagnostics cytosensor and Threshold System (Dill et al. 1999; Purvis et al., 2003). On the other hand, potentiometric transducers for Salmonella detection in food are rarely cited in the literature. A notable report is a LAPS used in the threshold system for Salmonella detection in chicken carcass capable of detecting levels as low as 119 CFU mL-1 (Dill et al. 1999).
Conclusion
Immunosensors offer an exciting alternative to the more traditional assay methods, allowing rapid “real-time” and multiple analyses that are essential for the detection of Salmonella and other microorganisms in food, especially in perishable or semi-perishable foods. Although conventional methods in the detection of Salmonella and other microbial contaminants can be very sensitive and inexpensive, they require several days to yield results. Thus, immunosensors represent a promising and faster alternative tool to ensure food safety. It is worth noting that the device performance and the commercial prospect for future sensor systems may vary depending on transducer’s properties, improvements in optimization responses, and operational parameters. Future immunosensors with increased sensitivity, lower costs, and easier handling are highly desirable. Such immunosensors will be very useful for the rapid and routine detection of Salmonella in foods both in the field and in laboratories.
References
Afonso A, Perez-Lopes B, Faria RC, Mattoso L, Hernandez-Herrero M, Roig-Sagues A, Maltez-Da CM, Merkoci A (2013) Electrochemical detection of Salmonella using gold nanoparticles. Biosens Bioelectron 40:121–126
Alonso-Lomillo MA, Domínguez-Renedo O, Arcos-Martínez MJ (2010) Screen-printed biosensors in microbiology; a review. Talanta 82:1629–1636
Anandan V, Gangadharan R, Zhang G (2009) Role of SAM chain length in enhancing the sensitivity of nanopillar modified electrodes for glucose detection. Sensors 9:1295–1305
Andrews WH, Jacobson A, Hammack T (2015) Food and Drug Administration. Bacteriological Analytical Manual (BAM). Chapter 5 Salmonella Available at: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149. htm (accessed 06.01.16)
Arora P, Sindhu A, Dilbaghi N, Chaudhury A (2011) Biosensors as innovative tools for the detection of food borne pathogens. Biosens Bioelectron 28:1–12
Babacan S, Pirarnik P, Letcher S, Rand AG (2000) Evaluation of antibody immobilization methods for piezoelectric biosensor application. Biosens Bioelectron 15:615–621
Bally M, Voros J (2009) Nanoscale labels: nanoparticles and liposomes in the development of high-performance biosensors. Nanomed 4:447–467
Brandão D, Liébana S, Campoy S, Cortés P, Alegret S, Pividori MI (2013) Electrochemical magneto-immunosensing of Salmonella based on nano and micro-sized magnetic particles. J Phys: Conf Series 421(012020):1–7
Brzeska M, Panhorst M, Kamp PB, Schotter J, Reiss G, Pühler A, Becker A, Brückl H (2004) Detection and manipulation of biomolecules by magnetic carriers. J Biotechnol 112:25–33
Burcu BE, Kemal SM (2015) Applications of electrochemical immunosensors for early clinical diagnostics. Talanta 132:162–174
Canbaz MÇ, Sezgintürk MK (2014) Fabrication of a highly sensitive disposable immunosensor based on indium tin oxide substrates for cancer biomarker detection. Anal Biochem 446:9–18
Cao Y, Sun X, Guo Y, Zhao W, Wang X (2015) An electrochemical immunosensor based on interdigitated array microelectrode for the detection of chlorpyrifos. Bioprocess Biosyst Eng 38:307–313
Centers for Disease Control and Prevention (2012) Pathogens causing US foodborne illnesses, hospitalizations, and deaths, 2000–2008 http://www.cdc.gov/foodborneburden/PDFs/pathogens-complete-list-01-12.pdf. Accessed 10 December 2015
Chumyim P, Rijiravanich P, Somasundrum M, Surareungchai W (2014) Detection of Salmonella enterica serovar Typhimurium in milk sample using electrochemical immunoassay and enzyme amplified labeling. Int Conf Agric Environ Biol Sci (AEBS-2014) Phuket (Thailand) 24–25
Clark LC Jr, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Am NY Acad Sci 31:29–45
Coen MC, Lehmann R, Groning P, Bielmann M, Galli C, Schlapbach L (2001) Adsorption and bioactivity of protein A on silicon surfaces studies by AFM and XPS. J Colloid Interf Sci 233:180–189
