Abstract
A three-dimensional biofilm-electrode reactor (3DBER) was integrated with sulfur autotrophic denitrification (SAD) to improve nitrogen removal performance for wastewater reclamation. The impacts of influent carbon/nitrogen (C/N) ratio, electric current, and hydraulic retention time (HRT) were evaluated. The new process, abbreviated as 3DBER-SAD, achieved a more stable denitrification compared to the recently studied 3DBER in literature. Its nitrogen removal improved by about 45 % as compared to 3DBER, especially under low C/N ratio conditions. The results also revealed that the biofilm bacteria community of 3DBER-SAD contained 21.1 % of the genus Thauera, 19.3 % of the genus Thiobacillus and Sulfuricella, as well as 5.3 % of the genus Alicycliphilus, Pseudomonas, and Paracoccus. The synergy between these heterotrophic, sulfur autotrophic, and hydrogenotrophic denitrification bacteria was believed to cause the high and stable nitrogen removal performance under various operating conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wastewater reclamation has been recognized as an important measure to address water resources shortage problem. However, the reclaimed water may still contain a relatively high concentration of inorganic nitrogen (e.g., NO3 −−N and NO2 −−N) when using conventional treatment process (Hao et al. 2013a, b). The eutrophication of lakes and rivers or decreased groundwater quality may be resulted when the water is used for river/lake replenishing or groundwater recharge. Therefore, it is of critical importance to effectively reduce the nitrogen content in the reclaimed water. A variety of methods have been used for removing nitrogen in water, including physicochemical, chemical reduction (e.g., metal and catalytic reduction), and biological denitrification (Lu et al. 2014). Among them, biological denitrification uses bacteria to reduce NO3 −−N into harmless N2 gas with the presence of an electron donor, and has received the greatest interests (Lu et al. 2014).
The biological method includes autotrophic and heterotrophic denitrification. The autotrophic denitrification can use sulfur, sulfur-reduced compounds, H2 or Fe2+ as the electron donor, and is suitable for the treatment of organic carbon (OC) deficient wastewater (Sahinkaya et al. 2014). A few applications using pyrite, arsenite, or thiocyanide also exist in literatures (Shao et al. 2010; Pu et al. 2014). In general, autotrophic denitrification is associated with a number of advantages, such as lower biomass accumulation, reduced turbidity of effluent, and lower sludge production (Sun and Nemati 2012; Zhao et al. 2012). On the other hand, heterotrophic denitrification uses OC as the electron donor, and has been widely studied and applied due to its high denitrification rate, high treatment capacity, and low operating cost. Its denitrification efficiency can be affected by many factors such as pH, temperature, hydraulic retention time (HRT), and influent C/N ratio. Among them, C/N ratio is the most important parameter (Qiao et al. 2012). If insufficient OC was supplied for denitrification, the accumulation of intermediate NO2 −−N species and low denitrification rate could be observed (Lu et al. 2014). This is a challenging problem for wastewater reclamation because the effluent of wastewater treatment plant (WWTP) usually contains little OC source for bacteria to utilize (Hao et al. 2013a). Under such situation, some external organic carbon sources (e.g., methanol, ethanol, and acetate) need to be used as electron donors (Lu et al. 2014). However, if these external sources were over supplied, the effluent after denitrification may contain a great amount of OC which could lead to secondary pollution, and may also increase system operation cost (Sun and Nemati 2012). In order to address such problems, the coupled heterotrophic and autotrophic denitrification has received increasing attention in recent years (Zhao et al. 2012; Zou et al. 2014; Sahinkaya and Kilic 2014).
