Abstract
Influence of pH on the dominant microbial community structure in bioelectrochemical system (BES) for nitrate removal is poorly understood. Here, the dynamics and variations of microbial communities were investigated with pH varied from 6.0 to 9.0 in a novel three-dimensional BES (3D-BES). The maximum nitrate removal efficiencies 97.58 and 96.36 % were obtained at pH 7.0 and 8.0, due to the main contributions of bacterial phylum Firmicutes and class Clostridia. The abundances of dominant phyla and classes tended to decrease under pH 6.0 and 9.0 conditions. Additionally, phylum Proteobacteria and class Gammaproteobacteria preferred acid environment in the BES, while phylum Chloroflexi and class Bacilli and Betaproteobacteria preferred alkaline environment. Furthermore, the excellent nitrate removal ability of the 3D-BES was ascribed to the presences of genera Exiguobacterium, Proteiniclasticum, Pseudomonas, Planococcus, Thauera, Azoarcus, Thiobacillus, etc. These genera facilitated the combined autotrophic denitrification process so that this system achieved excellent nitrate degradation efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because excessive nitrate in waters always leads to human diseases such as gastrointestinal cancer, blue baby syndrome, and methemoglobinemia (Zhang et al. 2014a), besides high amounts of nitrate in natural waters often cause eutrophication (Wang et al. 2014), numerous kinds of technologies such as reverse osmosis; ion exchange; electrodialysis; biological denitrification; and physicochemical, chemical, and electrochemical processes (Epsztein et al. 2015; Kondaveeti et al. 2014) are conducted to eliminate nitrate in waters. However, considering the operating cost and removal efficiency, bioelectrochemical process is deemed as an effective and economical method for nitrate remediation (Kondaveeti et al. 2014).
Nowadays, bioelectrochemical systems have been widely used for contaminant degradation. Lim and Kim (2015) developed an integrated ion exchange and bioelectrochemical system for organic matter and total nitrogen removal and obtained excellent removal efficiencies. Guo and coworkers (Guo et al. 2015) established a bioelectrochemical reactor to degrade 4-chloronitrobenzene and achieved 93.7 % removal efficiency at initial concentration 20 mg/L with 0.5 V voltage. Bonmati et al. (2013) prepared a continuous bioelectrochmical reactor to removal oxalate and demonstrated that anode microbial community showed a shift during the start-up phase. In the research of Zhang and coworkers (Zhang et al. 2014b), a new bioelectrochmical reactor was prepared for nutrient removal, which showed that phosphate was removed by ion exchange and nitrogen was removed by current generation. Li et al. (2014) developed a fluidized bed membrane bioelectrochemical reactor for energy-efficient wastewater treatment process and obtained satisfied effects. Huang et al. (2013) discussed the effect of C/N ration on nitrogen removal using a bioelectrochemical reactor and evaluated the removal rates and anode transformation efficiency. Wang and coworkers (Wang et al. 2015a) summarized the developments and advantages of bioelectrochemical systems for environmental remediation. In the study of Ghafari and coworkers (Ghafari et al. 2008), numerous bioelectrochemical systems for nitrate removal were discussed and analyzed. Kong et al. (2014) used bioelectrochemical system for 4-chlorophenol dechlorination process and obtained excellent removal efficiency. Although numerous researches had investigated the pollutant removal efficiencies, fewer studies had explored the bacterial communities and structures to deeply discuss the relationship between pollutant removal and dominant bacterial community in bioelectrochemical system (BES).
In the present study, a novel three-dimensional bioelectrochemical denitrification system was developed to achieve high concentration of nitrate removal and evaluate the microbial phylogenetic and dominant communities under different pH condition through Illumina Miseq pyrosequencing. The novel BES system possessed not only the advantage of balanced pH environment but also the excellent nitrate removal efficiency due to the reason that H+ produced from sulfur-based autotrophic denitrification process would be utilized by hydrogen-based autotrophic denitrification process, and the concurrence of two autotrophic denitrification processes significantly improved nitrate removal ability. The exploration of this work would profoundly reveal the removal mechanisms of BES.
Materials and methods
Experimental setup
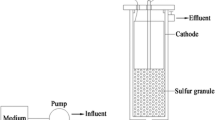
The continuous three-dimensional BES (3D-BES) (working volume 3.0 L) was developed using carbon fiber felt as cathode, graphite rod (diameter 8 mm) as anode, and sulfur granule (diameter 5–8 mm) as particle carrier (Fig. 1). The cathode and anode were connected by a direct current power.