Croci L, Delibato E, Volpe G, Palleschi G (2001) A rapid electro-chemical ELISA for the detection of Salmonella in meat samples. Anal Lett 34:2597–2607
Delibato E, Volpe G, Stangalini D, De Medici D, Moscone D, Palleschi G (2006) Development of SYBR-green real-time PCR and a multichannel electrochemical immunosensor for specific detection of Salmonella enterica. Anal Lett 39:1611–1625
Derkus B, Kc E, Mazi H, Emregul E, Yumak T, Sinag A (2014) Protein a immunosensor for the detection of immunoglobulin G by impedance spectroscopy. Bioprocess Biosyst Eng 37:965–976
Dev Das R, Roychaudhuri C, Maju S, Das S, Saha H (2009) Macroporous silicone based simple and eficiente trapping platform for electrical detection of Salmonella Typhimurium pathogens. Biosens Bioelectron 24:3215–3222
Dill K, Stanker LH, Young CR (1999) Detection of Salmonella in poultry using a silicon chip-based biosensor. J Biochem Biophys Methods 41:61–67
Dominguez-Benetton X, Srikanth S, Satyawali Y, Vanbroekhoven K, Pant D (2013) Enzymatic electrosynthesis: an overview on the progress in enzyme-electrodes for the production of electricity, fuels and chemicals. J Microb Biochem Technol S6:007. doi:10.4172/1948-5948. S6-007
Dong J, Xu M, Maa Q, Ai S, Zhao H (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-Chi nanocomposite modified glassy carbon electrode for detection of Salmonella Typhimurium in milk. Food Chem 141:1980–1986
European Food Safety Agency (2014) EFSA explains zoonotic diseases—Salmonella. http://www.efsa.europa.eu/en/search/doc/factsheetsalmonella.pdf. Accessed 20 October 2015
Feng P (2010) Rapid methods for detecting foodborne pathogens. In: Food and Drug Administration. Bacteriological Analytical Manual (BAM). Appendix 1. Available at: http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070149.htm (accessed 06.12.14)
Ferreira NS, Sales MGF (2014) Disposable immunosensor using a simple method for oriented antibody immobilization for label free real time detection of an oxidative stress biomarker implicated in cancer diseases. Biosens Bioelectron 53:193–199
Food and Drug Administration (2012) Bad bug book, foodborne pathogenic microorganisms and natural toxins. Second edition. [Salmonella species, pp. 9–13]
Freitas M, Viswanathan S, Nouws H, Oliveira M, Delerue-Matos C (2014) Iron oxide/gold core/shell nanomagnetic probes and CdS biolabels for amplified electrochemical immunosensing of Salmonella Typhimurium. Biosens Bioelectron 51:195–200
Gehring AG, Crawford CG, Mazenko RS, Van Houten LJ, Brewster JD (1996) Enzyme-linked immunomagnetic electrochemical detection of Salmonella Typhimurium. J Immunol Methods 195:15–25
Gil ES, Mello GR (2010) Electrochemical biosensors in pharmaceutical analysis. Braz J Pharm Sci 46:375–391
Hayes JJ, Kennmore M, Badley A, Cullen DC (1998) AFM study of antibody adsorption to polystylene microtitre plates. Nanobiotechnol 4:141–151
Holford TRJ, Davis F, Higson SPJ (2012) Recent trends in antibody based sensors. Biosens Bioelectron 34:12–24
Hu CM, Dou W, Zhao G (2014) Enzyme immunosensor based on gold nanoparticles electroposition and streptavidin-biotin system for detection of S. Pullorum & S. Gallinarum. Electrochim Acta 117:239–245
Jaffrezic-Renault N, Dzyadevych SV (2008) Conductometric microbiosensors for environmental monitoring. Sens 8:2569–2588
Kaur J, Singh KV, Schmid AH, Varshney GC, Suri R, Raje M (2004) Atomic force spectrsocpy-based study of antibody pesticide interactions for characterization of immunosensor surface. Biosens Bioelectron 20:284–293
Kim S, Choi SJ (2014) A lipid-based method for the preparation of a piezoelectric DNA biosensor. Anal Biochem 458:1–3
Kim G-H, Rand AG, Letcher SV (2003) Impedance characterization of a piezoelectric immunosensor part II: Salmonella Typhimurium detection using magnetic enhancement. Biosens Bioelectron 18:91–99
Kirsch J, Siltanen C, Zhou Q, Revzin A, Simonian A (2013) Biosensor technology: recent advances in threat agent detection and medicine. Chem Soc Rev 42:8733–8768
Lawrence AJ, Moores GR (1972) Conductimetry in enzyme studies. European J Biochem 24:538–546
Lee W, Oh B-K, Bae YM, Paek S-H, Lee WH, Choi J-W (2003) Fabrication of self-assembled protein A monolayer and its application as an immunosensor. Biosens Bioelectron 19:185–192
