Abstract
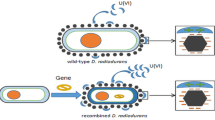
The aim of the present work was to engineer bacteria for the removal of Co in contaminated effluents. Radioactive cobalt (60Co) is known as a major contributor for person-sievert budgetary because of its long half-life and high γ-energy values. Some bacterial Ni/Co transporter (NiCoT) genes were described to have preferential uptake for cobalt. In this study, the NiCoT genes nxiA and nvoA from Rhodopseudomonas palustris CGA009 (RP) and Novosphingobium aromaticivorans F-199 (NA), respectively, were cloned under the control of the groESL promoter. These genes were expressed in Deinococcus radiodurans in reason of its high resistance to radiation as compared to other bacterial strains. Using qualitative real time-PCR, we showed that the expression of NiCoT-RP and NiCoT-NA is induced by cobalt and nickel. The functional expression of these genes in bioengineered D. radiodurans R1 strains resulted in >60 % removal of 60Co (≥5.1 nM) within 90 min from simulated spent decontamination solution containing 8.5 nM of Co, even in the presence of >10 mM of Fe, Cr, and Ni. D. radiodurans R1 (DR-RP and DR-NA) showed superior survival to recombinant E. coli (ARY023) expressing NiCoT-RP and NA and efficiency in Co remediation up to 6.4 kGy. Thus, the present study reports a remarkable reduction in biomass requirements (2 kg) compared to previous studies using wild-type bacteria (50 kg) or ion-exchanger resins (8000 kg) for treatment of ~105-l spent decontamination solutions (SDS).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spent decontamination solutions are generated from power-generating nuclear reactors during the chemical decontamination process (Ayres 1970; Taylor 1976; Cohen 1980; Lejon et al. 1994; Urch 2013). Radioactive cobalt, mainly as an isotopic mixture of 59Co, a stable isotope of elemental cobalt, and 60Co, an activation product of 59Co, are present at trace chemical concentrations (8.5–34 nM of total cobalt) in such solutions (Charlesworth 1971; Kurnaz et al. 2007). Apart from 60Co, the spent decontamination solution can also include radionuclides such as 51Cr, 54Mn, 59Fe, and 58Co, which are relatively minor contributors to the radiation field due to these solutions. The radiation field arising from these solutions is mainly controlled by 60Co due to its long half-life of 5.27 years and the high energy (1.17 and 1.33 MeV) gamma ray emission from its nucleus. Hence, the removal of this cobalt at trace chemical concentrations will make the solution benign from the radiation field control point of view. The conventional method of employing cationic exchange column is mainly for regenerating the complexants in the decontamination solution by way of metal ion removal. However, the sorption of soluble Co (as Co2+) from the spent decontamination formulation by the cation resin and its subsequent displacement from the resin due to the sorption of other metal ions like Fe, Cr, and Ni, which are present at much higher chemical concentrations but which are only minor contributors to the radiation field problem, create the requirement of a large number of cation columns to avoid the Co elution. This necessitates an inefficient use of large amount of cation-exchange resin. The large amount of solid radioactive waste thus generated also requires prolonged safe storage (for tens of years) in tile holes/trenches (Venkateswaran et al. 2003; Prakash et al. 2013; Frišták et al. 2014a, b).
Recent developments in microbiology have facilitated the use of fungi and bacteria for bioremediation of toxic metals and radionuclides from soils and waters (Akthar et al. 1996; Daly and Minton 1996; Brim et al. 2000; Amachi et al. 2010; Green et al. 2012). Microorganisms take up metal ions by two broad mechanisms referred to as biosorption and bioaccumulation. Biosorption is a rapid process of metal ion binding to surface of the cell and is independent of cell metabolism. In comparison, bioaccumulation is a slower energy-dependent process involving cell membrane transporters. Both approaches have been employed in bioremediation strategies, for the removal of heavy metals and radionuclide from simulated and natural environments (Gadd and White 1989; Akthar et al. 1996; Rama Rao et al. 1996; Naveena et al. 2005; Satinder et al. 2006; Kumar et al. 2007). Therefore, bioremediation of radionuclides or radioactive waste by using methods such as biotransformation, bioaccumulation, biosorption, biostimulation, and bioaugmentaion is only limited. Bioremediation of trace cobalt from simulated spent decontamination solutions of nuclear power reactor aims at the use of biological agents as such or genetically engineered (Gunjan et al. 2005; Lloyd and Renshaw 2005; Kumar et al. 2007; Prakash et al. 2013; Shih and Shen-Long 2014). This can be a viable and relatively inexpensive cleanup option. However, such organisms are generally radiosensitive and cannot be used effectively for bioremediation of radionuclides from the spent decontamination solutions. To overcome this problem, we have selected a highly radiation resistant organism known as Deinococcus radiodurans strain R1, a gram-positive nonpathogenic bacterium that can grow at a chronic exposure of 60 Gy/h (a dose rate that exceeds those in most solidified spent decontamination waste). D. radiodurans can also survive acute exposure to more than 10–20 kGy without undergoing mutations and have been successfully bioengineered for environmental biotechnology (Daly and Minton 1995, 1996; Battista 1997; Lange et al. 1998; Brim et al. 2000, 2003; Daly 2000; Kulkarni et al. 2013; Shih and Shen-Long 2014).