At the initial phase, the continuous-flow reactor was started for 60 days at current 10 mA, hydraulic retention time (HRT) 16 h, pH 7.0, and temperature 25 °C condition with anaerobic sludge (MLSS 3500 mg/L) collected from Erlangmiao Municipal Wastewater Treatment Plant, Wuhan, China. The medium was NO3 --N 50 mg/L, HCO3 −70 mg/L, MgCl2 10 mg/L, ZnCl2 0.50 mg/L, CoCl2 2.00 mg/L, MnSO4 1.00 mg/L, NiCl2 0.30 mg/L, CuCl2 0.30 mg/L, FeSO4 0.20 mg/L, CaCl2 0.50 mg/L, and Na2MoO4 0.30 mg/L.
Reactor operation
The BES was operated for 240 days by adjusting pH from 6.0 to 9.0 to evaluate the effect of pH on the BES. The initial nitrate concentration was maintained at high value 200 mg/L with current 100 mA, HRT 16 h, and temperature 25 °C during the experiment. The operational procedure of the reactor was summarized in Table 1. The concentrations of NO3 −, NO2 −, N2, and SO4 2− were measured to assess nitrate removal efficiency of this system under different pH environment.
Analytical methods
The pH value in the reactor was adjusted using NaOH and HCl. The samples during the experiments were filtered by 0.30-μm membrane by a suction filter machine. NO3 --N was measured by ultraviolet spectrophotometric method using spectrophotometer at 220–275 × 2 nm. NO2 --N was measured by N-(1-naphthyl) ethylenediamine dihydrochloride spectrophotometric method using spectrophotometer at 540 nm. Nitrogen gas (N2) was measured by an Agilent HP4890D gas chromatography. NH4 +-N was measured by Nessler’s reagent spectrophotometer method at 420 nm. SO4 2--S concentration was measured by ion chromatograph (881 Compact IC Pro, Metrohm, Switzerland). The pH was measured using a pH meter (PHS-3C, Kexiao Instrument, China). The water temperature was measured by a thermometer (TM827, Zhugongda Instrument, China).
DNA extraction and Miseq pyrosequencing
Four biofilm samples, D1, D2, D3, and D4 at pH 6.0, 7.0, 8.0, and 9.0 conditions, were collected, and the bacterial genomic DNA was extracted by PowerSoil DNA Extraction Kit (MO BIO Laboratories, Inc., Carlsbad, CA). The PCR amplification, pyrosequencing procedure, and sequence analyses followed the method described in our previous study (Chen et al. 2015). Especially, the primer sequences were 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The 16S rRNA gene sequences of the four samples are available at NCBI Sequence Read Archive (accession number SRX1611191).
Results
BES performances with different pH environment
The concentrations of NO3 --N, NO2 --N, N2, and SO4 2--S in the effluent were given in Fig. 2. The average nitrate removal efficiencies were 77.12, 97.58, 96.36, and 88.48 % at pH 6.0, 7.0, 8.0, and 9.0, respectively, which indicated that 200 mg/L nitrate was almost completely degraded under pH 7.0–8.0 condition in the BES. Besides, the contents of intermediate product nitrite were near zero at pH 7.0–8.0 condition, while the accumulated nitrite (maximum value 8.51 mg/L) was observed at pH 6.0 and 9.0. In addition, when pH varied from 6.0 to 9.0, the produced N2 concentrations reached 99.79, 133.08, 131.33, and 116.42 mg/L, which confirmed that the main ultimate product was nitrogen gas in this novel 3D-BES. Moreover, the average SO4 2−S concentrations were 68.23, 87.02, 87.62, and 100.91 mg/L with pH increase from 6.0 to 9.0, which was lower than the limited value 250 mg/L (China EPA). The concentrations of NH4 +-N were varied from 0.02 to 0.64 mg/L.
In this 3D-BES, the main nitrate degradation process was displayed in Eqs. (1) and (2).
Sulfur-based autotrophic denitrification process (S-process)
Hydrogen-based autotrophic denitrification process (H-process)
On the one hand, a portion of nitrate was converted to nitrogen gas by consuming S and then generated SO4 2− and H+ ions. On the other hand, the produced H2 on the cathode was used as electron donor to reduce the other part of nitrate to nitrogen gas, and H+ ion produced by S-process was consumed during the H-process. And because of this, the novel 3D-BES possessed promising nitrate removal efficiency and sustainable operation.
