Abstract
Microorganisms play a key role in removal of pollutants in constructed wetlands (CWs). The aim of this study was to investigate the composition and diversity of microbes in a full-scale integrated constructed wetland system and examine how microbial assemblages were shaped by the structures and physicochemical properties of the sediments. The microbial assemblages were determined using 16S rRNA high-throughput sequencing. Results showed that the microbial phenotypes were more diverse in the system than in single CWs. The genera of Zoogloea, Comamonas, Thiobacillus, Nitrosospira, Denitratisoma, Azonexus, and Azospira showed relatively high abundances, which contributed to the removal of organic matter and nitrogen. The interactions among the three CWs in series acted a key role in the increase of phylogenetic diversity and high percentage of shared operational taxonomic units. In the system, some core microbes always existed even with the changing environment. Redox potential and NH4-N were the important factors affecting the overall microbial community patterns. Total organic carbon had a relatively high impact on some denitrifiers. The results from this study should be useful to better understand the microbial mechanism of wastewater treatment in integrated constructed wetland systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Constructed wetlands (CWs) can be used for advanced treatment of wastewater with great ecological and economic benefits. In recent years, CWs have been developed from single types to integrated systems. An integrated constructed wetland (ICW) is composed of two or more types of CWs to take the advantages of individual CWs and complement each other (Vymazal 2013).
Microorganisms in the sediment play a key role in removal of pollutants in CWs (Elsayed et al. 2014). The treatment performance of a CW may depend on the microbial diversity and phylotype richness. Previous studies on microorganisms have been focused on single types of wetlands (Coban et al. 2015; Ligi et al. 2014; Truu et al. 2009). However, the microbial community structure and the relationship between the community and the physicochemical properties of wetlands are still not clear (Adrados et al. 2014). A higher microbial diversity has been observed in the upper sediment layer of a horizontal subsurface flow constructed wetland (HSFCW) than in the deeper layer (Truu et al. 2005). Ji et al. (2012) obtained similar results in two vertical subsurface flow constructed wetlands (VSFCWs). However, Sleytr et al. (2009) reported that the microbial diversity and phylotype richness did not change with sediment depth in a VSFCW. These inconsistent results may be attributed to different wetland structures as well as physicochemical properties of sediment. In some studies, the bacterial community has been related to physicochemical properties and the denitrification potential in CWs (Ligi et al. 2014; Yu et al. 2012). Nevertheless, Adrados et al. (2014) did not find any relation between microbial assemblage and sediment substrate. Therefore, it is necessary to further study the correlations between microbial community composition and diversity and physicochemical properties of CWs (Bodelier and Dedysh 2013).
Most of studies on wetland microorganisms have been carried out in single types of wetlands or pilot-scale systems rather than in full-scale ICWs (Liu et al. 2013). Microbial communities in sediment of ICW should exhibit new characteristics due to diverse structures and transfer of physicochemical properties among different CWs. High-throughput sequencing technologies, such as next-generation DNA sequencing and GeoChip, which generate large amounts of genetic information, allow more in-depth and comprehensive assessment of microbial communities in CWs (Ansola et al. 2014).
In this study, we presented a comprehensive study of microbial assemblages in a full-scale ICW system, which has been operated to treat sanitary and industrial mixed wastewater for more than 7 years. Microbial assemblages were determined with Illumina high-throughput sequencing of the 16S ribosomal RNA (rRNA) gene and then taxonomically analyzed. Specifically, we aimed to elucidate the composition and diversity of microbes in the full-scale ICW system and examine how microbial assemblages were shaped by the ICW structures and physicochemical properties of the sediments.
Materials and methods
System description and sampling
The ICW system was constructed in the northwest of Foshan City, Guangdong Province in Southern China (112°58′16.74″E, 23°12′37.70″N) and began to operate in April of 2008. The ICW system was composed of a VSFCW, a free water surface CW (FWSCW), and a HSFCW in series. The system currently receives about 1000 m3 day−1 of sanitary and industrial mixed wastewater from a flat panel display factory. The wastewater was pretreated with a hydrolysis-acidification tank and aeration biofilm tank mechanically. Then, the pretreated wastewater flowed into the wetland system by gravity. After the tertiary treatment through the system, the water was discharged into Xinan River (Fig. 1). A detail of the ICW design is presented in the Supplementary Material (SM).