Previous studies from our laboratory screened and tested several wild type and mutant fungal strains as well as wild-type bacteria for removal of total cobalt *Co (60Co + 59Co) from spent decontamination solutions. These studies demonstrated significant *Co removal ranging from 20 to 40 ng/g biomass by fungi and 1 μg/g biomass bacteria from simulated spent decontamination effluents containing 34 nM (2 ppb) of total cobalt (Rashmi et al. 2004, 2007). Genetically bioengineered E. coli, with Ni/Co transporter (NiCoT) genes from Rhodopseudomonas palustris CGA009 (RP) and Novosphingobium aromaticivorans F-199 (NA), were successfully used for removal of trace cobalt (*Co) from aqueous solutions (Raghu et al. 2008; Duprey et al. 2014). These recombinant E. coli strains showed significant *Co pickup capacities of up to 12 μg/g biomass under optimal conditions. However, bacteria did not survive beyond 20-Gy exposure to 60Co γ rays. The present study aims at developing microbes capable of surviving in high-radiation environments. Therefore, we chose an inherently radioresistant, solvent tolerant, and nonpathogenic D. radiodurans for this purpose. Moreover, its genome is capable of expressing foreign genes with no effect on either its growth or survival at highly extreme radiation doses (Carroll et al. 1996; Daly 2000; Misra et al. 2014).
Overall, we engineered recombinant D. radiodurans strains expressing NiCoT genes of R. palustris CGA009 and N. aromaticivorans F-199. The recombinant strains could remove trace cobalt in the presence >105-fold concentrations of Fe, Cr, and Ni from simulated decontamination solutions, and its capacity to remove cobalt was unaffected up to 6.4 kGy of γ dose.
Material and methods
Bacterial strains and growth conditions
D. radiodurans strain R1 was grown aerobically in TGY (1 % Bacto Tryptone, 0.1 % glucose, and 0.5 % yeast extract) liquid medium over 48 h of incubation at 32 ± 1 °C under agitation (180 ± 5 rpm). E. coli (DH5α) and E. coli (ARY023) (Rodrigue et al. 2005) cells were grown in Luria-Bertani (LB) medium over 24 h of incubation at 37 ± 1 °C under agitation (180 ± 5 rpm). Bacterial growth was assessed by measuring turbidity (optical density at 600 nm [OD600]) or by determining the number of colony-forming units (CFU)/ml on TGY agar plates (1.5 % Bacto Agar). The antibiotic concentration used for the selection of transformants was 3 μg ml−1of chloramphenicol for Deinococcus (Meima and Lidstrom 2000) and 100 μg ml−1of ampicillin for E. coli.
Cloning of groESL promoter and NiCoT-RP and NA genes into E. coli-Deinococcus shuttle vector-pRAD1
The shuttle vector pRAD1 was chosen because it can replicate both in D. radiodurans R1 and in E. coli. pRAD1 is 6.3 kb in size and contains an extended multiple cloning site which will be useful for general-purpose cloning. Since, Deinococcus does not often optimally recognize foreign promoters, the NiCoT genes were cloned and expressed under the influence of a deinococcal PgroESL promoter. The primers designed were based on the published groESL promoter sequences of D. radiodurans R1 and NiCoT genes of R. palustris CGA009 and N. aromaticivorans F-199. Hence, the groESL promoter was PCR-amplified from D. radiodurans R1 (DR0606) with BamHI and EcoRI sites, and the NiCoT genes of RP and NA were PCR-amplified from pCH675RP and pCH675NA (Hebbeln and Eitinger 2004) by using gene-specific primers with EcoRI and HindIII restriction sites (Table 1). The cohesive end NiCoT-RP and NA PCR products (1.05 and 1.10 kb) were ligated to the groESL promoter (0.256 kb), and final ligated products (1.306 and 1.356 kb) were ligated to the BamHI/HindIII sites of shuttle vector pRAD1 to obtain pRADgroESL-RP and pRADgroESL-NA. The E. coli (DH5α) transformants carrying plasmid pRADgroESL-RP and pRADgroESL-NA were selected on LB containing ampicillin (100 μg ml−1), plasmids were isolated, and the inserts therein were confirmed by restriction digestion. Plasmids were isolated from a few of the positive clones and used to transform competent E. coli (ARY023), which is a ΔrcnA mutant (Rodrigue et al. 2005), and D. radiodurans R1 cells as described earlier (Meima and Lidstrom 2000). The transformants were plated on LB agar containing ampicillin (100 μg ml−1) and TGY agar containing chloramphenicol (3 μg ml−1). Further, D. radiodurans R1 transformed with pRADgroESL-RP and pRADgroESL-NA transformants were screened by PCR using gene-specific primers. The expected sizes of DNA fragment products of promoter groESL (0.256) and NiCoT genes of RP and NA are 1.05 and 1.1 kb, respectively. The same plasmids were then used for the gene sequencing and PCR reactions using pRAD1 primers P5 and P6 which, respectively, bind upstream and downstream of the multiple cloning site. The expected DNA inserts (promoter groESL plus NiCoT-RP and NA) indicate the presence of gene upstream of the groESL promoter.
RNA Isolation from bioengineered Deinococcus expressing NiCoT genes of R. palustris CAG009 and N. aromaticivorans F-199 and quantitative RT-PCR
Overnight-grown recombinant Deinococcus (DR-NA and DR-RP) cultures (10 ml) in the absence or presence of 10 μM (Co+2 or Ni+2) were subjected to centrifugation at 9000 g for 5 min. The pellet (size ~100 μl) was washed twice in chilled ethanol, followed by one to two washes in DEPC-treated distilled water to remove all the ethanol. The pellet was next resuspended in 0.5 ml of RNAWiz (Ambion) and subjected to four cycles of freeze (in liquid nitrogen) and thaw (37 °C). The frozen cell suspension was stored at −70 °C overnight. The cell suspensions were then thawed on ice; then, 0.5 ml of autoclaved glass beads (~500 μ) and 0.5 ml of RNAWiz were added. The mixture was subjected to vigorous vortexing for 3 min followed by 3 min on ice. Total RNA derived from each sample was treated with 10 units DNase I (Ambion, Austin, TX, USA) and purified using RNeasy Minikit columns (QIAGEN, Valencia, CA, USA). Then, absorbance of the isolated RNA at 260 nm (1 OD = 40 μg of RNA) was taken. For checking the quality of RNA, electrophoresis was carried out with a 2 % agarose gel with 1× TAE as the running buffer. The total RNA (2 μg) was reverse transcribed to complementary DNA (cDNA) using Revert Aid™ H minus M-MuLV reverse transcriptase and random hexamer primers (Fermentas Life Sciences). The target genes selected in this study were NiCoT-NA and NiCoT-RP, and the primer pairs that were used for qRT-PCR were designed using the primer3 program (Rozen and Skaletsky 2000) and are shown in Table 1. The above synthesized cDNAs were quantitatively measured, and real-time PCR was performed with an Applied Biosystems 7500 Fast machine using Real Master Mix SYBR ROX from 5 PRIME, USA. Quantitative real-time PCR conditions employed were as follows: initial denaturation at 95 °C for 60 s followed by 40 cycles of denaturation at 95 °C for 20 s, annealing at 52 °C for 20 s, and extension at 68 °C for 20 s. Reaction specificity was determined for each reaction using the melt-curve analysis of the PCR product. To calculate the fold change in gene expression, the 2-∆∆Ct method was used (Livak and Schmittgen 2001). Expression levels of the target genes (NiCoT-NA and NiCoT-RP) were normalized to the housekeeping 16S rRNA gene (AE000513) of D. radiodurans R1 (Lin et al. 1999). Analyses were performed in three different independent experiments and in triplicate for both target and normalize reactions.