Equations (1) and (2) also show that pH played a vital role in the 3D-BES because the nitrate removal process would generate H+ ions and consume H+ ions. Furthermore, pH always affected the BES process through influencing the electric conductivity by decomposing the carbonate ions (Mook et al. 2013). Under alkaline environment, OH− could react with carbonate ions and then generated carbonate ion, while carbon dioxide was produced under acid environment so that the inorganic carbon for combined autotrophic denitrification process was decreased and thus decreasing the nitrate removal efficiency. Additionally, the unsuitable pH would inhibit the activity of autotrophic microorganism, thus affecting the denitrification efficiency (Mousavi et al. 2012). However, in this novel BES, when initial pH was 7.0–8.0, the effluent pH was maintained at 6.8–7.8 (Fig. 3), suggesting that this BES could control the system pH at neutral condition and thus facilitating the denitrification efficiency. Besides, the average effluent pH were 6.6 and 8.3 at initial pH 6.0 and 9.0.
Moreover, the variation of effluent SO4 2− concentrations showed that pH value would influence the proportions of S-process and H-process in the BES. At acid environment, the S-process was weakened because excessive H+ ions would reduce the S-process (Eq. (1)) whereas enhance the H-process (Eq. (2)) so that SO4 2− concentrations were relatively lower. However, the S-process efficiency was improved and the SO4 2− concentrations were higher at pH 9.0 due to the increasing OH− ions.
Bacterial diversity and community composition
In order to further investigate the microbial community diversity, phylogenetic analyses of the gene sequences were performed in phylum, class, genus, and species levels. The identified phyla of sample D1–D4 were shown in Fig. 5a. Overall, most of the identified phyla of the four samples were Proteobacteria, Firmicutes, and Actinobacteria. For D1 at pH 6.0 condition, the abundances of the three major phyla were Proteobacteria (40.05 %), Firmicutes (39.64 %), and Actinobacteria (12.93 %). At pH 7.0, the main phyla were Firmicutes (68.60 %), Proteobacteria (23.71 %), and Actinobacteria (3.48 %), while the proportions of the prominent phyla were Firmicutes (72.39 %), Proteobacteria (19.54 %), and Actinobacteria (3.87 %) under pH 8.0 condition. Besides, the phyla distribution turned to Firmicutes (48.09 %), Proteobacteria (35.80 %), and Actinobacteria (8.20 %) when pH was 9.0. Because the best nitrate removal efficiencies were achieved at pH 7.0–8.0 environment in the BES, phylum Firmicutes was considered to be the most dominant community in this novel BES.
Using the Miseq high-throughput pyrosequencing 30,244, 35,572, 40,329, and 42,483 high-quality reads for samples D1, D2, D3, and D4 were obtained. Besides, the corresponding number of operational taxonomic units (OTUs), Ace, Chao, Shannon, Simpson, and Coverage were calculated and given in Table 2. The Coverage values of four samples were greater than 99 %, suggesting that the diversities of microbial communities in the BES were covered by the sequences (Shu et al. 2015). The OTU numbers of D1 and D4 were 646 and 603, which were greater than that of D2 429 and D3 358, which not only demonstrated that the BES possessed abundant bacterial communities but also indicated that the community abundances under pH 6.0 and 9.0 were higher than that of pH 7.0–8.0. In addition, the values of Ace, Chao, Shannon, and Simpson of D1–D4 showed the similar trends as OTU, which also proved that there were more communities in the BES at pH 6.0 and 9.0 environments. The reason for this phenomenon might be that more communities would appear under extreme pH environment in this bioelectrochemical system, which also indicated that this system possessed abundant communities under extreme pH environment. Figure 4 displayed the Venn diagram of the four samples, which showed that the four samples shared only 248 OTUs (12.18 % of the total OTUs) in the total of classified 2036 OTUs, indicating that the microbes in the four samples were rather different from each other in the BES at different pH condition. Based on the richness and abundance analyses, it could be concluded that the novel BES contained abundant bacteria and pH condition was important for the bacteria to degrade nitrate.
