Abstract
Numerous studies have demonstrated that targeting immunogens to FcγR on antigen-presenting cells (APCs) can selectively uptake and increase cellular immunity in vitro and in vivo. Therefore, the present study was conducted to evaluate immunogenicity of a novel multistage tuberculosis vaccine, a combination of an early and a dormant immunogenic protein, ESAT6 and HspX, fused to Fcγ2a fragment of mouse IgG2a to target all forms of tuberculosis. Codon-optimized genes consisting of ESAT6, a linker, and HspX fused either to mouse Fcγ2a (ESAT6:HspX:mFcγ2a) or 6× His-tag (ESAT6:HspX:His) were synthesized. The resulting proteins were then produced in Pichia pastoris. The fusion proteins were separately emulsified in dimethyldioctadecylammonium bromide(DDA)–trehalose-6,6-dibehenate(TDB) adjuvant, and their immunogenicity with and without bacille Calmette–Guérin (BCG) was assessed in C57BL/6 mice. Th1, Th2, Th17, and T-reg cytokine patterns were evaluated using the ELISA method. Both multistage vaccines induced very strong IL-12 and IFN-γ secretion from splenic cells; the Fc-tagged subunit vaccine induced a more effective Th1 immune response (IFN-γ, 910 pg/mL, and IL-12, 854 pg/mL) with a very low increase in IL-17 (∼0.1 pg/mL) and IL-4 (37 pg/mL) and a mild increase in TGF-β (543 pg/mL) compared to the BCG or ESAT6:HspX:His primed and boosted groups. The production of IFN-γ to ESAT6:HspX:Fcγ2a was very consistent and showed an increasing trend for IL-12 compared to the BCG or ESAT6:HspX:His primed and boosted groups. Fcγ2a used as a delivery vehicle supported the idea of selective uptake, inducing cross-presentation and forming a proper anti-tuberculosis response in context of Th1/Th2 and Th17/T-reg balances, which is important for protection and prevention of damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) is the second leading cause of morbidity and mortality from infectious disease worldwide. According to the latest report by the WHO in 2013, about 9 million people had developed TB that had resulted in 1.5 million deaths (WHO 2014). This situation is exacerbated by co-infection with HIV, which has considerably increased the emergence of multidrug-resistant (MDR) Mtb strains and the appearance of extremely drug-resistant (XDR) Mtb strains (WHO 2014). This further complicates the life-threatening nature of this reemerging disease. An important feature of Mtb as a pathogen is its ability to survive for long periods of time in an intracellular habitat as a latent state of infection that later may develop into active TB (O’Garra et al. 2013).
The TB vaccine, bacille Calmette–Guérin (BCG), is the only available licensed vaccine against tuberculosis; it has been widely used for decades. It provides highly variable efficacies against adult pulmonary TB that ranges from 0 to 80 % protection (Ohara 2012). This vaccine also fails to adequately protect against reactivation of latent infections in adults; thus, there is an urgent need for a novel, safe, and effective TB vaccine and vaccination strategy that prevents all forms of tuberculosis, particularly latent TB infection.
Most novel TB subunit vaccines currently in various stages of clinical trials or under preclinical studies are designed as prophylactic vaccines based on early antigens secreted by replicating bacilli for prevention of active tuberculosis, but not to prevent reactivation of latent TB infection. It has been suggested that a novel vaccine should include antigens from multistages such as dormant antigens and early antigens from replicating bacteria; however, the construct was fused to a monoclonal antibody to form immune complexes and enhances antigen uptake (Pepponi et al. 2014). Simultaneous vaccination with multiple antigens from Mtb may improve the protective effects against all forms of TB (Xin et al. 2013). The present study constructed multistage fusion proteins using ESAT-6 as an acute phase antigen fused to heat shock protein X (HspX) as a latent phase immunodominant antigen fused to mouse Fcγ2a fragment. This is a way to deliver the immunogene to the dendritic cell (DC) cytosol via FcγRI to induce cell-mediated immunity (CMI) responses.
The early secreted antigenic target of 6 kDa (ESAT-6) is one of the most immunodominant and highly Mtb-specific target antigens containing multiple immunogenic T cell epitops that are able to increase CMI responses. Previous studies have demonstrated that Mtb ESAT-6 is an important early antigen candidate for a TB vaccine. The TB vaccines containing ESAT-6 provide stronger protection than BCG in mouse, guinea pig, and non-human primate models (Brodin et al. 2004).
HspX is a 16-kDa protein also known as α-crystallin of Mtb. It shows strong immunogenicity and can induce a potent Th1 immunity in Mtb-exposed patients. This protein as an antigen is mainly secreted by non-replicating bacilli that could induce a stronger immune response in latently infected persons than in existing TB patients (Taylor et al. 2012).
Studies have demonstrated that targeting immunogens to Fcγ receptors (FcγR) on antigen-presenting cells (APCs) such as myeloid and plasmacytoid dendritic cells (DCs), monocytes, and macrophages can enhance the immune response in vitro and in vivo (Loureiro et al. 2010; Konduru et al. 2011; Lu et al. 2011; Kaplan 2012). This method is effective because it also increases the half-life of the antigen and facilitates its uptake by APCs via FcγR and consequently increases the efficiency of cross-presentation for inducing a potent Th1 immune response (Levin et al. 2015).
FcγRI mediates selective uptake of antigens by DCs, resulting in delivery to the cytoplasm, where epitops in cytosolic pathway are loaded onto major histocompatibility cells (MHCs) class I and presented to CD8+ cells (Levin et al. 2015). Cytotoxic T lymphocytes (CTLs) are an effective arm of CMI for killing intracellular pathogens. CTL activation in an FcγR targeted antigen delivery system destroys Mtb-infected cells and is an important source of IFN-γ production that activates infected macrophages for killing intracellular bacteria. The present study selected the Fc fragment of mouse IgG2a (Fcγ2a) as a tag protein to produce ESAT-6:HspX fusion recombinant protein (ESAT6:HspX:Fcγ2a) to introduce the Mtb fusion immunogenes to DCs selectively. This method of Ag internalization can stimulate Th1 responses and generate appropriate macrophage and CTL activation toward protection response. The immunogenicity of Fc-tagged protein was compared with that of ESAT6:HspX-His.