Geographic location, layout, and scheme of the integrated constructed wetland system. HAT, ABT, PS, VSFCW, FWSCW, HSFCW, and DT represent hydrolysis-acidification tank, aeration biofilm tank, pumping station, vertical subsurface flow constructed wetland, free-water surface constructed wetland, horizontal subsurface flow constructed wetland, and disinfection tank, respectively. Arrows show water flow direction in the system and stars represent the sampling zones
Water samples of inflow and effluent of the CWs were collected on March 20, 2015 at the inlets and outlets of the VSFCW, FWSCW, and HSFCW, respectively. All water samples were transferred immediately to the lab and stored at 4 °C before analysis.
Sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the VSFCW, at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the FWSCW, and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the HSFCW. Three samples were taken at each depth with horizontal distances about 1.2 m among them (Fig. 1); thus, totally 18 samples were collected. The three samples in each zone were numbered with 1, 2, and 3. The samples were stored in bags and placed on ice immediately after sampling. In the laboratory, the samples were homogenized manually, and visible root or plant materials were manually removed. Then, the samples were stored at −80 °C before use.
Physicochemical analysis
The water samples were used to measure pH, dissolved oxygen (DO), redox potential (E h ), chemical oxygen demand (COD), ammonium nitrogen (NH4-N), nitrate nitrogen (NO3-N), total nitrogen (TN), total phosphorus (TP), and sulfate (SO4 2−). The sediment samples were used to measure pH, E h , total organic carbon (TOC), NH4-N, NO3-N, TN, TP, and SO4 2−. The measurements were repeated for three times for each sample. The methods used to measure the physicochemical indices of the samples were described in SM.
DNA extraction, PCR amplification, and high-throughput sequencing
Genomic DNAs of the sediment samples were extracted using the MO-BIO PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, USA) following the manufacturer’s directions. Extracted DNA concentrations were determined through Nanodrop Spectrophotometer ND-1000 (Thermo Fisher Scientific, USA).
Primers for sequencing were 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CHV GGG TWT CTA AT-3′), with different barcodes for the V4 region of bacterial and archaeal 16S rRNA gene (Bates et al. 2011). The details of PCR mixture and PCR procedure can be seen in SM. A mixture of the amplicons was then used for sequencing on the Illumina MiSeq platform (paired-end 250-bp mode) at the Guangzhou Magigen Biotechnology Co. Ltd. The sequences have been deposited in the NCBI Sequence Read Archive under the accession number SRR3165867.
High-throughput sequencing data analysis
Reads with a quality less than 30 at the 3′ end were trimmed. The quality sequences were clustered into operational taxonomic units (OTUs) at the 97 % similarity level. Taxonomic assignment was determined at the 80 % threshold. The detail of high-throughput sequencing data analysis can be seen in SM.
Relative abundance (%) of individual taxa within each community was estimated by comparing the number of sequences assigned to a specific taxon versus the number of total sequences obtained for a sample. Calculations of alpha-diversity (including Phylotypes, Faith’s phylogenetic diversity (PD), No. of OTUs, Chao1, Shannon, and abundance-based coverage estimators (ACE)) and beta-diversity (Bray−Curtis distance) metrics were based on a subset of 24,684 randomly selected sequences from each sample. Bray–Curtis-based principal coordinate analysis (PCoA) was used to show the differences among the sediment samples of the three types of CWs. Beta diversity was also evaluated with the bacterial community data to examine the differences of community patterns related to CW structures and sampling locations via the Venn diagram.
Statistical analyses
All statistical analyses were implemented using SPSS 18.0 software and R vegan package (http://www.r-project.org). The independent sample t test and one-way ANOVA test were used to examine significant differences in physiochemical properties and relative abundances of dominant lineages among the types of CWs and significant differences between community dissimilarities (Bray–Curtis distances) among the sediments of CWs (P < 0.05) (Yatsunenko et al. 2012). The details of other statistical analyses are shown in SM.
Results
Physicochemical properties of water and sediment
Physicochemical properties of wastewater at the inlet and outlet of each CW are shown in Table S1 (in SM). Only the NO3-N concentration at the outlet of the VSFCW was higher than that at the inlet, whereas concentrations of COD, NH4-N, and TP decreased from the inlet to the outlet in each CW. The values of DO and E h suggested a primary aerobic-anaerobic process occurring in the VSFCW, a primary aerobic process in the FWSCW, and a primary anaerobic process in the HSFCW. Values of DO (P < 0.0001), E h (P < 0.0001), COD (P < 0.0001), NH4-N (P < 0.0001), TN (P = 0.025), and SO4 2− (P = 0.036) were significantly different at the inlets among the three units of CWs, whereas pH, NO3-N, and TP were not significantly different (P > 0.05). At the outlets, DO, E h , COD, NH4-N, NO3-N, and TN were significantly different among the three types of CWs (P < 0.05), but pH, TP, and SO4 2− were not significantly different (P > 0.05).