On class level, the major classes of the four samples were Clostridia, Alphaproteobacteria, Gammaproteobacteria, Betaproteobacteria, Actinobacteria, and Bacilli (Fig. 5b). For D1, the abundances of the major classes were Clostridia (32.32 %), Alphaproteobacteria (19.51 %), Gammaproteobacteria (11.93 %), Actinobacteria (7.52 %), Betaproteobacteria (7.08 %), Bacilli (5.45 %), and Erysipelotrichia (1.81 %). For D2, the abundances were Clostridia (56.54 %), Gammaproteobacteria (10.51 %), Bacilli (7.93 %), Alphaproteobacteria (7.04 %), Betaproteobacteria (6.00 %), and Actinobacteria (2.30 %). The proportions of the main classes were Clostridia (51.47 %), Bacilli (20.75 %), Gammaproteobacteria (8.91 %), Alphaproteobacteria (5.75 %), Betaproteobacteria (4.67 %), and Actinobacteria (2.86 %) for D3 under pH 8.0 condition. While, under pH 9.0, the main components were Bacilli (27.51 %), Betaproteobacteria (21.10 %), Clostridia (19.25 %), Alphaproteobacteria (8.79 %), Gammaproteobacteria (4.75 %), and Actinobacteria (4.25 %). Based on the class abundance results, Clostridia and Bacilli were the dominant classes due to the reason that the maximum nitrate removal efficiency was obtained at pH 7.0–8.0, and the most abundant classes were Clostridia and Bacilli at pH 7.0–8.0 as well.
Discussion
As reported by some literatures, Firmicutes and Proteobacteria often participated in the denitrification process. In the research of Wang et al. (2015b), the dominant phyla of the denitrification system contained Proteobacteria and Firmicutes, which confirmed that Proteobacteria and Firmicutes exhibited potential nitrate removal abilities. Liu and coworkers (Liu et al. 2014) discovered that Proteobacteria, Firmicutes, and Actinobacteria in a bioelectrochemical system harbored nitrate reductive genes and thus possessed denitrification abilities. In the study of Liu et al. (2015), Proteobacteria phylum was proved to be the most abundant denitrification bacteria in the nitrogen removal system. Zhang and coworkers (Zhang et al. 2015) demonstrated that Proteobacteria was the dominant phylum in a sulfur-based autotrophic system. In the present study, the most important contributor for nitrate removal through combined sulfur-based and hydrogen-based autotrophic denitrification process was phylum Firmicutes in this novel BES due to the fact that Firmicutes was the most abundant phylum when highest nitrate removal efficiency was achieved at pH 7.0–8.0 in this system. In addition, Proteobacteria and Actinobacteria also showed good nitrate removal abilities because 77.12 and 88.48 % nitrate removal efficiencies were obtained under pH 6.0 and 9.0 condition. The above analyses also indicated that Firmicutes was the dominant community for denitrification process in the BES and worked best under pH 7.0–8.0 environment. Otherwise, Proteobacteria and Actinobacteria preferred acid or alkaline environment in the BES.
Clostridia and Alphaproteobacteria might have significant contributions to nitrate removal. Lee et al. (2013) demonstrated that class Clostridia was the main community in autotrophic denitrification process and proved that Clostridia exhibited denitrifying activity in the bioelectrochemical denitrification system. Mao et al. (2013) indicated that most reported hydrogenotrophic denitrification bacteria belonged to α-, β-, and γ-proteobacteria classes, and α-proteobacteria was the dominant class in the hydrogen-oxidizing autotrophic denitrifying system. In the study of Kondaveeti et al. (2014), class Alphaproteobacteria played a vital role in the nitrate reduction process in a bioelectrochemical denitrification system. In this BES, Clostridia were the most important contributor for highest nitrate removal under pH 7.0–8.0 condition, followed by the class Bacilli, Gammaproteobacteria, Alphaproteobacteria, etc. Additionally, class Bacilli tended to prefer alkaline environment so that the most abundant class for pH 9.0 condition was Bacilli, while Gammaproteobacteria was the most abundant class under pH 6.0 environment, meaning that acid environment was more suitable for class Gammaproteobacteria in the BES. The above analyses suggested that pH 7.0–8.0 was most suitable for effective class Clostridia, and thus facilitating autotrophic denitrification efficiency, which also demonstrated that class Clostridia was the most important and dominant communities for achieving highly effective nitrate degradation in this novel BES.