*Co removal studies
In the present study, different physicochemical, chemical, and biological factors have been optimized for *Co removal from simulated effluent. They are pH of simulated effluent (2.8, 5.8, and 7.0); Fe-to-Co molar concentration ratio of 1.25, 2.5, 5, and 10 × 105; and duration of treatment (15, 30, 60, 90, and 120 min). To determine dose response to viability for Deinococcus, selected doses were 3.2, 4.8, 6.4, 8.0, 9.6, 12.8, 16.0, and 19.2 kGy. Different culture densities (0.5, 1.0, 2.0, 3.0, and 4.0) were also tested. Typically, the simulated decontamination effluent (SE) used to suspend the pelleted bacterial cultures was composed of iron (9.3 mM of Fe as FeSO4 (NH4)2SO4 · 6H2O), chromium (3 mM of Cr as CrCl3 · 6H2O), nickel (0.93 mM Ni as NiSO4 · 7H2O), and cobalt in the concentration range of 4.25–34 nM (0.25–2 ppb) Co as CoCl2 traced with 60Co to yield a solution specific activity of 40 Bq/ml (1.08 nCi/ml), and the tagged cobalt (represented as *Co) was used in the presence of disodium EDTA (13.5 mM). The initial pH of SE was adjusted to 2.8, 5.8, or 7.0 with dilute HCl/NaOH as required. An optimum culture density of 1 OD unit at 600 nm was used in the kinetic experiments. D. radiodurans cultures were grown in TGY medium for 48 h with 3.0 μg/ml chloramphenicol. The bacterial cells were pelleted by centrifugation at 7000 g for 5 min and resuspended in 20-ml volume of SE to give a density of o1 OD unit (0.5-mg dry weight) and incubated in rotary shaker incubator (120 rpm at 37 °C) for various periods up to 2 h. The cells were then harvested by centrifugation at 14,000 g, at 22 °C, for 10 min. *Co remaining in clear supernatant was determined by an integral γ-counter coupled to a well type (2 × 2 in. NaI (Tl) detector.
Post-irradiation and during irradiation *Co removal studies
Early-stationary-phase cultures of Deinococcus clones were washed twice and resuspended in fresh TGY and SE at an OD600 of 1.0 for the above mentioned studies. Then, the cultures were exposed to 3.2 to 19.2 kGy and 1.6 to 8 kGy of 60Co gamma rays at a dose rate of 3.2 kGy h−1 for post-irradiation and during irradiation studies, respectively (60Co Gamma Cell 5000 irradiation unit; HIRUP, Bhabha Atomic Research Centre, Mumbai, India). An aliquot of the culture kept outside the radiation source served as the control. The irradiated and control cells were washed, serially diluted, plated in triplicate on TGY plates containing chloramphenicol in the case of Deinococcus clones, and incubated under optimum growth conditions. Colonies were counted, and the survival curve was plotted. Irradiated cells were also assayed for their *Co removal studies as described earlier.
Statistical analysis
Data shown in most of the experiments are average values of triplicates from three independent batch experiments (± SD).
Results
Bioengineering of D. radiodurans R1 with NiCoT genes of R. palustris CGA009 and N. aromaticivorans F-199
The present study aims to generate recombinant clones capable of *Co bioremediation in radioactive environments. Thus, the above mentioned NiCoT-RP and NiCoT-NA genes, coding for highly specific cobalt transporters from R. palustris CGA009 (RP) and N. aromaticivorans F-199 (NA), were successfully cloned and expressed under the control of T7 promoter in E. coli and a strong deinococcal groESL promoter in both E. coli and D. radiodurans R1 (Table 1).
Quantitative real time-PCR analysis of NiCoT genes of R. palustris CGA009 and N. aromaticivorans F-199 expression in bioengineered D. radiodurans R1
The expression of two NiCoT gene (NiCoT-NA and NiCoT-RP) messenger RNA (mRNA) in bioengineered D. radiodurans R1 was examined by reverse transcriptase PCR (data not shown) and quantitative real-time-PCR. There was no apparent difference between the levels of mRNA expressed in these two clones, and the expression pattern of the NiCoT-NA transcripts was similar to that of NiCoT-RP transcripts. mRNA levels were significantly enhanced (6.4–7.3 or 1.8–1.9-fold) upon addition of Co+2 or Ni+2 (10 μM), respectively (Fig. 1). The NiCoT-NA and NiCoT-RP transcripts were found to be higher in Co+2 than in Ni+2-treated cells (≈4-fold). The groESL promoter is thus induced by Ni or Co.