Physicochemical properties of sediment are summarized in Table S2 (in SM). Overall, values of E h from the VSFCW were the highest among the CWs. The average concentration of TOC from the FWSCW was the highest among the three types of CWs. Concentrations of N and P in sediment samples of the VSFCW and HSFCW were the highest and the lowest, respectively, among the three types of CWs. Values of SO4 2− from the FWSCW were highest among the CWs. Among the three types of CWs, concentrations of TOC, NH4-N, NO3-N, TN, and TP in the sediments were significantly different (P < 0.05), but pH and SO4 2− were not (P > 0.05).
Sediment microbial diversity and community composition
High-quality sequences of 692,215 were acquired from the 18 sediment samples, with a range of 24,686 to 55,705 sequences per community (Table S3 in SM). In total, 17,201 OTUs were identified, of which 12.5 % were detected in only a single sample. Rarefaction analysis indicated that the full extent of microbial diversity was generally high in the sediment samples of the CWs (Fig. S1 in SM).
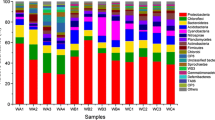
In sediment communities, Bacteria was dominated (> 92.0 %) with a low proportion of Archaea (< 8.0 %) in the VSFCW and HSFCW, but with a relatively high abundance of Archaea in the FWSCW (18.9–36.4 %) (Fig. 2a). Among the 31 bacterial phyla identified, Proteobacteria were predominant (20.8–40.0 %) across all the sediments, with other abundant phyla, such as Acidobacteria (4.8–13 %), Bacteroidetes (4.7–10 %), and Verrucomicrobia (2.0–12 %) (Fig. 2a). Remarkably, the majority of archaeal sequences were affiliated with Euryarchaeota, which reached the maximum abundance in the FWSCW. The abundance of Bacteroidetes was relatively stable among all samples, but the abundance of Nitrospira obviously fluctuated. Within a CW (V-I vs. V-O, F-I vs. F-O, H-I vs. H-O), the abundances of Proteobacteria, Euryarchaeota, Firmicutes, Planctomycetes, and Chloroflexi were relatively stable. Within the FWSCW (F-I vs. F-O), the abundances of almost all microbes fluctuated slightly along the water flow. Within the VSFCW and HSFCW (V-I vs. V-O, H-I vs. H-O), the abundances of Acidobacteria, Bacteroidetes, Verrucomicrobia, and Nitrospira fluctuated obviously along the water flow. Among the CWs, Euryarchaeota and Firmicutes, and Verrucomicrobia and Planctomycetes were most abundant in the FWSCW and HSFCW, respectively. Proteobacteria and Nitrospira were more abundant in the VSFCW and HSFCW (Fig. 2b).
a Phylum distributions of relative abundance (%) of dominant microbial taxa across all analyzed sediments revealed by 16S rRNA MiSeq sequencing at phylum level with mean relative abundance >1 % and b principal component analysis (PCA) of phylum abundance data. The sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the vertical subsurface flow constructed wetland, at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the free-water surface constructed wetland, and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the horizontal subsurface flow constructed wetland. The three samples in each zone were numbered with 1, 2, and 3
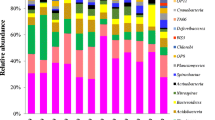
At the genus level, members from Nitrospira, Geothrix, Thiobacillus, Candidatus Brocadia, Sediminibacterium, Denitratisoma, and Azospira were most frequently detected (Fig. 3). Nitrospira showed the highest abundance (8.6 ± 3.0 %) in the VSFCW and the lowest abundance (0.5 ± 0.1 %) in the FWSCW. The Thiobacillus abundance decreased from 6.3 ± 0.7 % at the inlet of VSFCW to 0.3 ± 0.1 % at the outlet of HSFCW. Similarly, Candidatus Brocadia abundance decreased from the inlet of VSFCW to the outlet of HSFCW. The abundance of Geothrix was similar (2.2 ± 0.4 %) within the CWs (Fig. 3). Denitratisoma, Azospira, Dechloromonas, and Zoogloea were affiliated to the family Rhodocyclaceae, which was the most abundant family detected. Overall, the taxonomic classification revealed that the community composition at the phylum and genus levels in the FWSCW was significantly different from that of other CWs (Anosim, Adonis, and MRPP, all P < 0.05).