The similarity and differences of the four community structures were investigated using the microbial community heat map analysis (Fig. 6). Results showed that the most abundant genera of the four samples were different from each other, suggesting that pH had obvious effect on bacterial community in this BES. The major genus of D1–D3 was Peptostreptococcaceae_incertae_sedis, while Anaerobacillus was the most abundant genus in D4. In addition, Exiguobacterium, Proteiniclasticum, Pseudomonas, Planococcus, Thauera, Azoarcus, Thiobacillus, etc. also played vital roles during the combined autotrophic denitrification process in the BES. As reported, Peptostreptococcaceae was always excited in anaerobic environment (Lin et al. 2015), which might be essential for nitrate removal in the BES. Exiguobacterium was proven to be a potential and promising genus for denitrification process in the denitrifying sulfide system by the study of Huang et al. (2015). Similarly, Sahinkaya and coworkers (Sahinkaya et al. 2013) indicated that genus Exiguobacterium played a key role in a sulfur-based denitrification process. Thus, the genus Exiguobacterium in this BES could degrade nitrate and performed good sulfur-based autotrophic denitrification. Thauera and Thiobacillus were both considered as the main contributors for nitrate removal in the research of Huang and colleague (Huang et al. 2015). Yu et al. (2015) also suggested that Thiobacillus was an effective autotrophic denitrification bacterium in the bioelectrochemical system. Christianson and coworkers (Christianson et al. 2015) demonstrated that Azoarcus sp. (KH32C and BH72) and Thauera sp. MZ1T appeared more frequently in the sulfur-based autotrophic denitrification biofilters. In the research of Hosono and colleagues (Hosono et al. 2015), Pseudomonas and Thiobacillus contributed essential roles for denitrification process. In this novel BES, the observed excellent nitrate removal ability was ascribed to the presences of these reported genera Exiguobacterium, Proteiniclasticum, Pseudomonas, Planococcus, Thauera, Azoarcus, Thiobacillus, etc. These genera coexisted in the BES, facilitating the combined autotrophic denitrification process so that the BES achieved excellent nitrate degradation efficiency. Furthermore, Proteiniclasticum, Planococcus, and Anaerobacillus genera might be valuable for the BES and needed to conduct much deep research.
Based on the evaluation of nitrate removal, pH played a vital role in the 3D-BES. At acid environment, the S-process was weaker than H-process, while H-process was enhanced under alkaline environment. The dominant bacterial phyla and classes showed obvious migrations with pH varied from 6.0 to 9.0. The highest nitrate removal efficiency was achieved at pH 7.0–8.0, which was ascribed to the most abundant phylum Firmicutes and class Clostridia. Thus, phylum Firmicutes and class Clostridia were the most important contributors for nitrate degradation in this novel 3D-BES.
References
Bonmati A, Sotres A, Mu Y, Rozendal R, Rabaey K (2013) Oxalate degradation in a bioelectrochemical system: reactor performance and microbial community characterization. Bioresour Technol 143:147–153
Chen D, Wang HY, Ji B, Yang K, Wei L, Jiang Y (2015) A high-throughput sequencing study of bacterial communities in an autohydrogenotrophic denitrifying bio-ceramsite reactor. Process Biochem 50:1904–1910
Christianson L, Lepine C, Tsukuda S, Saito K, Summerfelt S (2015) Nitrate removal effectiveness of fluidized sulfur-based autotrophic denitrification biofilters for recirculating aquaculture systems. Aquac Eng 68:10–18
Epsztein R, Nir O, Lahav O, Green M (2015) Selective nitrate removal from groundwater using a hybrid nanofiltration-reverse osmosis filtration scheme. Chem Eng J 279:372–378
Ghafari S, Hasan M, Aroua MK (2008) Bio-electrochemical removal of nitrate from water and wastewater—a review. Bioresour Technol 99:3965–7394
Guo WQ, Guo S, Yin RL, Yuan Y, Ren NQ, Wang AJ, Qu DX (2015) Reduction of 4-chloronitrobenzene in a bioelectrochemical reactor with biocathode at ambient temperature for a long-term operation. J Taiwan Inst Chem E 46:119–124
Hosono T, Alvarez K, Lin IT, Shimada J (2015) Nitrogen, carbon, and sulfur isotopic change during heterotrophic (Pseudomonas aureofaciens) and autotrophic (Thiobacillus denitrificans) denitrification reactions. J Contam Hydrol 183:72–81