As an effective coupled denitrification process, the biofilm-electrode reactor (BER) has been studied by various researchers (He et al. 2016). Through water electrolysis, this process produces hydrogen gas (H2) which can be utilized by denitrifying bacteria as an electron donor (Zhao et al. 2012; Tong et al. 2013). In particular, the three-dimensional biofilm-electrode reactor (3DBER) developed in recent years represents a novel technology (Hao et al. 2013a). In 3DBER, activated carbon (AC) or AC mixed with another substance (e.g., limestone, sponge iron) is filled in the cathode part as a third electrode to provide surface area for biomass growth and attachment. It also increases H2 gas yield and produces more CO2 and thus requires less OC source for removing NO3 −−N (Zhou et al. 2009). We recently applied a 3DBER process for NO3 −−N removal from simulated WWTP effluent, and found that when the influent C/N ratio (i.e., COD/TN) ranged from 1.0 to 2.0, both heterotrophic and autotrophic denitrifying microorganisms played important roles in NO3 −−N removal, with about 85.0–90.0 % of NO3 −−N removal at a C/N ratio of 1.5 and a HRT of 10 h (Hao et al. 2013a). However, the 3DBER is still challenged by the low C/N ratio of influent when it is used for wastewater reclamation treatment, and its effluent is also associated with an increased pH value (Hao et al. 2013a). This process can be improved by combining with a sulfur autotrophic denitrification process. Compared to 3DBER, sulfur autotrophic denitrification generates SO4 2− as a by-product and consumes alkalinity to buffer the pH (Sahinkaya et al. 2014; Zhang et al. 2015). For example, Wang and Qu (2003) combined conventional BER with sulfur autotrophic denitrification process for drinking water treatment, with BER denitrification in the upper part and sulfur denitrification in the lower part of the reactor. They found that the H+ produced in the sulfur denitrification part could be consumed by hydrogen denitrification in the BER part, and the effluent from two parts was kept neutral at optimum operation conditions (Wang and Qu 2003). Wan et al. (2011) used a system consisting of a BER denitrification reactor based on proton-exchange membrane electrodialysis and a sulfur autotrophic denitrification column, and they observed a 95.8 % NO3 −−N removal without NO2 −−N accumulation and an effluent SO4 2− concentration of 202.1 mg/L. However, the previous studies of coupled denitrification reported the separation of sulfur autotrophic denitrification process from the BER process (Wang and Qu 2003; Wan et al. 2011). In fact, such separation is not conducive to create synergy between the sulfur autotrophic and hydrogenotrophic denitrification processes. It is also not easy to effectively buffer the system pH value when there is a simultaneous heterotrophic denitrification process. Moreover, despite the improved denitrification performance of 3DBER as compared to conventional BER (Hao et al. 2013a), few studies were reported to integrate this process with sulfur autotrophic denitrification.
The objective of this study is then to investigate the performance of an integrated process by coupling 3DBER with sulfur autotrophic denitrification, abbreviated as 3DBER-SAD. This process does not separate heterotrophic denitrification from autotrophic denitrification so that both 3DBER process and sulfur autotrophic denitrification occur within the same reactor. To the best of our knowledge, few studies have been reported about such 3DBER-SAD process (Hao et al. 2013a; Sahinkaya and Kilic 2014). The performance of this integrated process was examined for removing NO3 −−N from simulated WWTP effluent under different operating conditions (e.g., C/N ratio, electric current, and HRT), while the denitrifying bacteria community of the biofilm in 3DBER-SAD system were also analyzed. Such results can provide valuable information for designing more cost-effective denitrifying process for wastewater reclamation.
Materials and methods
Experimental apparatus
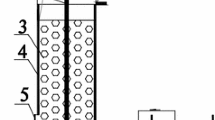
Figure 1 presents the experimental apparatus. The reactor made of plexiglass was cylindrical with a diameter of 25 cm and a height of 140 cm. The cathode (height of 96 cm) was double-layered nickel foam with its inside being polyacrylonitrile-carbon fibers. It was set surrounding the inner wall of the reactor column. The anode was a graphite rod (diameter of 5 cm and height of 130 cm) and was set in the center of the reactor. In this study, the denitrification performances of both 3DBER and 3DBER-SAD were examined. When the reactor was used as a 3DBER, the remaining space in the reactor was filled with granular activated carbon (AC) with particle size of 5–8 mm. When it was used as a 3DBER-SAD, the remaining space was filled with a mixture of granular AC and elemental sulfur (S0) granules (with particle size of 3–5 mm) at a mixing ratio of 8:1. The selection of this mixing ratio was based on previous literatures (Zhou et al. 2011) and the fact that we intended to establish an integrated system involving heterotrophic, hydrogenotrophic and sulfur autotrophic denitrification, where the heterotrophic and hydrogenotrophic denitrification play main roles because the simulated WWTP effluent contained a certain amount of organic carbon, while element sulfur plays a complementary role and helps to stabilize the pH of the system. The AC or AC mixture filler can serve not only as a biofilm carrier but also as the third electrode (Hao et al. 2013a). A layer of insulation cotton (thickness of 1.5 cm) was wrapped around the exterior of the reactor to maintain a relatively constant temperature (about 15–18 °C). The reactor had an effective volume of 22 L.