Materials and methods
Design of ESAT6:HspX:Fcγ2a and ESAT6:HspX:His constructs
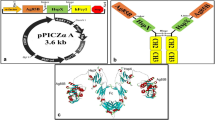
A DNA fusion construct consisting of the esxA (ESAT-6), a linker, hspX, and a constant region of the Fc fragment of mouse IgG2a was designed with an XhoI restriction site incorporated at the 5′ end and a NotI restriction site at the 3′ end (5′ Xho I-ESAT6-linker-HspX-Fcγ2a-Not I 3′) (GeneBank Accession No. KT213443). Figure 1 is a schematic of (a) ESAT6:HspX:His, (b) ESAT6:HspX:Fcγ2 fusion proteins, and (c) a map of the constructed vectors. The peptide sequence for the linker is the GGGGSGGGGS ((G4S)2) and Fcγ2a fragment containing the hinge, CH2, and CH3. The other DNA fusion construct consisting of esxA and hspX linked to a C-terminal 6× polyhistidine-tag (His) contained within the pPICZα A vector (CATCATCATCATCATCAT) was also designed with an XhoI restriction site incorporated at the 5′ end and a NotI restriction site at the 3′ end (5′ Xho I-ESAT6-HspX-6× His-tag-Not I 3′).
Schematic illustration of the Protein constructs. a Schematic illustration of the genetic fusion of Mycobacterium tuberculosis ESAT-6, HspX, and 6× His-tag to create a ESAT6:HspX:His fusion protein. b Schematic illustration of the genetic fusion of Mycobacterium tuberculosis ESAT-6, HspX, and murine Fcγ2a to create a ESAT6:HspX:Fcγ2a fusion protein. c Schematic map of the pPICZα:ESAT6:HspX:Fcγ2a and pPICZα:ESAT6:HspX:His. The insert was cloned into the XhoI and NotI restriction enzyme sites of pPICZαA vector downstream to the AOX1 promoter. 5′ AOX1, alcohol oxidase 1 promoter; AOX1 TT, transcriptional terminator from Pichia pastoris AOX1 gene; TEF1 promoter, transcriptional elongation factor 1 promoter from Saccharomyces cerevisiae; EM7 promoter, synthetic prokaryotic promoter; Zeocin, Zeocin resistance gene; CYC1 TT, transcriptional terminator from Saccharomyces cerevisiae CYC1 gene; pUCori, pUC origin of replication
Protein modeling of ESAT6:HspX:Fcγ2a in silico
ESAT6:HspX:Fcγ2a was modeled in silico using the homology molecular modeling program MODELLER 9v13 (http://salilab.org/modeller/) (Eswar et al. 2007). The first step for modeling was to search for a number of related sequences to find the template structure which had a high sequence identity with the target ESAT-6, HspX, and Fc fragments of mouse IgG2a sequences in the protein data bank (PDB) (www.rcsb.org). BLAST sequence homology searches (using the NCBI protein BLAST: http://www.ncbi.nlm.nih.gov/BLAST/) were performed to identify the template proteins. The ESAT-6-LIKE (PDB entry: 3FAV; chain D), HspX (PDB entry: 3W1Z; chain B), and IGG2A (PDB entry: 1IGT; chain B) were chosen as templates of ESAT-6, HspX, and Fc domains, respectively, to model the protein. The identity of the ESAT-6, Fc, and HspX domains with the selected templates are 100, 100, and 33 %, respectively (positive 54 %), with an E-value of <0.02. The sequences were aligned using ClustalW (output: PIR format) to make the ali file (Thompson et al. 1997). Structure optimization was performed using the molecular mechanic AMBER method (RMS gradient = 0.2) in HyperChem7.5 (Hypercube Inc., http://www.hyper.com/). The geometry of the modeled proteins was assessed using the ERRAT scores (UCLA, http://nihserver.mbi.ucla.edu/savs/) and Ramachandran plots using the ERRAT and RAMPAGE programs, respectively (Colovos and Yeates 1993; Lovell et al. 2003).
Gene construction
ESAT6:HspX:Fcγ2a and ESAT6:HspX:his genes were analyzed using Rare Codon Analysis Tool (GenScript: http://www.genscript.com). The optimized fused fragments were then sent to GenScript (USA) for construction. The fused DNA constructs were cloned in multiple cloning sites of the pUC57 with XhoI and NotI restriction sites at the 5′ and 3′ ends, respectively. Sequencing of both strands of DNA inserts in both directions confirmed that no errors had been introduced during synthesis. After confirmation, the constructs of interest were transformed to Top10F’ E. coli cells and grown in selective medium.
Subcloning and transformation
The fused ESAT6:HspX:Fcγ2a and ESAT6:HspX:His constructs were rescued from the pUC57 cloning vector by restriction digestion using XhoI and NotI (Thermo Scientific, USA) and ligated into the XhoI/NotI multiple cloning site of the digested pPICZα A expression vector (Invitrogen, USA). Recombinant vectors were purified from the transformed Top10F’ E. coli cells (Novagen) by selection of 25 μg/mL Zeocin antibiotics (Invitrogen, USA). Both expression vectors were then fully sequenced over the sites at which the fused DNA fragments were cloned to ensure that the ligation had generated appropriate and in-frame fusion constructs. Pichia pastoris (P. pastoris) GS115 cells (Invitrogen Life Technologies) were grown in yeast extract–peptone–dextrose (YPD) medium, and electrocompetent cells of P. pastoris GS115 were prepared according to manufacturer instructions for the Easy Select Pichia Expression Kit (Invitrogen, USA). The cells were washed several times in ice-cold water and finally resuspended in ice-cold 1 M sorbitol. The recombinant fusion plasmid (∼6 μg) was linearized with SacI restriction enzyme (Thermo Scientific, USA) and transformed into competent P. pastoris cells (80 μL) by electroporation. Transformants were selected on YPDS plates containing 100 μg/mL Zeocin after 72 h of incubation at 30 °C.
Colony selection
Right colonies bearing chromosomally-integrated copies of ESAT6:HspX:Fcγ2a and ESAT6:HspX:His were selected by polymerase chain reaction (PCR) with α-factor primer and 5′ AOX1 primer paired with 3′ AOX1 primer and were then stored for further processing. Then, for selection of yeast transformants with the highest expression levels, each PCR-positive colony (about 50 colonies) were cultured in 50 mL baffled flasks containing 5 mL of buffered minimal glycerol (BMGY) medium until they reached optical density (OD) 2 at 600 nm. The cells were then harvested and resuspended in 10 mL of buffered minimal methanol (BMMY) medium and cultured for 96 h. The supernatants were tested daily to monitor expression of the fused protein by enzyme-linked immunosorbent assay (ELISA).
Protein expression optimization and production
Then, expression optimization (temperature, methanol, time, sorbitol, and pH) was then carried out and analyzed by ELISA and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to select the ideal conditions for optimum protein expression. A single colony was initially grown in 500 mL BMGY at 30 °C in a shaking incubator (250 rpm) until the OD at 600 nm reached 2–6. The cells were then centrifuged and resuspended in 1000 mL of BMMY to an OD600 of 1 for induction of protein expression. During growth, incubation was maintained by adding 2 % methanol (100 %) every 24 h. The cells were incubated for 5 days at 30 °C.
