Abstract
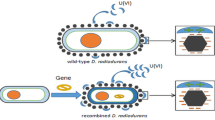
Biosorption by microorganisms is an environmentally friendly and efficient method to adsorb heavy metals and radionuclieds. The purpose of this study was to transform urease gene (Ure) from Sporosarcina pasteurii DSM33 into D.radiodurans to prepare a recombinant Deino-Ure strains. The urease was used to decompose urea to produce CO32−, promoting calcium carbonate precipitation in the presence of Ca2+. This enhances the biomineralisation and U(IV) enrichment of D. radiodurans, which provides a scientific basis for the treatment of low enriched uranium contaminated soil. During Deino-Ure mineralization of U(VI), uranium may co-precipitate with calcium, forming a stable U(VI)/U (IV)-calcite mineral precipitate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the most significant basements of human well-being, soil has been contaminated by the undue disposition of pesticides and industrial wastewater, waste gases, and residues, resulting in an increasingly severe pollution issue in the soil and gravely threatening public health and food security [1,2,3,4]. Thereinto, a hot and difficulty issue lie in the control and prevention of radionuclide pollution in soils, especially the remediation of soil in uranium mining and metallurgical areas, which urgently requires solving [5,6,7]. While the uranium in soil could be absorbed into plants by rhizomes and eventually ingested by the human body through the food chain. In addition, edaphic uranium could constantly migrate downward and spread during hydrological changes such as rainfall and irrigation, entering the groundwater cycle and causing more extensive pollution. If the diffused uranium is ingested or inhaled into the human body, it will trigger a permanent and strong internal radiation that is extremely harmful and may induce cancer [8, 9].
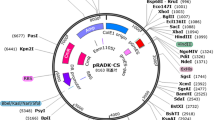
Contemporary remediation technology for contaminated soil consists of physical remediation, chemical remediation, and biological remediation [10, 11], and biomineralization is a inseparable part of biological remediation. Microbially induced carbonate precipitation (MICP), a remediation method to treat metallic contamination proposed in recent years [12,13,14], is mainly based on the decomposition of urea by urease secreted by carbonate-mineralizing bacteria to produce carbonate that could form precipitation in the presence of Ca2+ or other metal ions [15, 16]. Sporosarcina pasteurii (S. pasteurii) possesses favorable secretory ability of urease and a multitude of studies have shown that it also has satisfactory urease activity about 100 times that of soybean urease and 14 folds that of Jack bean urease [17, 18]. Thus, S. pasteurii is employed as a common strain for bio-induced calcium carbonate mineralization and has been progressively utilized in the fields of architecture, environment, and even medical treatment [19,20,21]. However, most of carbonate-mineralizing bacteria may be inherently devoid of tolerance for radionuclides, making it difficult to achieve pleasing results in practical applications. While D.radiodurans is known as the “most resistant bacterium in the world”, which can survive in a high-intensity radioactive environment, exhibiting great prospects for exploitation [22]. According to previous researches, the urease gene cluster of S. pasteurii can be successfully heterologously expressed in other hosts and obtain comparable urease activity to S. pasteurii [23]. Herein, we managed to transfer the urease gene of S. pasteurii into D.radiodurans for stable expression and link the optimized fragment to the E. coli shuttle plasmid (pRADK) to construct the pRADK-Ure plasmid vector, which was transferred into D.radiodurans in order to acquire a genetically engineered bacterium with both radiation resistance and prominent biomineralization ability, providing theoretical and experimental support for slowing down the migration of nuclear waste in the environment and the microbial treatment of uranium-containing soil.
Material and methods
Bacterial strains and growth conditions
Bacterial strains, primers and plasmids used in this study were listed in Table 1. E. coli shuttle plasmid (pRADK) was funded by Yuejin Hua group and D.radiodurans R1 was preserved in our laboratory. D.radiodurans R1 was cultured with TGY solid medium at 30 ± 1 °C and 220 rpm. TGY liquid medium was prepared with 0.5% tryptone, 0.1% glucose, and 0.3% yeast extract, while TGY solid medium was manufactured with liquid medium plus 1.5% agar.