Schematic diagram of 3DBER-SAD or 3DBER apparatus (1. DC regulated power supply; 2. anode; 3. cathode; 4. activated carbon particles for 3DBER and mixture of activated carbon particles with elemental sulfur granules for 3DBER-SAD; 5. sampling port; 6. supporting quartz sand layer; 7. water distribution plate; 8. effluent port; 9. back wash tube; 10. peristaltic pump. 11. influent tank)
Reactions in 3DBER-SAD system
The carbon electrolytic reaction could occur at the graphite anode in a 3DBER-SAD system, while hydrogen gas (H2) was produced at the cathode and thethird particle electrode (AC) (Zhao et al. 2012). The possible electrochemical reactions in the anodic area can include (Wang and Qu 2003):
Possible electrochemical reactions in the cathodic area and the third electrode area include (Wang and Qu 2003):
The biochemical reactions by biofilm in the cathodic and the third electrode area include (Wunderlich et al. 2012):
It can be found that CO2 is formed prior to O2 at the anode based on the electrode potentials as given in reactions (1) and (2). The production of CO2 could ensure an anoxic environment, buffer pH value, and serve as an inorganic carbon source for denitrifying bacteria of the 3DBER-SAD system. According to Nernst-Peters equation (Wang and Qu 2003; Zhao et al. 2012), both reactions (3) and (4) could take place simultaneously at the cathode. Reactions (5), (6) and (7) are theoretical equations for denitrification by heterotrophic, hydrogenotrophic and sulfur autotrophic denitrifying bacteria using organic carbon source CH3COONa, H2 and S0 as the electron donor, respectively. The H+ generated by sulfur autotrophic denitrification as shown in reaction (7) can promote the proceeding of reaction (3) (Wang and Qu 2003; Wan et al. 2011), and the produced H2 gas can then serve as the electron donor for further hydrogenotrophic denitrification as described in reaction (6).
Preparation of WWTP effluent
A simulated WWTP effluent was prepared by tap water amended with a stock solution of KNO3, KH2PO4, and CH3COONa (Hao et al. 2013a). Nitrogen was provided by KNO3, with a NO3 −−N concentration of 35 mg L−1 in the simulated effluent; phosphorus was provided by KH2PO4, with a PO4 3−−P concentration of 3.5 mg L−1 being maintained; CH3COONa was added to provide carbon source according to the desired C/N ratios (i.e., ratio of TOC to TN). The pH value was adjusted to 7.0–7.5 using HCl (1 mol L−1) and NaOH (1 mol L−1). No microelements were added since they were available in tap water (Hao et al. 2013a). Such simulated WWTP effluent was then used for the normal operation of 3DBER-SAD or 3DBER after its biofilm development. Using a peristaltic pump, the simulated WWTP effluent was pumped from the influent tank (150 L) into the reactor through its bottom inlet, and flew through the biofilm in the reactor, and then flew out from the upper outlet of the reactor (Fig. 1).
Biofilm development and experimental procedure
The biofilm development in the 3DBER-SAD or 3DBER followed similar procedures as in our previous study (Hao et al. 2013a). About 60 L of seed sludge (i.e., a mixture of wastewater and sludge) taken from an anaerobic-anoxic-oxic (A/A/O) process-based WWTP in Beijing, China was added to a plastic tank (100 L). A nutrient solution of KNO3, CH3COONa, and KH2PO4 was added into the tank to maintain a C: N: P (i.e., TOC: TN: P) = 10.7:10:1 (mass ratio), with a NO3 −−N concentration of 60 mg L−1 and pH of 7.0–7.5. When CH3COONa was used as the organic carbon for heterotrophic denitrification, the theoretical stoichiometric C/N ratio is 1.07:1 according to reaction (5). The desired N/P ratio for microbial growth is normally between 10:1 and 5:1, but the WWTP effluent usually contains less than 1 mg/L of P. As a result, a C: N: P = 10.7:10:1 was selected to represent the situation of sufficient organic carbon source and the consideration of phosphorus in WWTP effluent. The sludge-water was driven by a peristaltic pump to continuously circulate between the reactor and tank for 7 days (Hao et al. 2013a), while the nutrient solution was supplemented to the tank once every day based on the above C:N:P ratio. After this step, the immobilization and acclimatization of biofilm were conducted. Amended once every 3 days, the nutrient-containing water (140 L), prepared according to the above NO3 −−N concentration and C:N:P ratio, with pH being adjusted between 7.0–7.5 using HCl (1 mol L−1) and NaOH (1 mol L−1), was added to the 150-L influent tank. The water was then pumped from the influent tank to flow through the reactor, and was finally drained to the laboratory drainage system, with a HRT of 8 h. To make denitrifying microorganisms adapt to the environment, electric current in the reactor was gradually changing from 0 to 60 mA, from 60 to 100 mA, and from 100 to 140 mA, with each current level lasting for 4 days. This phase lasted for about half a month, and the effluent and influent concentration of NO3 −−N and TN were monitored every 3 days. For 3DBER-SAD reactor, more than 80 % of TN and 90 % of NO3 −−N was steadily removed; for 3DBER, more than 70 % of TN and 85 % of NO3 −−N was steadily removed after this phase. The effluent was seen with no suspended sludge, indicating that the biofilm had been well immobilized and developed (Hao et al. 2013a).