Relative abundance (%) of top 30 microbial genera across the three wetlands revealed by 16S rRNA MiSeq sequencing. The sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the vertical subsurface flow constructed wetland, at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the free-water surface constructed wetland, and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the horizontal subsurface flow constructed wetland. The three samples in each zone were numbered with 1, 2, and 3
The richness and diversity indexes ACE, no. of OTUs, and Faith’s PD ranged from 6670 to 8276, from 3712 to 4831, and from 161 to 190, respectively (Table S3 in SM), indicating significant increases in sediment microbial diversity in the CWs in series according to the ANOVA analysis (P < 0.005). The microbial diversity was significantly higher at H-O than at H-I in the VSFCW (P < 0.05), whereas the microbial diversity between the inlets and outlets in the FWSCW and HSFCW was not significantly different. Among the CWs, the lowest and highest diversities were observed at V-I and H-O, respectively (Table S3 in SM).
Results of the Bray–Curtis-based PcoA and sample clustering showed that the samples from the three CWs distributed in different parts of the data space (Figs. S2 and S3 in SM), indicating significant differences in sediment community compositions among the CWs. The significantly different community compositions were also supported by the three nonparametric, multivariate statistical tests (Anosim, Adonis, and MRPP, all P < 0.005). The results of the Venn diagram indicated that 538 OTUs (68.9 %) were presented in the wetlands (Fig. 4). The bacterial communities in the VSFCW and the HSFCW shared the most OTUs (82.8 %). A total of 688 OTUs (85.6 %) were shared by two communities in the VSFCW. Similarly, 655 OTUs (84.0 %) and 679 OTUs (83.3 %) were shared by two communities in the FWSCW and HSFCW, respectively.
Venn diagrams to show the unique and shared OTUs (0.03 phylogenetic distance) a for three types of CWs (V VSFCW, F FWSCW, H HSFCW), b two sampling zones in the VSFCW, c two sampling zones in the FWSCW, and d two sampling zones in the HSFCW. The calculation of the Venn diagram was based on results of three samples in each zone. The sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the vertical subsurface flow constructed wetland (VSFCW), at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the free-water surface constructed wetland (FWSCW), and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the horizontal subsurface flow constructed wetland (HSFCW)
Relative influences of sediment physicochemical properties on microbial community
The stepwise linear regression indicated that the microbial diversity indexes of Shannon, Chao1, ACE, and Faith’s PD were significantly correlated to NH4-N concentration with coefficients of determination of 0.666, 0.733, 0.737, and 0.701, respectively (P < 0.005). The overall community dissimilarities of the samples were mainly attributed to the environmental variable E h with the maximum correlation of 41.2 %. However, the environmental variables affecting community dissimilarities in each type of CW were different. The community dissimilarities were mainly attributed to TOC and NH4-N with the maximum correlation of 87.9 % in the VSFCW, to E h , NH4-N, and SO4 2− with the maximum correlation of 86.8 % in the FWSCW, and to TOC, NH4-N, and NO3-N with the maximum correlation of 76.4 % in the HSFCW. The significance of these relationships was also confirmed by the Monte Carlo permutation test. From the redundancy analysis (RDA) (Fig. 5), E h , NH4-N, and TOC were identified as the most correlated variables to the overall community variances, explaining 33.0 % of the OTUs.
Species diversity diagram of all OTUs (circles, response variables) across the three wetlands from 16S rRNA MiSeq sequencing with sediment physicochemistry (vectors, explanatory variables). The sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the vertical subsurface flow constructed wetland, at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the free-water surface constructed wetland, and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the horizontal subsurface flow constructed wetland. The three samples in each zone were numbered with 1, 2, and 3
At the genus level, variables of E h , NH4-N and TOC were most correlated to the community variances. The RDA revealed that E h , NH4-N, and TOC explained 62.8 % of the dominant genera (represented by 30 most dominant genera identified). As shown in Fig. 6, the transition among the species groups was related to the changes in sediment physicochemical properties. Zoogloea, Candidatus Brocadia, and Nitrospira were more associated with E h , and Nitrosospira, Azoarcus, and Denitratisoma were more associated with NH4-N, TC and NO3-N. In addition, the RDA identified the methane-producing Archaea and Thiobacillus to be more associated with E h and SO4 2−, respectively.