Lee K-M, Runyon M, Herrman TJ, Hsieh J, Phillips R (2015) Review of Salmonella detection and identification methods: aspects of rapid emergency response and food safety. Food Control 47:264–276
Liébana S, Lermo A, Alegret S, Pividori MI, Campoy S, Cortés MP (2009) Rapid detection of Salmonella in milk by electrochemical magneto-immunosensing. Biosens Bioelectron 25:510–513
Liu Y, Che Y, Li Y (2001) Rapid detection of Salmonella Typhimurium using immunomagnetic separation and immuno-optical sensing method. Sens Actuators: B Chem 72:214–218
Liu X, Wang X, Zhang J, Feng H, Liu X, Wong DKY (2012) Detection of estradiol at an electrochemical immunosensor with a Cu UPD| DTBP-Protein G scaffold. Biosens Bioelectron 35:56–62
Ma X, Jiang Y, Jia F, Chen J, Wang Z, Yu Y (2014) An aptamer-based electrochemical biosensor for the detection of Salmonella. J Microbiol Methods 98:94–98
Mantzila AG, Maipa V, Prodromidis MI (2008) Development of a faradaic impedimetric immunosensor for the detection of Salmonella Typhimurium in milk. Anal Chem 80:1169–1175
Margot H, Stephan R, Mahony E, Iversen C (2013) Comparison of rapid cultural methods for the detection of Salmonella species. International J Food Microbiol 163:47
Martín-Yerga D, González-García MB, Costa-García A (2013) Biosensor array based on the in situ detection of quantum dots as electrochemical label. Sens Actuators: B Chem 182:184–189
Meyer MHF, Hartmann M, Krause H-J, Blankenstein G, Mueller-Chorus B, Oster J, Miethe P, Keusgen M (2007) CRP determination based on a novel magnetic biosensor. Biosens Bioelectron 22:973–979
Morales MD, Serra B, Guzmán-Vázquez de Prada A, Reviejo AJ, Pingarrón JM (2007) An electrochemical method for simultaneous detection and identification of Escherichia coli, Staphylococcus aureus and Salmonella Choleraesuis using a glucose oxidase-peroxidase composite biosensor. Analyst 132:572–578
Mortari A, Lorenzelli L (2014) Recent sensing technologies for pathogen detection in milk: a review. Biosens Bioelectron 60:8–21
Muhammad-Tahir Z, Alocilja EC (2003) A conductometric biosensor for biosecurity. Biosens Bioelectron 18:813–819
Murugaiyan S, Ramasamy R, Gopal N (2014) Biosensors in clinical chemistry: an overview. Adv Biomed Res 3:67
Nandakumar S, Woolard SN, Yuan D, Rouse BT, Kumaraguru U (2008) Natural killer cells as novel helpers in anti-herpes simplex virus immune response. J Virol 82:10820–10831
Nandakumar V, Bishop D, Alonas E, Labelle J, Joshi L, Alford TI (2011) A low- cost electrochemical biosensor for rapid bacterial detection. IEEE Sensors J 11:210–216
Nguyen P-D, Tran TB, Nguyen DTX, Min J (2014) Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of Salmonella Typhimurium. Sens Actuators: B. Chemical 197:314–320
Niraj MMGASP (2012) Histamine biosensor: a review. Int J Pharm Sci Res 3:4158–4168
Olsen EV, Pathirana ST, Samoylov AM, Barbaree JM, Chin BA, Neely WC, Vodyanoy V (2003) Specific and selective biosensor for Salmonella and its detection in the environment. J Micorbiol Methods 53:273–285
Özel RE, Ispas C, Ganesana M, Leiter JC, Andreescu S (2014) Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments. Biosens Bioelectron 52:397–402
Park I-S, Kim W-Y, Kim N (2000) Operational characteristics of an antibody-immobilized QCM system detecting Salmonella spp. Biosens Bioelectron 15:167–172
Pathirana ST, Barbaree J, Chin BA, Hartell MG, Neely WC (2000) Rapid and sensitive biosensor for Salmonella. Biosens Bioelectron 15:135–144
Pimenta-Martins MG, Furtado RF, Heneine LG, Dias RS, Morges MF, Alves CR (2012) Development of an amperometric immunosensor for detection of Staphylococcal enterotoxin type A in cheese. J Microbiol Methods 91:138–143
Pohanka M, Skládal P (2008) Electrochemical biosensors—principles and applications. J Appl Biomed 6:57–64
Pournaras AV, Koraki T, Prodromidis MI (2008) Development of an impedimetric immunosensor based on electropolymerized polytyramine films for the direct detection of Salmonella Typhimurium in pure cultures of type strains and inoculated real samples. Anal Chim Acta 624:301–307
Prashar D (2012) Self assembled monolayers—a review. Int J Chem Tech Res 4:258–265
Prodromidis MI (2010) Impedimetric immunosensors—a review. Electrochim Acta 55:4227–4233