Protein purification
The ESAT6:HspX:Fcγ2a recombinant fusion protein was easily purified because of the presence of Fc-tag at the N-terminus by application of affinity chromatography on the HiTrap rProtein A FF affinity columns (GE Healthcare, USA). For purification of ESAT6:HspX:His recombinant fusion protein, affinity chromatography on the Ni-NTA agarose columns (Qiagen, USA) was chosen because of its high selectivity for the 6× His-tag at the N-terminus.
SDS-PAGE and western blot analysis
The resulting purification solutions were analyzed by SDS-PAGE using 12.5 % polyacrylamide gel with a Mini-PROTEAN II apparatus (Bio-Rad). Western blotting was performed based on the identification of Fc-tag in the ESAT6:HspX:Fcγ2a and His-tag in ESAT6:HspX:His fusion proteins. The membranes were washed four times with phosphate-buffered saline with Tween 20 (PBST) and incubated with their specific horseradish peroxidase (HRP)-conjugated antibodies, goat anti-Fcγ2a antibody conjugated with HRP for Fc-tag, and His probe antibody (H-3) HRP for His-tag (Santa Cruz, USA). The visualization was carried out using the ECL+ method according to manufacturer instructions (Amersham Biosciences).
Subunit vaccines: preparation and immunogenicity
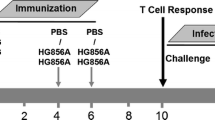
After desalting using Vivaspin20 columns (Sartorius Stedim, Germany), the fusion proteins ESAT6:HspX:Fcγ2a and ESAT6:HspX:His were suspended in PBS (0.2 mg/mL). Dimethyldioctadecylammonium bromide (DDA) (Sigma-Aldrich, UK) and trehalose-6,6-dibehenate (TDB) (VacciGrade; IvivoGen, USA) were dissolved in chloroform/methanol (9:1). The organic solvent was evaporated under a vacuum pump, resulting in a thin lipidic film containing DDA:TDB complex. The lipid film was rehydrated by adding Tris base buffer (10 mM; pH 7.4) and heating at 60 °C for 20 min. The final concentrations of DDA and TDM were 5.0 and 1.0 mg/mL, respectively. Female C57BL/6 mice 6 to 8 weeks of age were purchased from Pasteur Institute (Tehran, Iran). The mice were immunized by subcutaneous injection with a total volume of 200 μL/mouse and divided into six groups (seven mice in each group) receiving (i) 200 μL PBS, (ii) BCG of 5 × 105 CFU, (iii) 20 μg of ESAT6:HspX:Fcγ2a in 100 + 50 μL of DDA and 50 μL of TDB, and (iv) 20 μg of ESAT6:HspX:His in 100 + 50 μL of DDA and 50 μL of TDB. BCG- or PBS-immunized groups received a one-time inoculation at 0 week. Other groups received three inoculations, one each at 0, 4, and 6 weeks. Two other groups were primed with BCG plus each recombinant protein and then boosted two times at intervals of 14 days using each subunit vaccine with DDA/TDB for (v) ESAT6:HspX:Fcγ2a and (vi) ESAT6:HspX:His.
ELISA for IFN-γ, IL-12, IL-4, IL-17, and TGF-β production
Four weeks after final immunization, the mice were killed. The spleens from the vaccinated mice were removed aseptically and were gently homogenized through a 70-μm cell strainer. A cell suspension was prepared and the erythrocytes were lysed with lysis buffer (ammonium chloride). Freshly isolated spleen cells were plated in duplicate in 12-well plates at 5 × 106 cell per well in 500 μL of RPMI 1640 supplemented with penicillin/streptomycin solution (Penstrep, Biosera, UK) and 10 % fetal bovine serum (GIBCO, UK). The spleen cells were divided into three treatment groups in microtiter plates and incubated with (i) 10 μg/mL ESAT6:HspX:Fcγ2a, (ii) 10 μg/mL ESAT6:HspX:His, and (iii) 5 μg/mL PHA. Culture supernatants were harvested after 72 h of incubation. The concentration of IFN-γ, IL-12, IL-4, TGF-β, and IL-17 in the culture supernatant was measured using mouse cytokine ELISA kits (eBioscience, Austria) according to the manufacturer’s instructions.
Statistical analysis
The results were presented as mean ± SEM. The results were compared by analysis of variance (one-way ANOVA) with Tukey Kramer post hoc tests using SPSS13.0 software. The results were considered statistically significant at p ≤ 0.05.
Results
Molecular modeling of ESAT6:HspX:Fcγ2a recombinant protein
Initially, 30 models were generated using Modeler software, and the one with the best ERRAT score was selected to structure refinements. Several models at various refinement levels were generated. The modeling process modeled proteins converted to dimeric form; the disulfide bond between Cys256, Cys259, and Cys261 in the constructed chains was added, and molecular mechanics geometry optimization was performed to form the modified dimeric structures of the modeled proteins (Fig. 2a, d). Solvent surface and solid ribbon presentations of the final model were outlined in Fig. 2b, c. The final model had an ERRAT score of 97 %. The Ramachandran plot confirmed an acceptable structure by validation of more than 99 % of residues in the favored and allowed regions (Fig. 2d).
Protein modeling. a Stick view of the modeled protein and the presentation of the disulfide bonds situations (yellow = sulfur; blue = nitrogen; gray = carbon; red = oxygen). b Solvent surface and (c) Solid ribbon views of the modeled proteins. d The Ramachandran plot of the modeled protein. Number of residues in favored region (∼98.0 % expected): 869 (91.1 %). Number of residues in allowed region (∼2.0 % expected): 77 (8.1 %). Number of residues in favored region: 8 (0.8 %) (Color figure online)
Construction of recombinant fusion plasmids
The codon-optimized constructs were rescued from the pUC57 vector using restriction digestion using XhoI and NotI and ligated into the XhoI/NotI multiple cloning site of the digested pPICZα A expression vector (Fig. 3a, b).
Construction of recombinant ESAT6-HspX:Fcγ2a and ESAT6-HspX:His fusion proteins pPICZα A expression vectors. a The codon-optimized synthetic gene, ESAT6:HspX:His, was then digested by XhoI and NotI. Lane 1: undigested plasmid and Lane 2: digested pPICZαA:ESAT6:HspX:His vector. b The DNA fragments, ESAT6:HspX:Fcγ2a, was cloned in frame to XhoI and NotI digested sites of pPICZαA Pichia pastoris expression vector. Lane 1: undigested plasmid and Lane 2: digested pPICZαA:ESAT6:HspX:Fcγ2a vector. Both products were sequenced over the sites at which the tags and ESAT6-HspX genes were cloned, to ensure that the ligation had generated an in-frame construct. Lane M contain a 100-kb ladder, with size (bp) indicated. Samples were subjected to electrophoresis through a 1.2 % agarose gel stained with ethidium bromide and the products were analyzed using long wave UV light
The presence of ESAT6:HspX:Fcγ2a and ESAT6:HspX:His in the recombinant plasmids was confirmed using colony PCR for approximately 50 colonies (Fig. 4a, b). Of the 50 P. pastoris colonies screened for expression of each recombinant protein, the colony with the highest expression was identified as the best transformant by ELISA and was selected for further optimization of high protein production.