Biplot of RDA of the top 30 microbial genera (crosses, response variables) across the three wetlands from 16S rRNA MiSeq sequencing with sediment physicochemistry (vectors, explanatory variables). The genera are Anaeroln Anaerolinea, Anaeromx Anaeromyxobacter, Caldilin Caldilinea, Can Bro Candidatus Brocadia, Can Methy Candidatus Methylopumilus, Comamons Comamonas, Dechlorm Dechloromonas, Denitrat Denitratisoma, Gemmatim Gemmatimonas, Geobactr Geobacter, Georgfuc Georgfuchsia, Luteolib Luteolibacter, Lysobact Lysobacter, Methanob Methanobacterium, Methanol Methanolinea, Methanor Methanoregula, Methanos Methanosaeta, Nitrosos Nitrosospira, Nitrospr Nitrospira, Sediminb Sediminibacterium, Steroidb Steroidobacter, Syntroph Syntrophobacter, Thermomn Thermomonas, and Thiobacl Thiobacillus. The sediment samples were collected at depths of 10 cm of the inlet zone (V-I) and 40 cm of the outlet zone (V-O) in the vertical subsurface flow constructed wetland, at depths of 10 cm of both the inlet zone (F-I) and outlet zone (F-O) in the free-water surface constructed wetland, and at depths of 40 cm of both the inlet zone (H-I) and outlet zone (H-O) in the horizontal subsurface flow constructed wetland
Discussion
The microbial composition and diversity in the ICW
This study is the first report to depict microbial community in an ICW at an in-depth phylogenetic level. Proteobacteria is frequently observed as the dominant phylum in CWs and natural wetlands, including genera of Zoogloea, Comamonas, Thiobacillus, Nitrosospira, Denitratisoma, Azonexus, and Azospira. The abundance of these genera is usually considered to play a vital role in the removal of organic matter, nitrogen, and sulfur compounds (Arroyo et al. 2015; Zhong et al. 2015). The obvious fluctuation of Nitrospira as a nitrification bacterium across the CWs and the high abundance of Euryarchaeota in the FWSCW were attributable to the different structures and physicochemical properties of the CWs. The highest relative abundance of Chloroflexi in the FWSCW indicated the rapid growth of filamentous bacteria and their potential role in carbon cycling during litter decomposition and provided available carbon source to the HSFCW (Chen et al. 2015). It is worthy to pay more attention to the microbes responsible for nitrogen removal, which is the most important task for CWs. First, the abundance of nitrite-oxidizing bacteria (Nitrospira) was far more than that of ammonia-oxidizing bacteria (Nitrosospira and Nitrosomonas) in the ICW (Fig. 3). A new process of nitrification has been reported recently, in which complete oxidation of NH4-N to NO3-N is achieved by certain Nitrospira species (van Kessel et al. 2015). The complete ammonia oxidation process most probably occurred in the ICW due to the high relative abundance of Nitrospira and its frequent occurrence in engineered systems (Daims et al. 2015). Second, the majority of possible heterotrophic denitrifiers in the ICW belonged to β-Proteobacteria, such as Comamonas in the Comamonadaceae family and Azospira, Denitratisoma, Dechloromonas, and Thauera in the Rhodocyclaceae family. The result was similar to Zhong et al. (2015). The relative abundance of the denitrifying bacteria was not completely coupled with the treatment efficiency of nitrate, suggesting the importance of physiological function of denitrifiers (Manis et al. 2014). Thirdly, the anaerobic ammonium oxidizing (ANAMMOX) process in the ICW was encouraging with a large proportion of Candidatus Brocadia at V-I, whereas Chen et al. (2015) showed very limited amount of ANAMMOX bacteria in the CWs. The abundance of ANAMMOX bacteria probably aided to decrease N2O emission (Zhu et al. 2011). In addition, the high abundance of Thiobacillus at V-I should be responsible for the good treatment performance of reductive sulfur compounds in the wastewater (Fukushima et al. 2013).