After biofilm immobilization and acclimatization, the reactor was operated using simulated WWTP effluent as described above. The impacts of influent C/N ratio, current intensity and HRT on the performance of 3DBER-SAD were tested by changing the value of a single factor in sequence while maintaining constant values of other factors. Table 1 lists the parameter values used in the experiments. Capua et al. (2015) reported that the desired electric current changed with BER reactor size, such as 1–10 mA for a 0.2-L reactor and 80–960 mA for a 36-L reactor. Park et al. (2005) reported that the maximum nitrate reduction rate occurred at an electric current of 200 mA in a 2.5-L BER reactor. In this study, we intended to examine the impact of extremely high electric current on denitrification and microbial growth in a large-size 3DBER or 3DBER-SAD reactor (i.e., 22 L), and thus a current of up to 800 mA was used. The operation time for each factor value ranged from about 10 to 20 days when the system was stabilized with NO3 −−N and TN removal (He et al. 2016), and the average TN and NO3 −−N removal rates were used to examine the impact of that factor. At every 24-h interval, 100 mL of effluent sample was taken for the analysis of NO3 −−N, NO2 −−N, TN, SO4 2−, and TOC concentrations. The influent and effluent pH were also monitored.
Microbial and chemical analysis
The 3DBER-SAD process is affected by various biological denitrication mechanisms due to the complex biofilm microbial community, and thus it is of great importance to understand the information of the involved denitrification bacteria during treatment. Although the use of 16S ribosomal DNA (rDNA) analysis can provide information of various functional microorganisms, it could miss the information of some denitrifying bacteria and thus result in difficulty of revealing the denitrification mechanisms. In comparison, the use of denitrification functional genes (e.g., nirS) has been recognized as a valuable tool for particularly investigating denitrifying organisms in the environment (Zafiriadis et al. 2011). As a result, the relevant denitrifying bacterial community in the biofilm of 3DBER-SAD was analyzed in this study using nirS-based clone library technique (Zafiriadis et al. 2011; Hao et al. 2013a). About 50 mL of AC and S0 granule mixture sample with biofilm was taken from the biofilm sampling port on the bottom of the reactor (Fig. 1) operating at the C/N mass ratio of 0.36:1, current intensity of 60 mA, and HRT of 12 h. Following similar sample treatment procedure as described in Hao et al. (2013a), the sample was put in a 200-mL beaker to be stirred by using a glass rod to strip off the biofilm from AC and S0 granules. The obtained water-biofilm mixture was then subjected to centrifugation (12,000 rpm for 5 min) to separate biofilm from the mixture, and the biofilm sample was stored in a 50-mL centrifuge tube at −20 °C before analysis. The sample was then used for the characterization of denitrifying bacterial community using DNA extraction, polymerase chain reaction (PCR) amplification, clone library construction, colony PCR, nirS sequencing and phylogenetic analysis (Zafiriadis et al. 2011; Hao et al. 2013a).
In terms of chemical analysis, the NO3 −−N, NO2 −−N and SO4 2− in water samples were measured using an ion chromatography (Metrohm 861, Switzerland). The TN and TOC were measured using a TOC/TN meter (Jena multi N/C 3100, Germany). pH was measured using a pH meter (Sanxin pH-2s-3c, China). The temperature of water sample was measured using a thermometer that was fixed on the wall of the reactor. The denitrification rate of NO3 −−N or TN (η) was calculated using the following formula:
Where C 0 is the initial concentration of NO3 −−N or TN (mg L−1), and C t (mg L−1) is the remaining concentration of NO3 −−N or TN at time t.