Confirmation of transformed clones of P. pastoris by colony PCR with the 5′ AOX1 primer and the α-factor primer paired with the 3′ AOX1 primer. a Confirmation of ESAT6:HspX:Fcγ2a transformed clone. A1: PCR with the 5′ AOX1 and 3′ AOX1 primers: Lane 1 and 6: DNA Marker (100 bp Plus) with size (bp) indicated. Lanes 2: Untransformed colony of GS115 as a negative control (clone exhibiting a faint band of 2.2-kb GS115 AOX1 gene). Lane 3: Clones exhibiting an amplified thick band of ∼2-kb ESAT6:HspX:Fcγ2a gene. A2: PCR with the 5′ α-factor primer paired with the 3′ AOX1 primer: Line 4: Clones exhibiting an amplified thick band of ∼1.7-kb ESAT6:HspX:Fcγ2a gene. Lane 5: Untransformed colony of GS115 as a negative control. b Confirmation of ESAT6:HspX:His transformed clone. Lane 1 and 6: DNA Marker (100 bp Plus) with size (bp) indicated. B1: PCR with the 5′ α-factor primer paired with the 3′ AOX1 primer: Lanes 2, 3: Clones exhibiting an amplified thick band of 1.1-kb ESAT6:HspX:His gene. B2: PCR with the 5′ AOX1 primer paired with the 3′ AOX1 primer: Line 4 and 5: Clones exhibiting an amplified thick band of ∼1.4 ESAT6:HspX:His gene and a faint band of 2.2-kb GS115 AOX1 gene
Optimization of recombinant protein production
To find the best environment for higher protein production, the highest ESAT6:HspX:Fcγ2a and ESAT6:HspX:His engineered clone producers were introduced to different concentrations of methanol and sorbitol. They were then tested at different temperatures, pH values, and times of incubation. SDS-PAGE and ELISA analysis revealed that 2 % methanol and 1 % sorbitol were the optimal conditions for the highest recombinant protein production. The data also showed that the optimal conditions for ESAT6:HspX:Fcγ2a and ESAT6:HspX:His protein expression were 30 °C and 96- to 120-h induction time. The induction of protein expression at different initial pH indicated that the highest protein expression occurred at an initial pH of 6.5.
Recombinant fusion protein purification and identification
The HiTrap rProtein A FF affinity column was chosen for its high selectivity for IgG2a. SDS-PAGE analysis indicated that the purified ESAT6:HspX:Fcγ2a and ESAT6:HspX:His fusion proteins had a single band with molecular weights of 51 kDa (Figs. 5a and 6) and 30 kDa (Fig. 5c), respectively, which is similar to the theoretical molecular weight of the each fusion protein. This secreted protein was analyzed in culture by the Western blot, which showed that the ESAT6:HspX:Fcγ2a fusion protein can be recognized using goat anti-mouse IgG HRP and that ESAT6:HspX:His fusion protein can be recognized using His probe Antibody (H-3) HRP antibodies (Fig. 5b, d).
Purification and expression of the recombinant ESAT6:HspX:Fcγ2a and ESAT6:HspX:His fusion proteins. a Analysis of the recombinant ESAT6:HspX:Fcγ2a protein by 12 % SDS-PAGE stained with Coomassie Blue. Lane 1, 2, 3 and 4: fractions of the eluted recombinant protein of approximately 51 kDa. Lane M: protein marker. b The expression of the recombinant ESAT6:HspX:Fcγ2a protein was analyzed by immunoblot using anti mouse IgG-HRP. c Analysis of the recombinant ESAT6:HspX:His protein by 12 % SDS-PAGE stained with Coomassie Blue. Lane 1, 2, 3: fractions of the eluted recombinant protein of approximately 30 kDa. Lane 4: mouse serum as a positive control. Lane M: protein marker. d The expression of the recombinant ESAT6:HspX:His protein was analyzed by immunoblot using anti His tag-HRP. Lane M: protein marker
SDS-PAGE analysis of the expressed ESAT6:HspX:Fcγ2a fusion protein under reducing and Unreducing condition. Samples were electrophoresed on 12 % SDS-PAGE. Proteins were visualized by Coomassie blue G-250 staining. Lane M: molecular weight markers (kDa); Lane 1: the monomeric form of recombinant protein under reducing conditions; Lanes 2: the dimeric form of recombinant protein under non-reducing conditions
Th immune response pattern
The prototype cytokine for each pattern was evaluated to assess the immune response pattern toward Th1, Th2, Th17, and T-reg under different immunization conditions. IL-12, IFN-γ, IL-4, IL-17, and TGF-β were measured in the culture supernatants of each immunization group (seven mice in each group) under different stimulators.
Th1 immune response
C57BL/6 mice were immunized with ESAT6:HspX:Fcγ2a or ESAT6:HspX:His emulsified in DDA/TDB, and the production of IFN-γ and IL-12 from activated splenic lymphocytes in vitro with ESAT6:HspX:Fcγ2a, ESAT6:HspX:His, (10 μg/mL) or PHA (5 μg/mL) was evaluated. ESAT6:HspX:Fcγ2a-stimulated splenic cells from mice immunized with ESAT6:HspX:Fcγ2a had significantly higher levels (p < 0.001) of IFN-γ and IL-12 secretion than the ESAT6:HspX:His, BCG, or PBS immunized groups (Fig. 7a, b). Mice immunized with ESAT6:HspX.His also had a significantly higher level of IFN-γ than BCG and PBS (p < 0.01). These results indicated that recombinant fusion Fcγ2a-tagged protein can stimulate the higher specific Th1 responses than the other immunization groups; however, the recombinant protein with the His tag had acceptable response.