NiCoT gene expression in recombinant D. radiodurans R1-NA/RP by quantitative RT-PCR analysis: NiCoT-NA, NiCoT-RP relative gene expression in DR-NA and DR-RP. Total RNAs extracted from DR-NA and DR-RP cultured in the absence or presence of 10 μM Co+2 or Ni+2 as its chlorides. Samples were reverse transcribed to cDNA and mRNA expression was measured by qRT-PCR. The expression of 16S rRNA was used as the internal control
*Co removal efficiency by transformed E. coli and Deinococcus cells
The Co removal efficiency of recombinant D. radiodurans strains was optimized using overnight-grown cultures. The cells were incubated in presence of SE containing 8.5 nM of Co. The results showed that *Co removal reached a maximum (>60 %) at 60-min incubation in SE for E. coli recombinants, whereas 90 min was required for getting the similar efficiency removal for Deinococcus transformants (Fig. 2a). Beyond 90 min the Co removal efficiency was decreased to 30–40 %. In case of vector controls, about 5 or 25 % of Co removal was achieved for E. coli (ARY023) and Deinococcus, respectively. The 20–30 % Co removal capacity exhibited by pRAD-V (DR1) indicates that the basic organism itself seems to be containing specific (i.e., ABC cobalt transporter) and or nonspecific transporters (i.e., CDF and CorA transporters), some cobalt binding proteins unlike in the case of pRAD-V (ARY023). Increased incubation time even up to 24 h (usual decontamination run duration) had no further effect on *Co removal (data not shown). Toxic effects of other metal ions present in simulated effluent could lead to cell lysis and subsequent release of Co from the biomass into the solution. Hence, in a separate experiment, the chemical toxicity caused by SE to the bacteria during different periods of incubation was determined by a viable counting method (Fig. 2b). Up to 90-min incubation in SE, ≈20 % cell lysis was noted, but beyond 90 min, there was a drastic decrease in cell viability. Long incubation periods result thus in severe chemical toxicity due to other metal ions and chelators present in the decontamination solutions (Fig. 2b).
a Cobalt removal kinetics in control as well as bioengineered E. coli and D. radiodurans with NiCoT-RP and NA: filled circles on solid line indicate E. coli ARY023 (pRAD-V), empty circles on solid line indicate E. coli ARY023 (pRAD-RP), filled inverted triangles on solid line indicate E. coli ARY023 (pRAD-NA), empty triangles on solid line indicate DR1 (pRAD-V), filled squares on solid line indicate DR1 (pRAD-RP), and empty squares on solid line indicate DR1 (pRAD-NA). b Bioengineered E. coli and D. radiodurans viability with incubation time in SE (concentration of Co is 8.5 nM): filled circles on solid line indicate pRAD-V, empty circles on solid line indicate pRAD-RP, and filled inverted triangles on solid line indicate pRAD-NA
In subsequent experiments of *Co removal, the influence of concentrations of Co (i.e., 4.25, 8.5, 17, and 34 nM in SE) was studied. It may be noted that SE also contains large excess of iron (9.3 mM), chromium (3 mM), and nickel (0.93 mM). The results shown in Fig. 3a clearly depicted that the percentage of Co removal efficiency was 20–25 %, 50–60 %, 35–40 %, and 32–33 % at 4.25, 8.5, 17, and 34-nM initial Co concentrations, respectively. However, the specific Co removal capacities of bioengineered DR1 strains were 40–50, 260–280, 190–200, and 180–200 nmol g−1 of biomass at 4.25, 8.5, 17, and 34-nM initial Co concentrations, respectively (Table 2). The chemical process exhibited by the bioengineered D. radiodurans in picking up *Co from simulated spent decontamination solutions is expected to be concentration dependent. Starting with an initial ≥8.5-nM *Co solution, the decrease in Co concentration observed is >5 nM. However, the sharp decrease in pickup of Co at a concentration of 4.25 nM under similar duration of exposure implies a change in the kinetics. The rate constant seems to get lowered by more than a factor of 5, and at <4.25 nM the rate constant seems to become independent of *Co concentration; a zero-order kinetics comes into play. The availability of cobalt ions in solution becomes critical, and this critical concentration seems to be 8–10 nM. Thus, the NiCoT-RP and NiCoT-NA genes known as high-affinity NiCoT transporter genes have a functional expression at an optimal cobalt concentration in the range of 8–10 nM (Hebbeln and Eitinger 2004); this could be the possible reason for the sudden drop in cobalt removal capacity observed in the present study. This suggests that the efficiency of Co removal is optimum when the initial concentration is around 10 nM. However, the amounts of removed Co (0.93, 5.03, 6.09, and 9.93 nmol) increased with the increasing initial concentration of Co. The strain with the vector removed constant amounts of Co independently of the initial Co concentration. This suggests that, in this case, passive mechanisms are responsible for Co capture. Co removal capacities were also established by using different culture densities of D. radiodurans transformants (Fig. 3b). About 18–22 % removal was achieved at 0.5 OD units; it increased to more than 65–75 % removal efficiency at ≥1.0 OD units. Beyond 1 OD unit, no significant change in *Co removal was observed; hence, 1 OD unit seems to be optimum. Both DR-RP and DR-NA showed similar *Co removal capacities. DR alone showed similar removal capacities all along the tested OD range. At 1.0 OD unit, maximum removal was obtained; this condition was employed in further experiments. This shows that there is an optimal solution volume to culture density (in the present experiment, it is ≈ 20), beyond which it is not beneficial to use high culture densities. The increase in culture density was obtained by using the same growth phase culture. The improvement of cobalt removal from solution with a culture density shows a saturation effect at ≥ 1 OD unit (about 65 % removal efficiency). In a heterogeneous system like bacteria culture/metal ion in solution, there can be different concentration gradients from the surface of the bacteria to bulk solution especially at high culture densities. Such a phenomenon could possibly explain the observed saturation pickups. Hence, culture density-solution volume parameter as observed in the present work is important from the application point of view. Finally, we checked the *Co removal at different pH of SE solution. The efficiency values were of 22, 60, and 40 % at pH 2.8, 5.8, and 7, respectively (Fig. 4). Hence, in all subsequent experiments, the optimal pH of 5.8 of simulated effluent was employed. Overall, the optimal conditions for *Co removal were 90-min incubation time with 1.0 OD unit culture density, in SE solution containing an initial concentration of 8.5 nM Co, at pH 5.8.