Microbial community shift with different structures of CWs
The wetland structure had a major impact on microbial community in the ICW, similar to the single type of wetlands (Ansola et al. 2014). The structure of VSFCW permitted wastewater flowing from top to bottom in a short path and carrying more air. Therefore, the aerobic microbes were abundant with the largest amount of Nitrospira. In the HSFCW, wastewater flowed through a relatively long pathway in subsurface substrate, which created a more anaerobic environment. The abundances of denitrifiers Denitratisoma and Azospira increased from the inlet to the outlet of the HSFCW. The existence of nitrifying bacteria might be due to the small amount of DO entering into the upper part of the bed (Adrados et al. 2014). The different abundances of nitrifiers, denitrifiers, and other decomposers between H-I and H-O locations suggested the changing microbial communities along the flow pathway, which contributed to the good treatment efficiencies of nitrogen and organic compounds in the HSFCW. With a relatively open water area, the FWSCW was similar to a natural wetland. More hydraulic disturbances happened with wind, which influenced the bacterial composition and dynamics (Bertin et al. 2014). Thus, either physicochemical properties of water and sediments or microbial clustering in the inlet and outlet zone of FWSCW was more closer than those in VSFCW and HSFCW (Table S1 and S2, Fig. S2 in SM). Water depth is a crucial factor to influence biochemical reactions by changing the redox status and dissolved oxygen level in CWs (Kjellin et al. 2007). The water depth of FWSCW (0.6–1.6 m) provided an anaerobic environment preferred by denitrifiers (Alley et al. 2013; Wu et al. 2015). The anaerobic condition also contributed to the highest abundance of Chloroflexi, which enhanced carbon cycling during litter decomposition to supply energy for the metabolism of denitrifiers (Chen et al. 2015). Therefore, the FWSCW promoted higher abundance of denitrifiers than the VSFCW and HSFCW. The E h value of FWSCW sediment was below −200 mV, which was beneficial to the anaerobic microorganism (e.g., methanogens) (Liu 2010). Thus, the structure of FWSCW also brought the high abundance of Euryarchaeota (mainly the methanogens) (Fig. 3).
The connections between the adjoining CWs in the ICW permitted transfer of DO and available carbon source and spreading of species though the water flow. Therefore, the increasing phylogenetic diversity from the VSFCW to HSFCW might be attributable to the interactions among the three CWs (Table S3 in SM). Moreover, the percentage of shared 538 OTUs (68.9 %) in ICW was higher than those in the single CWs (Ansola et al. 2014) (Fig. 4). More similar structures between the VSFCW and HSFCW contributed to the highest percentage of shared OTUs. The high percentage of shared OTUs indicated some core microbes always existed with varying relative abundances under the changed environment.
Linking physicochemical properties and bacterial community profile
The microbial communities were also influenced by some physicochemical conditions, and primarily by E h (Fig. 5), which was similar to the report of Dušek et al. (2008). E h was the key factor affecting the overall community dissimilarities of the sediments. The ecological effect of E h was also responded by some taxa aforementioned. A varying gradient of redox in the sediments was benefit for the oxidizing-reducing process. E h was generally positively correlated with DO in the CWs (Zhai et al. 2012), and aerobic and anaerobic microbes favor high and low E h conditions, respectively (Fig. 6). The exception of a few genera, such as Sediminibacterium, Candidatus Brocadia, Azoarcus, Denitratisoma, Anaerolinea, and Azospira, might be due to their physiological and metabolic characteristics (Coban et al. 2015; Dušek et al. 2008; Gao et al. 2010; Qu and Yuan 2008), the sufficient oxidizing-reducing environment in the ICW, and other favorable factors (e.g., more nutrients).
NH4-N concentration is a primary factor on nitrogen cycle in CWs when it is below the reported threshold (Baxter et al. 2012). In this study, the ecological effect of NH4-N was more responded by some taxa than the whole microbial communities. Nitrosospira, Azoarcus, and Denitratisoma increased with the NH4-N and NO3-N concentrations in the sediments (Fig. 6). The NH4-N concentration had a greater impact on the community composition than NO3-N, indicating the NH4-N concentration was below the threshold, but the NO3-N concentration was close to the threshold. Overall, the ICW has the potential to treat higher concentrations of NH4-N and NO3-N than the present study (Vymazal 2013).
TOC has been considered as a favorable factor to promote denitrification due to the lack of carbon source in CWs (Shen et al. 2014). Most of previous studies have shown that increases of TOC or organic matter can increase the diversity and abundance of the microbial community in CWs, especially for the denitrification bacteria (Chen et al. 2015; Yu et al. 2012). In this study, the ecological effect of TOC was more responded by some taxa than the whole microbial communities. TOC in the inlet zone of VSFCW was high, and TOC in the inlet zone of HSFCW increased with plant residuals. The TOC only affected the abundances of some denitrifying bacteria, which may be related to the competition from other heterotrophic communities (Johnson et al. 2012). Denitratisoma and Azonexus showed positive correlation with TOC, whereas Azospira and Candidatus Brocdia were negatively correlated with TOC (Fig. 6). The relatively sufficient carbon source in the ICW should meet the need for partial denitrifiers; thus, the abundances of some denitrifying bacteria were not correlated with TOC.