Cytokine releases from splenocytes of immunized mice. IFN-γ, IL-12, and IL-4 secretion in splenocytes was evaluated following stimulation with ESAT-6:HspX:Fcγ2a, ESAT6:HspX:His, and PHA. Mice were immunized with ESAT-6:HspX:Fcγ2a in DDA/TDM, and ESAT6:HspX:His in DDA/TDM, respectively. PBS and BCG were used as controls. Freshly isolated spleen cells were plated in duplicate at 5 × 106 cell per well in 24-well plate and incubated with ESAT6:HspX:Fcγ2a and ESAT6:HspX:His (10 μg/mL) and PHA (5 μg/mL) for 72 h at 37 °C, 5 % CO2. IFN-γ, IL-12, and IL-4 were detected using mouse IFN-γ, IL-12, and IL-4 ELISA kits. a, b ESAT6:HspX:Fcγ2a-stimulated splenic cells from immunized mice with ESAT6:HspX:Fcγ2a had the highest numbers of IFN-γ and IL-12 secretion than ESAT6:HspX:His, BCG, or PBS-immunized groups (p < 0.001). c Both ESAT:HspX:Fcγ2a- and ESAT6:HspX:His-immunized groups showed increased level of IL-4 production, as a marker of Th2, compare to BCG and PBS control groups p < 0.001. d Differential IFN-γ, IL-12 and IL-4 production in the vaccinated mice showed all immunizes groups were pushed toward Th1 responses. *There is no significant difference between the groups with at least one shared letter
Correlation analysis demonstrated that the highest positive relationship was observed between IFN-γ and IL-12 production (r ≥ 0.90 and p = 0.000) in BCG-, ESAT6:HspX:Fcγ2a-, and ESAT6:HspX:His-immunized groups. There was no correlation between cytokine releases in PBS groups.
Th2 immune response
Both ESAT6:HspX:Fcγ2a- and ESAT6:HspX:His-immunized groups showed significantly higher levels of IL-4 production as a marker of Th2 than for the BCG and PBS control groups (p < 0.001); however, the IL-4 concentration was very low compared to that for IFN-γ and IL-12 (Fig. 7c). The protein stimulated the Th1 immune response more vigorously than Th2. Correlation analysis showed that there was a negative correlation between Th1 and Th2 cytokine production (IFN-γ/IL-4) in the ESAT6:HspX:Fcγ2a-immunized group in which the splenic cells were stimulated by the same protein, ESAT6:HspX:Fcγ2a (r = −0.975, p = 0.005); however, there was no correlation between the other cytokine releases investigated. Figure 7d shows that all immunized groups were pushed toward the Th1 responses; however, the highest Th1/Th2 balance was seen for the BCG-primed and the ESAT6:HspX:Fcγ2a group, respectively.
Th17 immune response
The production of IL-17 in all groups was less than 1 pg/mL, which is relatively low. The findings show that ESAT6:HspX:Fcγ2a-stimulated splenic cells from mice immunized with ESAT6:HspX:Fcγ2a had a significantly higher level (p < 0.001) of IL-17 secretion than for the ESAT6:HspX:His-, BCG-, and PBS-immunized groups (Fig. 8a). The ESAT6:HspX:His-immunized mice showed significantly higher levels (p < 0.01) of IL-17 than for BCG and PBS. These results demonstrate that recombinant fusion Fcγ2a-tagged protein can stimulate Th17 responses that were stronger than for other immunization groups. There was no correlation between IL-17 production and the other cytokines investigated.
IL-17and TGF-β releases from splenic lymphocytes of immunized mice. Splenic lymphocytes (5 × 106 cells/well) from killed mice were stimulated with 10 μg/mL proteins (ESAT6:HspX:Fcγ2a and ESAT6:HspX:His) and 5 μg/mL PHA for 72 h. a IL-17 productions in groups were assayed and showed that ESAT6:HspX:Fcγ2a-stimulated splenic cells from immunized mice with ESAT6:HspX:Fcγ2a had the highest level of IL17 secretion than ESAT6:HspX:His-, BCG-, and PBS-immunized groups (p < 0.001). b TGF-β productions in groups were assayed and showed that there were no statistically significant differences between groups. c Differential IL-17 and TGF-β productions in the vaccinated mice showed which balance in this situation fully pushed toward T-reg without stimulatory effect on Th17. *There is no significant difference between the groups with at least one shared letter
T-reg immune response
The production of TGF-β as a marker of T-reg from activated splenic lymphocytes was evaluated. There were no statistically significant differences for TGF-β production between groups (Fig. 8b). The BCG-immunized group in which splenic cells were stimulated by PHA or ESAT6:HspX:His had higher levels of TGF-β than the mice immunized with ESAT6:HspX:His and ESAT6:HspX:Fcγ2a. The findings showed that there were significant correlations in the BCG-primed groups between T-reg cytokine and IL-4 (r = 0.90, p = 0.000), IL-12 (r = 0.90, p = 0.03), and IFN-γ (r = −0.975, p = 0.005).
Th17/T-reg balance
Figure 8c shows that the highest amount of IL-17 secretion was observed in the ESAT6:HspX:Fcγ2a-immunized group in which the splenic cells were stimulated with ESAT6:HspX:Fcγ2a (0.1 pg/mL) or PHA (0.05 pg/mL); however, the concentrations of TGF-β in those treatments indicated that the suppressor of this cytokine was significantly higher (543 vs 580 pg/mL). The lowest IL-17 level was seen in the BCG-immunized group in which splenic cells were stimulated by ESAT6:HspX:Fcγ2a or ESAT6:HspX:His (∼0 pg/mL); TGF-β production was very high (566 vs 686 pg/mL). The balance in this situation tipped toward T-reg without a stimulatory effect on Th17. The ESAT:HSP:Fcγ2a-immunized group in which splenic cells were stimulated by ESAT6:HspX:Fcγ2a or PHA were significantly tipped toward a very low level of Th17 and stronger level of T-reg stimulation.
Immune responses induced by BCG priming and subunit vaccine boosting
Two groups of mice were primed with BCG and then boosted two times with one of the recombinant fusion proteins, ESAT6:HspX:Fcγ2a or ESAT6:HspX:His emulsified in DDA/TDB. The production of IFN-γ, IL-12, IL-4, IL-17, and TGF-β was evaluated in vitro from the activated splenic lymphocytes with ESAT6:HspX:Fcγ2a, ESAT6:HspX:His (10 μg/mL), or PHA (5 μg/mL).
The IFN-γ and IL-12 production level was higher for the BCG-primed group boosted and stimulated with ESAT6:HspX:Fcγ2a than for the BCG-immunized and boosted group (p < 0.001) and the BCG-primed group boosted and stimulated by ESAT6:HspX:His (p < 0.1) (Fig. 7a, b). The latter was not statistically different at 95 % CI, and more treatments were needed to confirm the statistical difference. There was no significant difference between the BCG-primed group boosted and stimulated with ESAT6:HspX:Fcγ2a and the ESAT6:HspX:His group.