Results
Effect of influent C/N ratio
The effect of influent C/N ratio on denitrification performance was investigated by changing this ratio (i.e., 1.07:1, 0.71:1, and 0.36:1) under the condition of influent NO3 −−N = 35 mg/L, with no NO2 −−N or NH4 +-N, current intensity = 60 mA, HRT = 12 h, influent pH = 7.0~7.5, and temperature = 15–18 °C. As seen from Fig. 2, when the influent C/N ratio was 0.36:1, the TN removal rate was 40–50 % for 3DBER but 85–95 % for 3DBER-SAD, with a detritrification enhancement of about 45 %. It can be found that the denitrification performance of 3DBER was significantly affected by C/N ratio, with an average TN removal rate of 75, 65, and 45 %, and an average NO3 −−N removal rate of 88, 69, and 52 % at the C/N ratio of 1.07:1, 0.71:1, and 0.36:1, respectively. However, the influent C/N ratio showed little impact on nitrogen removal rate of 3DBER-SAD which had a relatively stable removal rate of >85 % for NO3 −−N and TN. This new integrated process had an average TN removal rate of 88.5, 86.9, and 86.4 %, and an average NO3 −−N removal rate of 96, 90, and 87 % at the C/N ratio of 1.07:1, 0.71:1, and 0.36:1, respectively. As shown in Fig. 3, the effluent pH of both reactors decreased with decreasing C/N ratio, but the 3DBER-SAD had a more stable effluent pH which is closer to influent pH than 3DBER, especially under low C/N ratio (i.e., 0.36:1) condition. It was found that the SO4 2− accumulation in the effluent of 3DBER-SAD increased with decreasing C/N ratio, and it showed a negative correlation with effluent pH (Fig. 3).
Effect of electric current intensity
The effect of current intensity was investigated in this study by gradually increasing it from 60 to 800 mA under the influent condition of NO3 −−N = 35 mg/L, C/N = 0.36:1, HRT = 12 h, pH = 7.0–7.5, and temperature = 15–18 °C. It can be found from Fig. 4 that the 3DBER-SAD maintained a relatively stable denitrification performance (i.e., a TN removal of about 80 % and a NO3 −N removal of about 85 %) for all the studied current intensities compared to 3DBER. The 3DBER had a generally increasing nitrogen removal with increasing current intensity because of the increasing production of H2. It had an average TN removal rate of 83, 79, and 81 %, as well as a NO3 −−N removal rate of 87, 90, and 93 % at a current intensity of 60, 300, and 800 mA, respectively. No significant difference of nitrogen removal was observed between electric current of 60 and 800 mA. In comparison, the 3DBER had an average TN removal rate of 38, 51, and 78 %, as well as a NO3 −−N removal rate of 42, 64, and 89 % under these three current intensity conditions, respectively. As shown in Fig. 5, the effluent pH for 3DBER generally decreased with current intensity, and it was always higher than the influent pH. However, for 3DBER-SAD, its effluent pH was less affected by current intensity and was relatively stable. The SO4 2− accumulation in the effluent of 3DBER-SAD decreased with current intensity, and a strong negative linear correlation (R 2 = 0.92) existed between them.
Influent and effluent pH of 3DBER-SAD and 3DBER as well as the effluent SO4 2− accumulation in 3DBER-SAD under different current intensities (a), and the relationship between current intensity and SO4 2− accumulation in 3DBER-SAD effluent (b). (reactor operating condition: NO3 −−N = 35 mg.L−1, N:P = 10:1, C/N = 0.36:1, HRT = 12 h, pH = 7.0–7.5, T = 15–18 °C)
Effect of hydraulic retention time
The effect of HRT on the performance of 3DBER-SAD was examined by changing this factor from 12 to 1 h under the influent condition of NO3 −−N = 35 mg/L, C/N = 0.36:1, current intensity =300 mA, pH = 7.0–7.5 and temperature = 15–18 °C. Figure 6 illustrates the TN and NO3 −−N removal rates under different HRT conditions. It also presents the result of TN removal loading (g · m−3 · d−1) which is defined as the removed TN amount per unit time per unit effective volume of a reactor. A decrease in nitrogen removal but an increased TN removal loading with HRT were observed (Fig. 6), with the average TN removal rate of 80.4, 73.3, 72.8, 63.9, and 54.6 %, and the average TN removal loading of 56.24, 76.93, 152.95, 268.56, and 458.40 g/(m3 d) at the HRT of 12, 8, 4, 2 and 1 h, respectively. A satisfactory correlation (R 2 = 0.98) was established between TN removal rate and HRT (Fig. 7b), and it was found that a relatively stable TN removal rate was reached when the HRT was above 4 h. However, the denitrification efficiency decreased significantly when the HRT was reduced from 4 to 1 h. A generally decreasing SO4 2− accumulation in the 3DBER-SAD effluent with HRT was observed (Fig. 7), with the average SO4 2− accumulation of 87.6, 84.2, 90.1, 81.4 and 58.3 mg L−1 at the HRT of 12, 8, 4, 2 and 1 h, respectively. A HRT longer than 1 h resulted in a comparatively higher SO4 2− accumulation, but the accumulation was relatively stable at a HRT of 4 h or above. As a result, a HRT ≥ 4 h should be maintained to achieve a satisfactory denitrification performance of 3DBER-SAD.