Prusak-Sochaczewski E, Luong JTT, Guilbault GG (1990) Development of a piezoelectric immunosensor for the detection of Salmonella Typhimurium. Enzyme Microbiol Technol 12:173–177
Purvis D, Leonardova O, Farmakovsky D, Cherkasov V (2003). An ultrasensitive and stable potentiometric immunosensor. Biosens and Bioelectron 18:1385–1390
Qureshi A, Gurbuz Y, Niazi JH (2012) Biosensors for cardiac biomarkers detection: a review.(Report). Sens Actuators: B Chem 171:62–76
Ricci F, Adornetto G, Palleschi G (2012) A review of experimental aspects of electrochemical immunosensors. Electrochim Acta 84:74–83
Rickert J, Gopel W, Beck W, Jung G, Heiduschka P (1996) A ‘mixed’ self- assembled monolayer for an impedimetric immunosensor. Biosens Bioelectron 11:757–768
Salam F, Tothill IE (2009) Detection of Salmonella Typhimurium using an electrochemical immunosensor. Biosens Bioelectron 24:2630–2636
Saleem M (2013) Biosensors a promising future in measurements. IOP Conf. Series: Mater Sci Eng 51(012012):1–10
Si S-H, Li X, Fung Y-S, Zhu D-R (2001) Rapid detection of Salmonella Enteritidis by piezoelectric immunosensor. Microchem J 68:21–27
Sibai A, Elamri K, Barbier D, Zaffrezic-Renault N, Souteyrand E (1996) Analysis of the polymer-antibody-antigen interaction in a capacitive immunosensor by FTIR difference spectroscopy. Sens actuators: B Chem 31:125–130
Singh R, Mukherjee MD, Sumana G, Gupta RK, Sood S, Malhotra BD (2014) Biosensors for pathogen detection: a smart approach towards clinical diagnosis. Sens actuators: B Chem 197:385–404
Skládal P, Kovar D, Krajicek V, Siskova P, Pribyl J, Svabenska E (2013) Electrochemical immunosensors for detection of microorganisms. Int J Electrochem Sci 8:1635–1649
Soto AMG, Jaffari SA, Bone S (2001) Characterisation and optimization of AC conductometric biosensor. Biosens Bioelectron 16:23–29
Su L, Jia W, Hou C, Lei Y (2011) Microbial biosensors: a review. Biosens and Bioelectron 26:1788–1799
Vashist SK, Zheng D, Al-Rubeaan K, Luong JHT, Sheu F-S (2011) Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnol Adv 29:169–188
Velusamy V, Arshak K, Korostynska O, Oliwa K, Adley C (2010) An overview of foodborne pathogen detection: in the perspective of biosensors. Biotechnol Adv 28:232–254
Vidal JC, Bonel L, Ezquerra A, Hernandez S, Bertolin JR, Cubel C, Castillo JR (2013) Electrochemical affinity biosensors for detection of mycotoxins: a review. Biosens Bioelectron 49:146–158
Vilarino N, Fonfria ES, Louzao MC, Botana LM (2009) Use of biosensors as alternatives to current regulatory methods for marine biotoxins. Sens 9(11):9414
Vo-Dihn T, Tromberg GD, Griffin KR, Ambrose MJ, Sepaniak MJ, Gardenhire EM (1987) Antibody-based fiber ptics biosensor for the carcinogen benzo (a) pyrene. Appl Spectro Sci 41:735–738
Wang J (2005) Nanomaterial based electrochemical biosensors. Analyst 130:421–426
Wang Y, Ye Z, Ying Y (2012) New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors 12:3449–3471
Wong YYNG, Ng MH, Si SH, Yao SZ, Fung YS (2002) Immunosensor for the differentiation and detection of Salmonella species based on quartz crystal microbalance. Biosens Bioelectron 17:676–684
Yang G-J, Huang J-L, Meng W-J, Shen M, Jiao X-A (2009) A reusable capacitive immunosensor for detection of Salmonella spp. based on grafted ethylene diamine and self-assembled gold nanoparticle monolayers. Anal Chim Acta 647:159–166
Zhao Y, Zheng Y, Kong R, Xia L, Qu F (2016) Ultrasensitive electrochemical immunosensor based on horseradish peroxidase (HRP)-loaded silica-poly (acrylic acid) brushes for protein biomarker detection. Biosens Bioelectron 75:383–388
Zhu D, Yan Y, Lei P, Shen B, Cheng W, Ju H, Ding SJ (2014) A novel electrochemical sensing strategy for rapid and ultrasensitive detection of Salmonella by rolling circle amplification and DNA-AuNPs probe. Anal Chim Acta 846:44–50
Acknowledgments
The authors acknowledge the financial support of the CNPq (Award number: 475174/2012-7) and CAPES Brazilian agencies. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Melo, A.M.A., Alexandre, D.L., Furtado, R.F. et al. Electrochemical immunosensors for Salmonella detection in food. Appl Microbiol Biotechnol 100, 5301–5312 (2016). https://doi.org/10.1007/s00253-016-7548-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7548-y