IL-4 production was lower in the BCG-primed group boosted with ESAT6:HspX:Fcγ2a with stimulated splenic cells; however, there was no meaningful difference between the treated groups (Fig. 7c). The BCG-primed group boosted and then stimulated with ESAT6:HspX:Fcγ2a had the highest Th1/Th2 balance of all groups (Fig. 7d). There was no significant difference for IL-17 production between the BCG-primed groups; however, TGF-β production was significantly higher for the BCG primed with ESAT6:HspX:Fcγ2a or ESAT6:HspX:His and boosted and stimulated with the same proteins or with PHA (Fig. 8a, b).
Discussion
The growing problems of multidrug-resistant TB (MDR-TB) and co-infection with HIV highlight the critical need for new effective and safe TB vaccines. BCG, the TB vaccine currently in use, is a live attenuated strain of Mycobacterium bovis and does not induce life-long protection against lung Mtb infection (WHO 2014). The global pipeline of tuberculosis vaccine candidates in clinical trials is more robust than at any previous period in history and includes recombinant BCGs such as VPM1002 and rBCG30, attenuated Mtb strains (MTB VAC), recombinant viral vectored platforms (Crucell MVA85A, Ad35, and TB/FLU-04L), protein/adjuvant combinations (M72/ASO1, hybrid 1/IC31 and HyVac4/IC31), and mycobacterial extracts such as RUTI, Mycobacterium vaccae, and Dar-901 (WHO 2014).
Most TB subunit vaccines currently in different stages of clinical trial have been designed as prophylactic vaccines based on antigens expressed in the replicating stage. The present study constructed a subunit TB vaccine using a combination of two highly immunogenic proteins, ESAT-6 and HspX, which are expressed in early and dormant stages of bacterial life cycle and target active and the latent asymptomatic forms of TB. The combined antigens genetically fused to mouse Fcγ2a as a delivery vehicle to make ESAT6:HspX:Fcγ2a that facilitates the selective antigen intake of the ESAT6:HspX construct by FcγRI on DCs and compared its immunogenicity with a similar construct fused to 6× His-tag (ESAT6:HspX-His). Although HspX has adjuvant properties, the immunogenicity of both constructs was assessed in the presence of DDA/TDB adjuvant in mice.
It is known that selective uptake of antigens by APCs is an important aspect of the TB vaccine strategy (Schuurhuis et al. 2006; Ottenhoff and Kaufmann 2012). Numerous studies have demonstrated that targeting immunogens to FcγR on APCs can selectively uptake and increase humoral and cellular immunity in vitro and in vivo (Loureiro et al. 2010; Lu et al. 2011; Krishnamurthy Konduru et al. 2013). These reports indicate that Fc in ligation with selected antigens such as HspX from Mtb can increase the immunogenicity of Fcγ-fused antigens and could be considered as a molecular adjuvant (Rath et al. 2013).
Historically, the advantage of a TB vaccine candidate is assessed by its ability to induce antigen-specific Th1 cells producing IFN-γ following vaccination (Pitt et al. 2013). The results showed that both fusion subunit vaccines induced strong antigen-specific Th1 immune responses, and IFN-γ and IL-12 production, in mice with a better response for Fc-tagged protein (Fig. 7a, b). Furthermore, the ESAT6:HspX:Fcγ2a vaccine increased BCG-primed immunity against Mtb in mice (Fig. 7a and b).
Th1 and Th2 are regulated by an epigenetic event, which is an interaction between the Th microenvironment and its potential response. In a suitable microenvironment, Th responses differentiate toward Th1, which produces IFN-γ and protects against intracellular bacterial infections. Th2 cells secret IL-4, IL-5, and IL-13 to combat extracellular pathogens (Ansel et al. 2003; Wilson et al. 2009). The microenvironment at the site of the infection/vaccination determines the outcome of the immune response by activation of suitable transcription factors for production of protective responses. In the presence of an appropriate cytokine (IL-12), the naive T cells differentiate toward Th1; IL-4 differentiates Th0 toward Th2 cells. These changes require an antigen, but not cytokine stimulation (Ansel et al. 2003; Wilson et al. 2009). IL-12 production by APCs acts mainly on downstream of epigenetic changes in Th1 differentiation, which favors Th1 growth and survival. It prolongs the Ifng transcription factor for production of more IFN-γ and IL-2. IL-12 production is upregulated by T-bet dependence and downregulated by Th2 cell cytokines (Lighvani et al. 2001; Mullen et al. 2001).
In the Th1-mediated immune response to TB, IL-12 and IFN-γ constitute an IFN-γ-IL-12 immune axis in a positive feedback cycle. As the results show, IL-12 increased considerably in the ESAT6:HspX:Fcγ2a primed and boosted group in which splenic cells were stimulated with the same antigen (ESAT6:HspX:Fcγ2a). This outcome may be a reflection of its effect on DCs that induces significant IL-12 production.
As expected, ESAT6:HspX:His protein induced a high level of IL-12, but this was significantly less than Fc-tagged TB protein (Fig. 7b). It is known that ESAT-6 and HspX are strong immmunogens; however, the main difference between these fused recombinant subunit vaccines in the production of IL-12 is tagging of the proteins. Fc had a significant effect on the immunogenicity of ESAT6:HspX:Fcγ2a, most likely by affecting the DCs (Fig. 7a, b). The same results were observed for BCG-primed mice boosted either by ESAT6:HspX:Fcγ2a or ESAT6:HspX:His; however, as Fig. 7b shows, the concentration of IL-12 was much lower than the in the aforementioned experiment. The increment of IFN-γ as a marker of Th1 immune response to ESAT6:HspX:Fcγ2a has a high level of consistency with the increase in IL-12 compared to ESAT6:HspX:His (Fig. 7d). This is the basis for the activation of macrophages and CD8+ CTL. Moreover, IFN-γ is a promising treatment for drug-resistant TB or MDR-TB (Guo and Zhao 2012).
The present study showed that IFN-γ increased significantly in the ESAT6:HspX:Fcγ2a primed and boosted group in which splenic cells were stimulated with the same protein compared with the other groups and the controls (Fig. 7a). As expected, ESAT6:HspX:His protein induced a high level of IFN-γ, but significantly less than ESAT6:HspX:Fcγ2a. IFN-γ, like IL-12, increased in the BCG primed ESAT6:HspX:Fcγ2a boosted group, but this was significantly less than for the ESAT6:HspX:Fcγ2a primed and boosted group (Fig. 7d). Previous studies have shown that Mtb subunit vaccines like MVA85A provide greater protective efficacy than BCG in animal models and provide a less-convincing increase in protection over BCG when used in prime/boost regimes (Kagina et al. 2010; PCL Beverley et al. 2013).
Potentiation of IL-12 production in ESAT6:HspX:Fcγ2a primed and immunized group was more than ESAT6:HspX:His. Increasing BCG-primed immunity shows that the Fc fragment is also involved in the development and promotion of mature DCs, inducing cross-presentation.