Huang B, Feng H, Wang M, Li N, Cong Y, Shen D (2013) The effect of C/N ratio on nitrogen removal in a bioelectrochemical system. Bioresour Technol 132:91–98
Huang C, Li ZL, Chen F, Liu Q, Zhao YK, Zhou JZ, Wang AJ (2015) Microbial community structure and function in response to the shift of sulfide/nitrate loading ratio during the denitrifying sulfide removal process. Bioresour Technol 197:227–234
Kondaveeti S, Lee SH, Park HD, Min B (2014) Bacterial communities in a bioelectrochemical denitrification system: the effects of supplemental electron acceptors. Water Res 51:25–36
Kong FY, Wang AJ, Ren HY (2014) Improved 4-chlorophenol dechlorination at biocathode in bioelectrochemical system using optimized modular cathode design with composite stainless steel and carbon-based materials. Bioresour Technol 166:252–258
Lee S-H, Kondaveeti S, Min B, Park H-D (2013) Enrichment of Clostridia during the operation of an external-powered bio-electrochemical denitrification system. Process Biochem 48:306–311
Li J, Ge Z, He Z (2014) A fluidized bed membrane bioelectrochemical reactor for energy-efficient wastewater treatment. Bioresour Technol 167:310–315
Lim SJ, Kim TH (2015) Removal of organic matter and nitrogen in swine wastewater using an integrated ion exchange and bioelectrochemical system. Bioresour Technol 189:107–112
Lin L, Wen L, Chen S, Yang X, Liu X, Wan CL (2015) Effect of alkaline treatment pattern on anaerobic fermentation of swine manure. Process Biochem 50:1710–1717
Liu H, Yan Q, Shen W (2014) Biohydrogen facilitated denitrification at biocathode in bioelectrochemical system (BES). Bioresour Technol 171:187–192
Liu CS, Zhao DF, Yan LH, Wang AJ, Gu YY, Lee DJ (2015) Elemental sulfur formation and nitrogen removal from wastewaters by autotrophic denitrifiers and anammox bacteria. Bioresour Technol 191:332–336
Mao YP, Xia Y, Zhang T (2013) Characterization of Thauera-dominated hydrogen-oxidizing autotrophic denitrifying microbial communities by using high-throughput sequencing. Bioresour Technol 128:703–710
Mook WT, Aroua MKT, Chakrabarti MH, Noor IM, Irfan MF, Low CTJ (2013) A review on the effect of bio-electrodes on denitrification and organic matter removal processes in bio-electrochemical systems. J Ind Eng Chem 19:1–13
Mousavi S, Ibrahim S, Aroua MK, Ghafari S (2012) Development of nitrate elimination by autohydrogenotrophic bacteria in bio-electrochemical reactors—a review. Biochem Eng J 67:251–264
Sahinkaya E, Kilic A, Calimlioglu B, Toker Y (2013) Simultaneous bioreduction of nitrate and chromate using sulfur-based mixotrophic denitrification process. J Hazard Mater 262:234–239
Shu D, He Y, Yue H, Wang Q (2015) Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing. Bioresour Technol 186:163–172
Wang T, Lin JJ, Chen ZL, Megharaj M, Naidu R (2014) Green synthesized iron nanoparticles by green tea and eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J Clean Prod 83:413–419
Wang H, Luo H, Fallgren PH, Jin S, Ren ZJ (2015a) Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol Adv 33:317–334
Wang YY, Zhang ZJ, Qiu L, Guo Y, Wang XJ, Xiong XJ, Chen SH (2015b) Effect of temperature downshifts on biological nitrogen removal and community structure of a lab-scale aerobic denitrification process. Biochem Eng J 101:200–208
Yu LP, Yuan Y, Chen SS, Zhuang L, Zhou SG (2015) Direct uptake of electrode electrons for autotrophic denitrification by Thiobacillus denitrificans. Electrochem Commun 60:126–130
Zhang BG, Liu Y, Tong S, Zheng MS, Zhao YX, Tian CX, Liu HY, Feng CP (2014a) Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J Power Sources 268:423–429
Zhang F, Li J, He Z (2014b) A new method for nutrients removal and recovery from wastewater using a bioelectrochemical system. Bioresour Technol 166:630–634
Zhang LL, Zhang C, Hu CZ, Liu HJ, Bai YH, Qu JH (2015) Sulfur-based mixotrophic denitrification corresponding to different electron donors and microbial profiling in anoxic fluidized-bed membrane bioreactors. Water Res 85:422–431
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC; 51378400) and the National Science and Technology Pillar Program (2014BAL04B04 and 2015BAL01B02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, D., Wei, L., Zou, Z. et al. Bacterial communities in a novel three-dimensional bioelectrochemical denitrification system: the effects of pH. Appl Microbiol Biotechnol 100, 6805–6813 (2016). https://doi.org/10.1007/s00253-016-7499-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7499-3