Post-irradiation response and *Co removal efficiency of D. radiodurans R1 harboring NiCoT genes of RP and NA
The radiation toxicity was determined on the different Deinococcus strains by evaluating their D xy value, where x corresponds to 10 % survival, y corresponds to 90 % lethality, and D is the corresponding dose received (dose allowing 10 % survival and causing 90 % lethality). From the above relationship, this D value for DR clones was found to be close to 12.8 kGy (Fig. 5a). It indicates that cloned NiCoT genes of R. palustris CGA009 and N. aromaticivorans F-199 genes did not affect the radiation resistance of the host cell. There was no much change in the total Co removal efficiency of D. radiodurans clones (DR-RP and DR-NA) up to 6.4-kGy exposure. However, beyond 9.6-kGy dose, Co removal decreased progressively. The Co removal efficiency percentage was 55–60 %, 54–55 %, 52–59 %, 54–60 %, 52–53 %, 51–52 %, 39–42 %, 32–38 %, and 12–15 % at 0, 3.2, 4.8, 6.4, 8, 9.6, 12.8, 16, and 19.2 kGy of radiation dose, respectively (Fig. 5b). The studies on trace Co removal from simulated effluents containing a large excess (>105) of heterogeneous other metal ions (Fe, Cr, and Ni) using bioengineered radiation-resistant bacteria reported in this work are indeed very significant advancements over their previous efforts using several species of bacteria, fungi (Rashmi et al. 2007, 2004), and bioengineered E. coli (ARY023) with NiCoT gene of R. palustris and N. aromaticivorans (Raghu et al. 2008). The problem is that bioengineered E. coli hardly survives up to 20 Gy during the Co removal, because of γ-radiation toxicity, and the survival values for recombinant E. coli and DR at 20 Gy are ≈ 1.125 × 107 and ≈ 2.95 × 107 CFU ml−1, respectively (Raghu et al. 2008). In the present study, we successfully constructed recombinant D. radiodurans R1 strains capable of expressing NiCoT genes of RP and NA, which exhibit good capacity to remove trace cobalt from simulated effluents of nuclear power reactors even after exposure of 8 kGy of γ-radiation dose, without changing its radiation resistance.
a Radiation toxicity of different Deinococcus strains—D xy value evaluation where x is 10 % survival and y is 90 % lethality: filled circles on solid line indicate pRAD-V, empty circles on solid line indicate pRAD-RP, and filled inverted triangles on solid line indicate pRAD-NA. b Post-irradiation percentage Co removal by control and cloned DR1 species with absorbed dose: filled circles on solid line indicate pRAD-V, empty circles on solid line indicate pRAD-RP, and filled inverted triangles on solid line indicate pRAD-NA
*Co removal efficiencies of bio-engineered D. radiodurans R1 during irradiation
D. radiodurans R1 clones (DR-RP and DR-NA) were exposed to different doses along with the simulated effluent solution. The results depicted in Fig. 6a clearly point to the fact that there was no change in the Co removal capacity of Deinococcus clones up to 3.2-kGy exposure. Up to 6.4-kGy dose received, there was a negligible decrease (≈5 %) in *Co pickup, and beyond this dose, *Co pickup reduced from 60 to 40 % (Fig. 6b). The known advantage with D. radiodurans is that it can easily survive at higher doses of γ-radiation, which was also clearly depicted in the present study (Fig. 5a, b). In addition, the Co removal efficiency of irradiated D. radiodurans recombinants was more when compared to the unirradiated ones (Table 3).
a Survival of control and bioengineered D. radiodurans when irradiated in the presence of simulated spent decontamination solution to lower absorbed doses: filled circles on dashed line indicate pRAD-V, open circles on dashed line indicate pRAD-RP, and filled inverted triangles on dashed line indicate pRAD-NA. b Cobalt removal during γ-irradiation: percentage cobalt removal by control and cloned Deinococcus strains taken in simulated spent decontamination solution and exposed to different doses of γ-irradiation: filled circles on solid line indicate pRAD-V, empty circles on solid line indicate pRAD-RP, and filled inverted triangles on solid line indicate pRAD-NA
Discussion
Selective transport of molecules across the cell membrane is a fundamental process in all living organisms. Uptake of metals by various microorganisms broadly involves rapid process of biosorption to the cell wall followed by a relatively slower process of bioaccumulation into the cell (Rama Rao et al. 1996; Naveena et al. 2005). The later process involves both specific and nonspecific transporters located on the cell membrane. Recent advances in genetic engineering revealed that biological metal transporters assimilate and accumulate metals for bioremediation purposes (Maruthi Mohan et al. 2007). The cobalt transporter gene, which was first identified in Rhodococcus rhodochorus (nhlF) (Komeda et al. 1997), is the NiCoT family of secondary transporters. NiCoT genes have been characterized from various bacterial species such as R. palustris CGA009 (RP); N. aromaticivorans F-199 (NA) were shown to have preferential uptake for cobalt than for Ni (Hebbeln and Eitinger 2004; Raghu et al. 2008; Deng et al. 2013; Duprey et al. 2014). The tight homeostatic control mechanisms, which could limit metal influx or efflux of accumulated metals inside the cell, can be used for the improvement of bioremediation systems to remove or reduce the concentration of radionuclides from spent decontamination solutions (Liu and Duu-Jong 2014; Won et al. 2014). Recent developments in microbiology and molecular biology have been applied for metal bioremediation through construction of bioengineered microorganisms (Gadd and White 1989; Daly 2000; Gunjan et al. 2005; Lloyd and Renshaw 2005; Kumar