In conclusion, the ICW showed more diverse microbial phenotypes than the single types of CWs previously reported, with a significant increase in the three CWs in series. Results showed that the microbial phenotypes were more diverse in the system than in single CWs. The genera of Zoogloea, Comamonas, Thiobacillus, Nitrosospira, Denitratisoma, Azonexus, and Azospira showed relatively high abundances, which contributed to the removal of organic matter and nitrogen. The wetland structure acted a key role in shaping the microbial community patterns. The interactions among the three CWs in series resulted in increase of phylogenetic diversity and high percentage of shared OTUs, and some core microbes always existed even with the changing environment. E h and NH4-N were the important factors affecting the overall microbial community patterns, whereas TOC only affected the abundances of some denitrifying bacteria. The various environmental factors in the CWs affected the community dissimilarities differently.
References
Adrados B, Sanchez O, Arias CA, Becares E, Garrido L, Mas J, Brix H, Morato J (2014) Microbial communities from different types of natural wastewater treatment systems: vertical and horizontal flow constructed wetlands and biofilters. Water Res 55:304–312
Alley BL, Willis B, Rodgers J, Castle JW (2013) Water depths and treatment performance of pilot-scale free water surface constructed wetland treatment systems for simulated fresh oil field produced water. Ecol Eng 61:190–199
Ansola G, Arroyo P, Saenz de Miera LE (2014) Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci Total Environ 473–474:63–71
Arroyo P, Saenz de Miera LE, Ansola G (2015) Influence of environmental variables on the structure and composition of soil bacterial communities in natural and constructed wetlands. Sci Total Environ 506–507:380–390
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917
Baxter AM, Johnson L, Edgerton J, Royer T, Leff LG (2012) Structure and function of denitrifying bacterial assemblages in low-order Indiana streams. Freshw Sci 31:304–317
Bertin A, Alvarez E, Gouin N, Gianoli E, Montecinos S, Lek S, Gascoin S, Lhermitte S (2014) Effects of wind-driven spatial structure and environmental heterogeneity on high-altitude wetland macroinvertebrate assemblages with contrasting dispersal modes. Freshw Biol 60:297–310
Bodelier PL, Dedysh SN (2013) Microbiology of wetlands. Front Microbiol 4:1–4
Chen Y, Wen Y, Tang Z, Huang J, Zhou Q, Vymazal J (2015) Effects of plant biomass on bacterial community structure in constructed wetlands used for tertiary wastewater treatment. Ecol Eng 84:38–45
Coban O, Kuschk P, Kappelmeyer U, Spott O, Martienssen M, Jetten MS, Knoeller K (2015) Nitrogen transforming community in a horizontal subsurface-flow constructed wetland. Water Res 74:203–212
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, Bergen MV, Rattei T, Bendinger B, Nielsen PH, Wagner M (2015) Complete nitrification by Nitrospira bacteria. Nature 528:504–509
Dušek J, Picek T, Čížková H (2008) Redox potential dynamics in a horizontal subsurface flow constructed wetland for wastewater treatment: diel, seasonal and spatial fluctuations. Ecol Eng 34:223–232
Elsayed OF, Maillard E, Vuilleumier S, Imfeld G (2014) Bacterial communities in batch and continuous-flow wetlands treating the herbicide S-metolachlor. Sci Total Environ 499:327–335
Fukushima T, Whang LM, Chen PC, Putri DW, Chang MY, Wu YJ, Lee YC (2013) Linking TFT-LCD wastewater treatment performance to microbial population abundance of Hyphomicrobium and Thiobacillus spp. Bioresour Technol 141:131–137
Gao H, Schreiber F, Collins G, Jensen MM, Svitlica O, Kostka JE, Lavik G, de Beer D, Zhou HY, Kuypers MM (2010) Aerobic denitrification in permeable Wadden Sea sediments. ISME J 4:417–426
Ji G, Wang R, Zhi W, Liu X, Kong Y, Tan Y (2012) Distribution patterns of denitrification functional genes and microbial floras in multimedia constructed wetlands. Ecol Eng 44:179–188
Johnson LT, Royer TV, Edgerton JM, Leff LG (2012) Manipulation of the dissolved organic carbon pool in an agricultural stream: responses in microbial community structure, denitrification, and assimilatory nitrogen uptake. Ecosystems 15:1027–1038