Denitrifying bacteria community
Figure 8 presents the proportion of each denitrifying bacteria genus in the biofilm during the operation of 3DBER-SAD. The genus Thauera accounted for 21.1 % of the denitrifying bacteria, and they can make use of benzoate, phenylacetate, acetate, and ethanol as electron donors for denitrification under anoxic conditions (Liu et al. 2013). The genus Thiobacillus can take advantage of S0 as the electron donor for denitrification under anoxic conditions, and they possess the obligate autotrophic and facultative anaerobic metabolism types (Letain et al. 2007; Shao et al. 2010). The genus Sulfuricella is another kind of sulfur autotrophic denitrifying bacteria (Watanabe et al. 2012). The sum of the genus Thiobacillus and Sulfuricella accounted for 19.3 % of the denitrifying bacteria community in the biofilm of 3DBER-SAD. The genus Alicycliphilus, Pseudomonas, and Paracoccus accounted for 5.3 % of the bacterial community, and they can use H2 as the electron donor for denitrification (Vasiliadou et al. 2006).
Discussion
An integrated denitrification process (3DBER-SAD) was examined in this study by combining a three-dimensional biofilm-electrode with sulfur autotrophic denitrification within the same reactor. Unlike 3DBER, the influent C/N ratio and electric current intensity had little impact on the nitrogen removal efficiency of 3DBER-SAD. Its denitrification rate greatly improved over 3DBER for each investigated C/N ratio and current intensity, with a NO3 −−N removal rate of above 80 % under different operating conditions. The improvement was even more obvious under low C/N ratio and low current condition, with a denitrification enhancement of about 45 % being observed. This is of particular importance for the deep denitrification of WWTP effluent (i.e., low organic carbon source) in terms of wastewater reclamation (Hao et al., 2013a).
The improved performance of 3DBER-SAD can be caused by its stronger pH stabilization ability. Proper pH value is conducive to the growth of denitrifying bacteria for improving their nitrogen removal efficiency, and a desirable pH range of 7.0–8.0 was widely reported (Rezania et al. 2005). The strong pH buffer capability of 3DBER-SAD to the desired pH range (Fig. 3) can be attributed to the sulfur autotrophic dentrification process. In fact, the effluent SO4 2− due to sulfur utilization by autotrophic denitrifying bacteria as described in reaction (7), can reflect the occurrence and effect of sulfur autotrophic denitrification process. Sun and Nemati (2012) indicated that when acetate and S0 were supplied simultaneously for the denitrifying bacteria, acetate was the sole electron donor and S0 was hardly consumed, with the electron donor order of preference as acetate >S0. Zhou et al. (2007) reported that the denitrification primarily used H2 as an electron donor when the C/N ratio was less than the theoretical stoichiometric ratio (i.e., 1.05) in an electrode-biomembrane reactor, with the electron donor order of preference as H2 > acetate. As a result, the electron donor preference in 3DBER-SAD may follow the order of H2 > acetate > sulfur. When the 3DBER-SAD dealt with wastewater with relatively high C/N ratio (i.e., 1.07), the H2 (i.e., generated by electrochemical reaction) and organic carbon were firstly consumed, and OH− was produced by hydrogenotrophic and heterotrophic denitrification processes as described in reactions (5) and (6). The heterotrophic denitrification was dominant when there was H2 shortage, which could lead to the shortage of electron acceptor for sulfur autotrophic denitrifying bacteria. Therefore, the sulfur autotrophic denitrification was restrained, leading to limited SO4 2− and H+ to neutralize the generated OH−, and a higher effluent pH (but pH still close to 8) was then observed (Fig. 3). However, when the organic carbon was consumed and the wastewater still contained a large amount of nitrogen (e.g., C/N ratio of 0.36:1), the S0 was preferably used by denitrifying bacteria as the electron donor. This sulfur autotrophic denitrification process led to SO4 2− accumulation and H+ increase which could neutralize OH− in the system, leading to relatively low effluent pH which was close to that of the influent (Fig. 3). Consequently, the sulfur autotrophic denitrification process is complementary to the hydrogenotrophic and heterotrophic denitrification process in 3DBER-SAD, especially under low C/N ratio conditions.