et al. 2007; Prakash et al. 2013; Shih and Shen-Long 2014). Recently, the extremely radioresistant bacteria such as Deinococcus geothermalis and D. radiodurans have been bioengineered to reduce the organic solvents and uranium from nuclear waste (Lange et al. 1998; Appukuttan et al. 2006; Kulkarni et al. 2013; Misra et al. 2014). Interestingly, these bioengineered microbes survived against acute exposures of more than 10–20 kGy of γ-irradiation (Daly and Minton 1995; Battista 1997; Daly 2000; Appukuttan et al. 2006; Kulkarni et al. 2013; Misra et al. 2014). As Deinococcus does not often optimally recognize foreign promoters, the NiCoT genes were cloned and expressed in the present study under the influence of a deinococcal PgroESL promoter. Although the groESL promoter is involved in the heat shock response in most of the bacteria, the deinococcal groESL is a strong promoter at normal growth temperature of Deinococcus (Meima and Lidstrom 2000). Therefore, it was chosen to drive the expression of NiCoT genes of RP and NA. The NiCoT-RP and NiCoT-NA genes were fused to the groESL promoter and transformed into both E. coli and D. radiodurans using the shuttle vector pRAD1. These vectors are present in the cell at approximately the same copy number as the chromosome, which is present at 7 to 10 copies per cell (Meima and Lidstrom 2000); hence, there could be minimal possibility of plasmid curing or lost. During the time of the cobalt removal assay or of the process, there are no subcultures of D. radiodurans; hence, there will be no chance to lose the plasmid, because, first, the bacteria are grown in the presence of an antibiotic followed by harvesting and packing for the mean of remediation. Previous results showed that the mRNA expression of the groESL gene was induced by a wide range of divalent metal ions like Cu+2, Zn+2 (at concentrations ranging from 10 to 100 μM), and Cd+2 (as low as 1 μM) (Ybarra and Webb 1999). However, Co+2 or Ni+2 have not been tested previously on the induction of the groESL promoter. Hence, in the present study, the metal response of the groESL promoter of D. radiodurans R1 was studied at mRNA level and was found to be activated under Ni or Co stress. These findings indicate that Ni or Co present in SE solution can regulate the expression of both NiCoT-RP and NiCoT-NA in Deinococcus recombinants.
Efforts have been made in the past to remove *Co effectively from simulated effluent decontamination solution of nuclear power reactors (Ayres 1970; Venkateswaran et al. 2003; Tišáková et al. 2013; Urch 2013). The major objective of our study is to address the problem of solid waste generation in the hitherto adopted ion-exchange methodology, which exhibits only a limited selectivity in metal ion pickup during the treatment of spent decontamination solution arising from the decontamination of primary heat transport systems of nuclear power reactors of the water-cooled type. In a typical boiling water reactor (BWR) system, the decontamination solution volume is 1 × 105 l (Taylor 1976; Bradbury et al. 1986) with a total Co concentration of ≈ 8.5 nM (0.5 ppb) and a total exposure dose rate of >60 Gy h−1. A typical Co removal capacity of 200 –nmol g biomass−1 (i.e., 11.79 μg g−1 of biomass) would require about 4.2 kg of biomass for total Co bioremediation from a BWR system. At 1 OD culture density, application will require suspension of about 2 kg of biomass of D. radiodurans R1 expressing NiCoT genes of RP and NA in 1 × 105 l of reactor water, and hence, a two-stage treatment would be required to remove all the Co from reactor water.
The presently observed removal capacities are much higher than that observed in our previous studies using bioengineered E. coli with NiCoT genes of RP and NA (Raghu et al. 2008; Duprey et al. 2014) and mutant variety of simple bacteria and fungi (Rashmi et al. 2007, 2004). In conclusion, this study reports for the first time the efficient *Co removal by recombinant DR-RP and DR-NA (12.0 μg of cobalt) when compared to fungi Neurospora crassa, N. crassa CSM-9, and E. coli DH5α (0.02, 0.04, and 1.0 μg, respectively). NiCoT gene efficiency in various host systems like E. coliBL21, JM109, MC4100, and ARY023 and D. radiodurans showed removal efficiency values of 5.0, 6.0, 1.0, 12.0, and 12.0 μg/ g dry wt biomass, respectively. Though the ultimate total Co removal capacities observed with bioengineered D. radiodurans and E. coli is about the same, the radiation stability of D. radiodurans is a significant advantage over the E. coli species. In vitro D. radiodurans R1 had shown efficiency up to 6.4-kGy radiation. As a part of future work, we are in a process to clone and overexpress a suitable cytoplasmic cobalt metal binding protein in D. radiodurans R1, helps in excess metal sequestration entered through influx transporter, which, in turn, maintains the intracellular homeostasis and uncontrolled metal uptake. Further, it necessary to evaluate the efficiency of these engineered stains under field conditions enabling design of large-scale bioreactor for radioactive decontamination form nuclear reactors. The rationale of the present study was to demonstrate the advantages of using D. radiodurans for the bioremediation of *Co from spent decontamination solution in a radiation-ridden ambience. The batch experiments carried out in the present study successfully demonstrated the same. Nevertheless, more laboratory experiments especially under flow conditions and column parameters are essentially needed as well in order to translate into viable biotechnological applications of nuclear power reactors. Moreover, scaled-up studies are required before taking the methodology to actual application.