A main property of the Fc-fusion immunogen is its ability to bind Fcγ receptors. This property is a selective pathway for the uptake, processing, and presentation of antigens. The Fcγ fragment of this recombinant protein serves as a selective delivery system for endocytosis by way of the FcγRI. This type of endocytosis promotes antigen cross-presentation to CTLs and, consequently, activation of CMI (Baker et al. 2011; Levin et al. 2015). It is expected that immunization with ESAT6:HspX:Fcγ2a will induce cross-presentation and form CMI anti-TB responses. Therefore, Fc-fusion ESAT-6:HspX protein may promote the generation of specific CTLs that can kill Mtb-infected cells and also induces an Th1 immune response and CTL activation to produce an appropriate amount of IFN-γ to activate infected macrophages and improve their ability to destroy intercellular bacteria. ESAT6:HspX:Fcγ2a induced a Th1 cell response in mice, as evidenced by the activation of IFN-γ-positive cells in splenocytes stimulated with ESAT6:HspX:Fcγ2a. Interestingly, stimulation with ESAT6:HspX:His elicited a lower level of response than ESAT6:HspX:Fcγ2a, indicating that the Fc fragment contributed minimally to the cellular response against the fusion protein.
Th2 cytokines, including IL-4, IL-5, IL-10, and TGF-β secretion by T-reg, are suppressors of the excessive and protective response of Th1 and prevent pathological damage (Harris et al. 2007). Thus, Th1/Th2 imbalance is significant in the pathogenesis and development of TB (Reljic et al. 2009). As our results demonstrated, although the concentration of IL-4 increased, it was not comparable with IFN-γ (37 vs 900 pg/mL; Fig. 7d).
The level of Th1 and IFN-γ alone does not accurately reflect vaccine protection against Mtb infection. It has been reported that, similar to IFN-γ, IL-17 can act as an effector molecule against Mtb infection to protect against human TB (Khader et al. 2007). Qi Xin et al. demonstrated that, besides producing IFN-γ, mice receiving BCG prime and ESAT6-Ag85B-MPT64-Mtb8.4 (EAMM)-boost generated robust antigen-specific IL-17, and Mtb10.4-HspX (MH) vaccine-boosted mice generated less HspX-specific IFN-γ and IL-17 than EAMM-boost. The production of IFN-γ and IL-17 is consistent with the protective efficacy of EAMM and MH as boosters for BCG (Xin et al. 2013). Since both Th17 and T-reg cells are present in an Mtb infection, it is important for them to be balanced for a proper protective response.
A largely negative role for T-reg cells in TB has been suggested (Torrado and Cooper 2010). Their presence in the infected organs may be important for balancing protective and damaging immune responses as well. IFN-γ appears to be a key regulator of IL-17 responses in mycobacterial infections, both directly by differentiation of IL-17-producing cells and indirectly by inducing the release of tryptophan catabolic products through indoleamine 2,3 dioxygenase activation by epithelial and endothelial cells (Cruz et al. 2006).
In the present study, it can be concluded that, although the production of Th17 increased with Fc-tagged recombinant protein, it was much lower than for the IFN-γ in ESAT6:HspX:Fcγ2a groups. The increase in IL-17 compared to TGF-β as an inhibitor was very low (Fig. 8c). Taken together, our results demonstrate that the Fc-tagged subunit vaccine is more likely to induce an effective protective Th1 immune response with a very low increase in IL-17and IL-4, a slight increase in TGF-β, and a very strong response for Th1 (IL-12 and IFN-γ production) (Figs. 7 and 8).
Another advantage of the Fc-tag is dimerization of ESAT-6:HspX fusion protein after expression of an antibody-like molecule that provides high avidity interaction between a fusion partner and its receptor on APCs (Fig. 1b) (David et al. 2011). The flexible hinge region in Fcγ2a and the linker between ESAT6 and HspX facilitates proper folding of EAST6:HspX:Fcγ2a and helps preserve the function of both parts of the molecule separately (Figs. 1a, b and 6). Dimerization is an efficient way of increasing the valence and size or the hydrodynamic radius of a protein above the renal filtration threshold. Size is a factor that increases immunogenicity of the molecule and improves the pharmacokinetic properties. The effectiveness of therapeutic recombinant proteins strongly relies on their pharmacokinetic properties (David et al. 2011).
The Fc-tag may also have an adjuvant effect in mice from the interaction with Fcγ2a receptors on APCs (Czajkowsky et al. 2012; Rath et al. 2013).
The dominance of Fc-fusion proteins is largely a result of their biological and pharmacological properties, many of which reside in the immunoglobulin constant region of the Fc domain. Besides the nine Fc-fusion drugs approved by the FDA (Czajkowsky et al. 2012) and a number of studies on appropriate vaccines against infectious diseases such as Ebola (Konduru et al. 2011), HIV (Lu et al. 2011), and influenza (Loureiro et al. 2010), there has been no effort to construct an Fc-fusion vaccine against TB. The Fc-fusion TB vaccine in the present study creates a platform technology to be used for vaccination with and without BCG or for boosting. The ESAT6:HspX:Fcγ2a could be used as a subunit TB vaccine for human use. It is simple to produce in the P. pastoris expression system, easy to purify, and cost-effective (Braren et al. 2007; Guo et al. 2012). It will likely result in only limited adverse events, is safe, and is bio-compatible with the human body, like Fc-fusion therapeutic agents such as TNFR-Fc and CTLA4-Fc (Czajkowsky et al. 2012).
Further research is needed to determine whether ESAT6:HspX:Fcγ2a vaccination elicits appropriate protection in guinea pigs and monkey models for TB. These assessments are limitation of the present study. Colocalization of yeast-made Fc-Fusion protein and FcRs can help to better understand a cell-mediated response in such situations. The role of cross-presentation and CD8+T cell activation should be assessed in context of Mtb occultation to specify the role of CTLs in TB protective responses.