In the 3DBER-SAD process, the electric current intensity can directly affect the generation of H2 and thus the efficiency of nitrogen removal. The stimulation of weak electric current can also enhance biological metabolism and mass transfer process, and thus promote biological denitrification (Zhou et al. 2007, 2009). The 3DBER-SAD improved the nitrogen removal over 3DBER for all the investigated current intensities, especially under low current intensity (i.e., 60 mA) conditions. The denitrification enhancement reached 45 % at the current intensity of 60 mA, although the enhancement was small (i.e., 3–4 %) at the high intensity of 800 mA (Fig. 4). For 3DBER-SAD, the electron donors from organic carbon and H2 were limited at low C/N ratio and low current intensity, but it could still achieve and maintain a high nitrogen removal rate because the elemental sulfur can also be used as the electron donors for denitrification process. This is of practical importance from an energy consumption point of view. For 3DBER-SAD, its effluent pH was less affected by current intensity and was relatively stable. Such pH variation pattern can also be explained by the variation of SO4 2− accumulation. Under lower OC source condition (i.e., low C/N ratio), when less H2 was generated at low current intensity (e.g., 60 mA), sulfur autotrophic denitrification process played a leading role in 3DBER-SAD, causing high SO4 2− accumulation in effluent (e.g., 137 mg L−1) (Fig. 5). However, when more H2 was generated at high current intensity (e.g., 800 mA), the hydrogenotrophic denitrification process played a leading role by using H2 as the electron donor prior to S0, leading to the weakening sulfur autotrophic denitrification and thus less SO4 2− accumulation (e.g., 20 mg L−1). Meantime, the increased production of CO2 and H+ with current intensity as described in reaction (1) could neutralize the OH− generated by hydrogenotrophic denitrification, and thus a relatively stable effluent pH in the desired range for the growth of denitrifying bacteria can be remained.
The 3DBER-SAD process achieved a relatively stable nitrogen removal rate at a HRT ≥ 4 h, and the TN removal loading reached 152.95 g/(m3.d) at a HRT of 4 h. The higher and more stable denitrification efficiency of 3DBER-SAD under various operating conditions was also due to the synergy and existence of various sulfur autotrophic, heterotrophic and hydrogenotrophic denitrifying bacteria in the same reactor under the desired pH range for bacterial growth. Together with other bacteria (e.g., genus Dechloromonas, Azoarcus and uncultured bacterium), the mixed culture (Thauera, Thiobacillus, Sulfuricella, Alicycliphilus, Pseudomonas, and Paracoccus) in 3DBER-SAD biofilm could use different electron donor types. This could ensure a synergy and ecological stability of denitrifying bacteria (Lu et al. 2014), thus leading to a high and stable nitrogen removal under different operating conditions. A detailed analysis of the diversity and dynamics of bacterial community composition during wastewater treatment of 3DBER-SAD is required to further reveal the mechanisms of high denitrification performance of this new process, and to design more effective wastewater reclamation treatment system.
References
Capua FD, Papirio S, Lens PNL, Esposito G (2015) Chemolithotrophic denitrification in biofilm reactors. Chem Eng J 280:643–657
Hao RX, Li SM, Li JB, Meng CC (2013a) Denitrification of simulated municipal wastewater treatment plant effluent using a three-dimensional biofilm-electrode reactor: operating performance and bacterial community. Bioresour Technol 143:178–186
Hao RX, Li SM, Li JB, Zhang QK, Liu F (2013b) Water quality assessment for wastewater reclamation using principal component analysis. J Environ Inform 21:45–54
He Y, Wang Y, Song X (2016) High-effective denitrification of low C/N wastewater by combined constructed wetland and biofilm-electrode reactor (CW–BER). Bioresour Technol 203:245–251
Letain TE, Kane SR, Legler TC, Salazar EP, Agron PG, Beller HR (2007) Development of a genetic system for the chemolithoautotrophic bacterium Thiobacillus denitrificans. Appl. Environ. Microb. 73:3265–3271
Liu BB, Mao YJ, Bergaust L, Bakken LR, Frostegard A (2013) Strains in the genus Thauera exhibit remarkably different denitrification regulatory phenotypes. Environ Microbiol 15:2816–2828
Lu H, Chandran K, Stensel D (2014) Microbial ecology of denitrification in biological wastewater treatment. Water Res 64:237–254
Park HI, Kim JS, Kim DK, Choi YJ, Pak D (2005) Nitrate-reducing bacterial community in a biofilm-electrode reactor. Enzym Microb Technol 39:453–458