References
Akthar N, Sastry KS, Mohan PM (1996) Mechanism of metal biosorption by fungal biomass. Bimetals 9:21–28
Amachi S, Minami K, Miyasaka I, Fukunaga S (2010) Ability of anaerobic microorganisms to associate with iodine: 125I tracer experiments using laboratory strains and enriched microbial communities from subsurface formation water. Chemosphere 79:349–354
Appukuttan D, Rao AS, Apte SK (2006) Engineering of Deinococcus radiodurans R1 for Bioprecipitation of Uranium from Dilute Nuclear Waste. Appl Environ Microbiol 72:7873–7878
Ayres JA (1970) Decontamination of Nuclear reactors and equipments, Ronald Press Company NY. Libr Cong Cat Card Number 76:110543
Battista JR (1997) Against all odds: the survival strategies of Deinococcus radiodurans. Annu Rev Microbiol 51:203–224
Bradbury D, Smee TL, Williams MR (1986) Recent reactor decontamination experience with LOMI/CANDECON and related processes. In: Proceedings of international conference on water chemistry of nuclear reactor systems (4), British Nuclear Energy Society (BNES) London, 257
Brim H, McFarlan SC, Fredrickson JK, Minton KW, Zhai M, Wackett LP, Daly MJ (2000) Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat Biotechnol 18:85–90
Brim H, Venkateshwaran A, Kostandarithes HM, Fredrickson JK, Daly MJ (2003) Engineering Deinococcus geothermalis for bioremediation of high-temperature radioactive waste environments. Appl Environ Microbiol 69:4575–4582
Carroll JD, Daly MJ, Minton KW (1996) Expression of recA in Deinococcus radiodurans. J Bacteriol 178:130–135
Charlesworth DH (1971) Water reactor plant contamination and decontamination requirements - a survey. Proc Am Power Conf 33:749–756
Cohen A (1980) Water coolant technology of power reactors. American Nuclear Society, La Grange Park
Daly MJ, Minton KW (1995) Resistance to radiation. Science 270:1318
Daly MJ, Minton KW (1996) An alternative pathway of recombination of chromosomal fragments precedes recA-dependent recombination in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol 178:4461–4471
Daly MJ (2000) Engineering radiation-resistant bacteria for environmental biotechnology. Curr Opin Biotechnol 11:280–285
Deng X, Jinmei H, Ning H (2013) Comparative study on Ni2+-affinity transport of nickel/cobalt permeases (NiCoTs) and the potential of recombinant Escherichia coli for Ni2+ bioaccumulation. Bioresour Technol 130:69–74
Duprey A, Viviane C, Franck F, Clémence G, Yoann L, Philippe L, Fanny S, Valérie D, Agnès R, Corinne D (2014) “NiCo Buster”: engineering E. coli for fast and efficient capture of cobalt and nickel. J Biol Eng 8:19
Frišták V, Martin P, Michaela V, Juraj L, Marián R (2014a) Monitoring 60Co activity for the characterization of the sorption process of Co2+ ions in municipal activated sludge. J Radioanal Nucl Chem 299:1607–1614
Frišták V, Michaela V, Martin P, Juraj L (2014b) The Influence of chemical modification on the Co 2+ ion sorption process by anaerobic sludge. Pol J Environ Stud 23:705–712
Gadd GM, White C (1989) Heavy metal and radionuclide accumulation and toxicity in fungi and yeast. In: Poole RK, Gadd GM (eds) Metal-microbe interactions. IRI, Oxford, pp 19–38
Green SJ, Prakash O, Jasrotia P, Overholt WA, Cardenas E, Hubbard D, Tiedje JM, Watson DB, Schadt CW, Brooks SC, Kostka JE (2012) Denitrifying bacteria from the genus Rhodanobacter dominate bacterial communities in the highly contaminated subsurface of a nuclear legacy waste site. Appl Environ Microbiol 78:1039–1047
Gunjan P, Paul D, Jain RK (2005) Conceptualizing “suicidal genetically bioengineered microorganisms” for bioremediation applications. Biochem Biophys Res Commun 327:637–639
Hebbeln P, Eitinger T (2004) Heterologous production and characterization of bacterial nickel/cobalt permeases. FEMS Microbiol Lett 230:129–135
Komeda H, Kobayashi M, Shimizu S (1997) A novel transporter involved in cobalt uptake. Proc Natl Acad Sci 94:36–41
Kulkarni S, Anand B, Shree KA (2013) Bioprecipitation of uranium from alkaline waste solutions using recombinant Deinococcus radiodurans. J Hazard Mater 262:853–861
Kumar R, Singh S, Singh OV (2007) Bioremediation of radionuclides: emerging technologies. OMICS 11:295–304
Prakash D, Prashant G, Anuj K, Chandel ZR, Om VS (2013) Bioremediation: a genuine technology to remediate radionuclides from the environment. Microb Biotechnol 6:349–360
Kurnaz A, Kucukomeroglu B, Keser R, Okumusoglu NT, Korkmaz F, Karahan G, Cevik U (2007) Determination of radioactivity levels and hazards of soil and sediment samples in Firtina Valley (Rize, Turkey). Appl Radiat Isot 65:1281–1289
Lange CC, Wackett LP, Minton KW, Daly MJ (1998) Engineering a recombinant Deinococcus radiodurans for organopollutant degradation in radioactive mixed waste environments. Nat Biotechnol 16:929–933
Lejon J, Hermansson A, Bertholdt HO (1994) A full system decontamination of Oskarshamn 1 BWR. Proc Int Conf Water Chem Nucl Reactor Syst 1:203–210
Lin J, Qi R, Aston C, Jing J, Anantharaman TS, Mishra B, White O, Daly MJ, Minton KW, Venter JC, Schwartz DC (1999) Whole-genome shotgun optical mapping of Deinococcus radiodurans. Science 285:1558–1562
Liu X, Duu-Jong L (2014) Biosorption studies on bioremediation and biorecovery. J Taiwan Inst Chem Eng 45(2):1863–1864