References
Ansel KM, Lee DU, Rao A (2003) An epigenetic view of helper T cell differentiation. Nat Immunol 4(7):616–623
Baker K, Qiao SW, Kuo TT, Aveson VG, Platzer B, Andersen JT, Sandlie I, Chen Z, de Haar C, Lencer WI, Fiebiger E, Blumberg RS (2011) Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b + dendritic cells. Proc Natl Acad Sci U S A 108(24):9927–9932
Braren I, Greunke K, Umland O, Deckers S, Bredehorst R, Spillner E (2007) Comparative expression of different antibody formats in mammalian cells and Pichia pastoris. Biotechnol Appl Biochem 47(Pt 4):205–214
Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R (2004) ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 12(11):500–508
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2(9):1511–1519
Cruz A, Egidio Torrado SAK, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG (2006) cutting edge: IFN-γ regulates the induction and expansion of IL-17-producing CD4 T cells during Mycobacterial infection. J Immunol 177:1416–1420
Czajkowsky DM, Hu J, Shao Z, Pleass RJ (2012) Fc-fusion proteins: new developments and future perspectives. EMBO Mol Med 4(10):1015–1028
David NAM, Czajkowsky DM, Andersen JT, Shi J, El-Faham M, Doenhoff M, McIntosh RS, Sandlie I, He J, Hu J, Shao Z, Pleass RJ (2011) Polymeric human Fc-fusion proteins with modified effector functions. Sci Rep 1:124
Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Eramian D, Shen MY, Pieper U, Sali A (2007) Comparative protein structure modeling using MODELLER. Curr Protoc Protein Sci Chapter 2:Unit 2 9
Guo S, Zhao J (2012) Immunotherapy for tuberculosis: what’s the better choice? Front Biosci (Landmark Ed) 17:2684–2690
Guo Y, Kang W, Zhong Y, Li R, Li G, Shen Y, Hu S, Sun J, Xiao W (2012) Purification and characterization of human IL-10/Fc fusion protein expressed in Pichia pastoris. Protein Expr Purif 83(2):152–156
Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V (2007) T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity 27(3):505–517
Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA (2010) Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med 182(8):1073–1079
Kaplan G (2012) Filovirus vaccines. J Vaccines Vaccination 01(S1).
Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM (2007) IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8(4):369–377
Konduru K, Bradfute SB, Jacques J, Manangeeswaran M, Nakamura S, Morshed S, Wood SC, Bavari S, Kaplan GG (2011) Ebola virus glycoprotein Fc fusion protein confers protection against lethal challenge in vaccinated mice. Vaccine 29(16):2968–2977
Krishnamurthy Konduru AS, Bavari S, Kaplan G (2013) Evaluation of ebolavirus glycoprotein Fc fusion protein as a subunit vaccine (P4417). J Immunol 190:205–218
Levin D, Golding B, Strome SE, Sauna ZE (2015) Fc fusion as a platform technology: potential for modulating immunogenicity. Trends Biotechnol 33(1):27–34
Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ (2001) T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A 98(26):15137–15142
Loureiro S, Ren J, Phapugrangkul P, Colaco CA, Bailey CR, Shelton H, Molesti E, Temperton NJ, Barclay WS, Jones IM (2010) Adjuvant-free immunization with hemagglutinin-Fc fusion proteins as an approach to influenza vaccines. J Virol 85(6):3010–3014
Lovell SC, Davis IW, Arendall WB 3rd, de Bakker PI, Word JM, Prisant MG, Richardson JS, Richardson DC (2003) Structure validation by Calpha geometry: phi, psi and Cbeta deviation. Proteins 50(3):437–450
Lu L, Palaniyandi S, Zeng R, Bai Y, Liu X, Wang Y, Pauza CD, Roopenian DC, Zhu X (2011) A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J Virol 85(20):10542–10553
Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, Cereb N, Yao TP, Yang SY, Reiner SL (2001) Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science 292(5523):1907–1910
O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP (2013) The immune response in tuberculosis. Annu Rev Immunol 31:475–527
Ohara N (2012) Current status of tuberculosis and recombinant bacillus Calmette-Guérin vaccines. J Oral Biosci 54(2):92–95
Ottenhoff TH, Kaufmann SH (2012) Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog 8(5):e1002607
PCL Beverley SS, Lalvani A, Tchilian EZ (2013) Harnessing local and systemic immunity for vaccines against tuberculosis. Mucosal Immunol 7:20–26
Pepponi I, Diogo GR, Stylianou E, van Dolleweerd CJ, Drake PM, Paul MJ, Sibley L, Ma JK, Reljic R (2014) Plant-derived recombinant immune complexes as self-adjuvanting TB immunogens for mucosal boosting of BCG. Plant Biotechnol J 12(7):840–850
Pitt JM, Blankley S, McShane H, O’Garra A (2013) Vaccination against tuberculosis: how can we better BCG? Microb Pathog 58:2–16
Rath T, Baker K, Dumont JA, Peters RT, Jiang H, Qiao SW, et al. (2013) Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics. Crit Rev Biotechnol 35(2):235–254
Reljic R, Paul MJ, Arias MA (2009) Cytokine therapy of tuberculosis at the crossroads. Expert Rev Respir Med 3(1):53–66
Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F (2006) Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol 176(8):4573–4580
Taylor JL, Wieczorek A, Keyser AR, Grover A, Flinkstrom R, Karls RK, Bielefeldt-Ohmann H, Dobos KM, Izzo AA (2012) HspX-mediated protection against tuberculosis depends on its chaperoning of a mycobacterial molecule. Immunol Cell Biol 90(10):945–954
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Torrado E, Cooper AM (2010) IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev 21(6):455–462
WHO (2014) Global tuberculosis report 2014. WHO www.who.int/about/licensing/copyright_form/en/index.html
Wilson CB, Rowell E, Sekimata M (2009) Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol 9(2):91–105
Xin Q, Niu H, Li Z, Zhang G, Hu L, Wang B, Li J, Yu H, Liu W, Wang Y, Da Z, Li R, Xian Q, Wang Y, Zhang Y, Jing T, Ma X, Zhu B (2013) Subunit vaccine consisting of multi-stage antigens has high protective efficacy against Mycobacterium tuberculosis infection in mice. PLoS One 8(8):e72745
Acknowledgments
The authors are grateful to Reza Nasr for his valuable advice and help for designing the constructs, and Maliha Hassannia, Mastoureh Momen Heravi, Maliheh Moghadam, and Mehdi Aganj, Medical School, MUMS, for valuable help. This study was a part of a PhD dissertation by Saman Soleimanpour and has been supported by Vice Chancellor for Research, Mashhad University of Medical Sciences, Mashhad, Iran. This paper was financially supported by grants from the Mashhad University of Medical Sciences (grant number: 910072).
Conflict of interest
The authors declare no conflict of interest associated with the present manuscript.
Compliance with ethical standards
The experimental protocol was reviewed, approved, and supervised by the Institutional Animal Care and Use Committee of Mashhad University of Medical Sciences (permit number: 910072). During the experiments, the vaccinated mice were monitored every day. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Mice were killed by cervical dislocation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hadi Farsiani and Arman Mosavat contributed equally to this work.
Rights and permissions
About this article
Cite this article
Soleimanpour, S., Farsiani, H., Mosavat, A. et al. APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice. Appl Microbiol Biotechnol 99, 10467–10480 (2015). https://doi.org/10.1007/s00253-015-6952-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6952-z