Kjellin J, Hallin S, Worman A (2007) Spatial variations in denitrification activity in wetland sediments explained by hydrology and denitrifying community structure. Water Res 41:4710–4720
Ligi T, Oopkaup K, Truu M, Preem JK, Nõlvak H, Mitsch WJ, Mander Ü, Truu J (2014) Characterization of bacterial communities in soil and sediment of a created riverine wetland complex using high-throughput 16S rRNA amplicon sequencing. Ecol Eng 72:56–66
Liu Y (2010) Taxonomy of methanogens. In: Timmis KN (ed) Handbook of hydrocarbon and lipid microbiology. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. pp 547–pp 558
Liu WL, Pan XC, Zhang CB, Wang J (2013) Characterization of substrate microbial communities in vertical flow mesocosms as impacted by both planting pattern and species richness. Res Microbiol 164:941–948
Manis E, Royer TV, Johnson LT, Leff LG (2014) Denitrification in agriculturally impacted streams: seasonal changes in structure and function of the bacterial community. PLoS One 9:e105149
Qu JH, Yuan HL (2008) Sediminibacterium salmoneum gen. nov., sp. nov., a member of the phylum Bacteroidetes isolated from sediment of a eutrophic reservoir. Int J Syst Evol Microbiol 58:2191–2194
Shen Z, Zhou Y, Liu J, Xiao Y, Cao R, Wu F (2014) Enhanced removal of nitrate using starch/PCL blends as solid carbon source in a constructed wetland. Bioresour Technol 175C:239–244
Sleytr K, Tietz A, Langergraber G, Haberl R, Sessitsch A (2009) Diversity of abundant bacteria in subsurface vertical flow constructed wetlands. Ecol Eng 35:1021–1025
Truu J, Nurk K, Juhanson J, Mander UE (2005) Variation of microbiological parameters within planted soil filter for domestic wastewater treatment. J Environ Sci Heal A 40:1191–2000
Truu M, Juhanson J, Truu J (2009) Microbial biomass, activity and community composition in constructed wetlands. Sci Total Environ 407:3958–3971
van Kessel MA, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJ, Kartal B, Jetten MS, Lucker S (2015) Complete nitrification by a single microorganism. Nature 528:555–559
Vymazal J (2013) The use of hybrid constructed wetlands for wastewater treatment with special attention to nitrogen removal: a review of a recent development. Water Res 47:4795–4811
Wu H, Zhang J, Ngo HH, Guo W, Hu Z, Liang S, Fan J, Liu H (2015) A review on the sustainability of constructed wetlands for wastewater treatment: design and operation. Bioresour Technol 175:594–601
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI (2012) Human gut microbiome viewed across age and geography. Nature 486:222–227
Yu Y, Wang H, Liu J, Wang Q, Shen T, Guo W, Wang R (2012) Shifts in microbial community function and structure along the successional gradient of coastal wetlands in Yellow River Estuary. Eur J Soil Biol 49:12–21
Zhai J, Zou J, He Q, Ning K, Xiao H (2012) Variation of dissolved oxygen and redox potential and their correlation with microbial population along a novel horizontal subsurface flow wetland. Environ Technol 33:1999–2006
Zhong F, Wu J, Dai Y, Yang L, Zhang Z, Cheng S, Zhang Q (2015) Bacterial community analysis by PCR-DGGE and 454-pyrosequencing of horizontal subsurface flow constructed wetlands with front aeration. Appl Microbiol Biot 99:1499–1512
Zhu G, Wang S, Feng X, Fan G, Jetten MS, Yin C (2011) Anammox bacterial abundance, biodiversity and activity in a constructed wetland. Environ Sci Technol 45:9951–9958
Acknowledgments
This study was supported by grants from the National Science and Technology Major Project of the Ministry of Science and Technology of China (2015ZX07204-002-003) and from the Chinese National Natural Science Foundation (no. 41471181).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Yinghai Wu and Rui Han contributed equally to this work.
Electronic supplementary material
Supplementary material associated with this manuscript is also provided.
ESM 1
(PDF 410 kb)
Rights and permissions
About this article
Cite this article
Wu, Y., Han, R., Yang, X. et al. Correlating microbial community with physicochemical indices and structures of a full-scale integrated constructed wetland system. Appl Microbiol Biotechnol 100, 6917–6926 (2016). https://doi.org/10.1007/s00253-016-7526-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7526-4