Pu J, Feng C, Liu Y, Li R, Kong Z, Chen N, Tong S, Hao C, Liu Y (2014) Pyrite-based autotrophic denitrification for remediation of nitrate contaminated groundwater. Bioresour Technol 173:117–123
Qiao N, Gao B, Zhang L, Chen R (2012) Factors affecting the denitrification property of heterotrophic nitrification-aerobic denitrifier. Fresenius Environ. Bull. 21:3773–3778
Rezania B, Cicek N, Oleszkiewicz JA (2005) Kinetics of hydrogen-dependent denitrification under varying pH and temperature conditions. Biotechnol Bioeng 92:900–906
Sahinkaya E, Kilic A (2014) Heterotrophic and elemental-sulfur-based autotrophic denitrification processes for simultaneous nitrate and Cr(VI) reduction. Water Res 50:278–286
Sahinkaya E, Kilic A, Duygulu B (2014) Pilot and full scale applications of sulfur-based autotrophic denitrification process for nitrate removal from activated sludge process effluent. Water Res 60:210–217
Shao M, Zhang T, Fang HH (2010) Sulfur-driven autotrophic denitrification: diversity, biochemistry, and engineering applications. Appl. Microbiol. Biotechnol. 88:1027–1042
Sun YM, Nemati M (2012) Evaluation of sulfur-based autotrophic denitrification and denitritation for biological removal of nitrate and nitrite from contaminated waters. Bioresour Technol 114:207–216
Tong S, Zhang B, Feng C, Zhao Y, Chen N, Hao C, Pu J, Zhao L (2013) Characteristics of heterotrophic/biofilm-electrode autotrophic denitrification for nitrate removal from groundwater. Bioresour Technol 148:121–127
Vasiliadou IA, Siozios S, Papadas IT, Bourtzis K, Pavlou S, Vayenas DV (2006) Kinetics of pure cultures of hydrogen-oxidizing denitrifying bacteria and modeling of the interactions among them in mixed cultures. Biotechnol Bioeng 95:513–525
Wan DJ, Liu HJ, Liu RP, Qu JH (2011) Study of a combined sulfur autotrophic with proton-exchange membrane electrodialytic denitrification technology: sulfate control and pH balance. Bioresour Technol 102:10803–10809
Wang HY, Qu JH (2003) Combined bioelectrochemical and sulfur autotrophic denitrification for drinking water treatment. Water Res 37:3767–3775
Watanabe T, Kojima H, Fukui M (2012) Draft genome sequence of a psychrotolerant sulfur-oxidizing bacterium, sulfuricella denitrificans skB26, and proteomic insights into cold adaptation. Appl Environ Microb 78:6545–6549
Wunderlich A, Meckenstock R, Einsiedl F (2012) Effect of different carbon substrates on nitrate stable isotope fractionation during microbial denitrification. Environ. Sci. Technol. 46:4861–4868
Zafiriadis I, Ntougias S, Nikolaidis C, Kapagiannidis AG, Aivasidis A (2011) Denitrifying polyphosphate accumulating organisms population and nitrite reductase gene diversity shift in a DEPHANOX-type activated sludge system fed with municipal wastewater. J Biosci Bioeng 111:185–192
Zhang L, Zhang C, Hu C, Liu H, Qu J (2015) Denitrification of groundwater using a sulfur-oxidizing autotrophic denitrifying anaerobic fluidized-bed MBR: performance and bacterial community structure. Appl Microbiol Biot 99:2815–2827
Zhao YX, Zhang BG, Feng CP, Huang FY, Zhang P, Zhang ZY, Yang YN, Sugiura N (2012) Behavior of autotrophic denitrification and heterotrophic denitrification in an intensified biofilm-electrode reactor for nitrate-contaminated drinking water treatment. Bioresour Technol 107:159–165
Zhou MH, Fu WJ, Gu HY, Lei LC (2007) Nitrate removal from groundwater by a novel three-dimensional electrode biofilm reactor. Electrochim Acta 52:6052–6059
Zhou MH, Wang W, Chi ML (2009) Enhancement on the simultaneous removal of nitrate and organic pollutants from groundwater by a three-dimensional bio-electrochemical reactor. Bioresour Technol 100:4662–4668
Zhou W, Sun Y, Wu B, Zhang Y, Huang M, Miyanaga T, Zhang Z (2011) Autotrophic denitrification for nitrate and nitrite removal using sulfur-limestone. J Environ Sci 23:1761–1769
Zou SQ, Yao S, Ni JR (2014) High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Bioresour Technol 161:288–296
Acknowledgments
This research was supported by the Natural Science Foundation of China (No. 51378028). JL acknowledges the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant program that enables him to participate in the research collaboration. The authors thank the journal editor and the anonymous reviewers for their comments and suggestions that helped in improving the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
All authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Hao, R., Meng, C. & Li, J. An integrated process of three-dimensional biofilm-electrode with sulfur autotrophic denitrification (3DBER-SAD) for wastewater reclamation. Appl Microbiol Biotechnol 100, 7339–7348 (2016). https://doi.org/10.1007/s00253-016-7534-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7534-4