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔ CT method. Methods 25:402–408
Lloyd JR, Renshaw JC (2005) Bioremediation of radioactive waste: radionuclide-microbe interactions in laboratory and field-scale studies. Curr Opin Biotechnol 16:254–60
Meima R, Lidstrom ME (2000) Characterization of the minimal replicon of a cryptic Deinococcus radiodurans SARK plasmid and development of versatile Escherichia coli-D. radiodurans shuttle vectors. Appl Environ Microbiol 66:3856–3867
Misra CS, Rita M, Shree KA (2014) Harnessing a radiation inducible promoter of Deinococcus radiodurans for enhanced precipitation of uranium. J Biotechnol 189:88–93
Maruthi Mohan P, Kiranmayi P, Haritha A, Premsagar K, Tiwari A, Raghu G (2007) Bioremediation of toxic metal ions: a focused view of metal transportomes. In: Chopra VL, Sharma RP, Bhat SR, Prasanna BM (eds) Search for new genes, 1st edn 14. Academic Foundation in association with the National Academy of Agricultural Science (NAAS), New Delhi, pp 231-243
Naveena Lavanya Latha J, Rashmi K, Maruthi Mohan P (2005) Cell wall bound metal ions are taken up in Neurospora crassa. Can J Microbiol 51:1021–1026
Raghu G, Balaji V, Venkateswaran G, Rodrigue A, Maruthi Mohan P (2008) Bioremediation of trace cobalt from simulated spent decontamination solutions of nuclear power reactors using E. coli expressing NiCoT genes. Appl Microbiol Biotechnol 81:571–578
Rama Rao K, Sajani LS, Maruthi Mohan P (1996) Bioaccumulation and biosorption of cobalt ions by Neurospora crassa. Biotechnol Lett 18:1205–1208
Rashmi K, Naga Sowjanya T, Maruthi Mohan P, Balaji V, Venkateswaran G (2004) Bioremediation of 60Co from simulated spent decontamination solutions. Sci Total Environ 328:1–14
Rashmi K, Haritha A, Balaji V, Tripathi VS, Venkateswaran G, Maruthi Mohan P (2007) Bioremediation of 60-Co from simulated spent decontamination solutions of nuclear power reactors by bacteria. Curr Sci 92(10):1407–1409
Rodrigue A, Effantin G, Mandrand BM (2005) Identification of rcnA (yohM), a nickel and cobalt resistance gene in Escherichia coli. J Bacteriol 187(8):2912–2916
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132(3):365–386
Satinder KB, Verma M, Surampalli RY, Misra K, Tyagi RD, Meunier N, Blais JF (2006) Bioremediation of hazardous wastes -a review. Pract Periodical Hazard Toxic Radioactive Waste Manage 10 (2): 59-72 doi: 10.1061/(ASCE)1090-025X 10:2(59)
Shih TY, Shen-Long T (2014) Simultaneous silver recovery and bactericidal bionanocomposite formation via engineered biomolecules. R Soc Chem Adv 4:40994–40998
Taylor NK (1976) Review of available data on the release, transport and deposition of corrosion products in PWR, BWR and SGHWR Systems. United Kingdom Atomic Energy Authority Report, AERE-R8164
Tišáková L, Pipíška M, Godány A, Horník M, Vidová B, Augustín J (2013) Bioaccumulation of 137Cs and 60Co by bacteria isolated from spent nuclear fuel pools. J Radioanal Nucl Chem 295:737–748
Urch SD (2013) Radiochemistry. Annual Reports Section “A”(Inorg Chem) 109: 468-483
Venkateswaran G, Dey GR, Kerkar AS, Gokhale BK, Gokhale AS, Balaji V, Kumbhar AG, Nema MK, Anantharaman K, Kumar J, Ananthan P, Kumar S, Sathe SM, Sah DN, Sanyal DN, Nath R, Sahu RK, Ramu A, Kansara HN, Muraisharan K, Save CB, Patil DP, Padmanabhan SA, Shinde RP, Pisharody NN, Upadyaya TC, Sharma BL, Katiyar SC, Wagh PM (2003) Chemical decontamination of cleanup system of unit-2 Tarapur Atomic Power Station Phase 2 Task. BARC Report No. BARC/ 2003/ 012
Won SW, Pratap K, Wei W, Areum L, Yeoung-Sang Y (2014) Biosorbents for recovery of precious metals. Bioresour Technol 160:203–212
Ybarra GR, Webb R (1999) Effects of divalent metal cations, resistance mechanisms of the cyanobacterium Synechococcus sp. strain 7942. J Hazard Subst Res 2:1–9
Acknowledgments
The authors dedicate this manuscript to late Prof P. Maruthi Mohan. The authors thank Dr. Thomas Eitinger, Humboldt University, Germany, for providing the plasmids (pCH675-RP and pCH675-NA), K. W. Minton and M. J. Daly, Uniformed Services University of the Health Sciences, Bethesda, MD, for providing the D. radiodurans R1 strain, and M. E. Lidstrom, Departments of Chemical Engineering and Microbiology, University of Washington, Seattle, for providing the E. coli-Deinococcus shuttle vector pRAD1. We thank Deepti Appukuttan, who provided technical information related to Deinococcus transformation. We thank Dr. Venkata Prasuja Nakka, Department of Biotechnology and Bioinformatics, University of Hyderabad, Dr. Abdul Qadeer Mohammed, Department of Biochemistry, Osmania University, Hyderabad for their helpful suggestions and critical evaluation of the manuscript. The research work was supported by grants from the Department of Atomic Energy (No: 2004/37/17/BRNS), IFCPAR (3709-1), UGC-SAP (DRS-II), and INSPIRE Faculty Award (DST) IFA12-LSPA-11 to Raghu Gogada.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Gogada, R., Singh, S.S., Lunavat, S.K. et al. Engineered Deinococcus radiodurans R1 with NiCoT genes for bioremoval of trace cobalt from spent decontamination solutions of nuclear power reactors. Appl Microbiol Biotechnol 99, 9203–9213 (2015). https://doi.org/10.1007/s00253-015-6761-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6761-4