Construction of genetically engineered D.radiodurans containing urease gene clusters
The target gene was designed according to the urease gene cluster sequence UreABCDEFGD of S. pasteurii DSM33 strain in the NCBI database. Urease expression has been shown to be enhanced by adjusting the large subunit UreC in the urease gene cluster upstream of UreA and UreB [24, 25]. Therefore, in this experiment, before the large subunit UreC gene was moved to UreA and UreB genes, the codon and ribosome binding sites of the target gene sequence were optimized by Tsingke Biotech Co., Ltd. to synthesize the whole UreCABEFGD gene (hereinafter referred to as Ure gene) [26,27,28]. The synthesized Ure gene was used as a template, and the designed precursors of Ure-F and Ure-R were added to manipulate the polymerase chain reaction in order to amplify Ure gene fragment with NdeI and BstEII enzymatic cleavage sites. coli-D. The Ure gene was cloned downstream of the strong promoter of D.radiodurans to construct the recombinant plasmid pRADK-Ure. Finally, the recombinant plasmid pRADK-Ure was transformed into D.radiodurans cells, and positive clones were screened on TGY agar medium containing 3.4 μg/ml of chloramphenicol.
Determination of urease activity and calcium carbonate yield
In this experiment, urease activity was measured by the electrical conductivity method and the indophenol blue colorimetric method. Urea is non-conductive, while the urease produced by bacteria could hydrolyze urea to generate conductive NH4+ and CO32−. The increase in conductivity of the detected solution was positively correlated to the amount of hydrolyzed urea, and the relationship between the two was as given in Eq. (1) [29]:
The produced NH4+ in the urea hydrolysis process could react with phenol-sodium hypochlorite to form indophenol, which could increase the absorbance value at 625 nm. Thus the generated NH4+ could be quantitated by measuring the degree of the chromegenic reaction in order to calculate the microbial urease activity [30]. Moreover, 1 mL of Deino-Ure bacterial solution of different concentrations was added into a mixture containing 0.5 mol/L of urea and 0.5 mol/L of CaCO3, and the calcium carbonate yield was measured in different reaction time periods. The mixed solution was filtered with paper filters, washed with ultrapure water, and dried. Subsequently, 0.1 mol/L of HCl solution was added to wash multiple times until no bubbles were formed, washed with ultrapure water, and dried, then weighed, and the calcium carbonate yield was calculated as the difference between the two readings.
Experimental design using response surface methodology
To explore the effects of different Ni2+, Ca2+, and urea concentrations on the urease activity of the recombinant strain Deino-Ure, the optimal design using the Box-Behnken response surface model was analyzed by Design-Expert 12 software. Taking the urease activity and calcium carbonate yield of the recombinant strain as the response surfaces and values, concentrations of Ni2+ (A), urea (B), and Ca2+ (C) were designed to optimize the response surface, and the optimal conditions for the urease activity and calcium carbonate yield of the recombinant deino-ure strain were calculated through simulation analysis (Table 2).
Uranium mineralization enrichment experiment
A certain amount of bacterial solution was centrifuged, and the medium was discarded. Then an equal volume of 0.9% NaCl was added to resuspend the bacteria. Subsequently, the bacterial suspension was mixed with uranium solution, urea solution, Ca2+ solution, and Ni2+ solution, and the pH of the mixture was adjusted by NaOH and HCl. Furthermore, 0.9% NaCl was added to make the final concentrations of urea, Ca2+, and Ni2+ 30 g/L, 10 g/L, and 0.5 mol/L, respectively. Finally, the uranium mineralization enrichment experiment was carried out at 30 °C and 220 rpm. After the mineralization was completed, the concentration of uranium in the centrifugal supernatant was determined by the value change of absorbance of azoarsine (III) method at 652 nm [31] to calculate the enrichment rate (R) as given in Eq. (2)
R (%) represented the enrichment rate of uranium, C0 indicated the initial concentration of uranium, and Ct served as the remaining concentration of uranium in solution after the time of uranium enrichment.
Scanning electron microscopy (SEM), Energy dispersive spectrometer (EDS), X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD) of mineralised Deino-Ure cells
30 mL of the solution before and after the mineralization experiment was transferred to a 50 mL centrifuge tube and centrifuged at 4000 rpm for 10 min. Then the supernatant was discarded, and the precipitate was dried at 65 °C for 24 h to remove water. After cooling down, the dried solids were scraped off and ground into powder in a mortar, weighed, and placed in a 1.5-mL EP tube for later SEM, EDS, XPS, and XRD analysis experiments.
Results and discussion
Identification of the recombination strain Deino-Ure
Single colony of D.radiodurans was picked from TGY solid medium plate with 3.4 μg/mL chloramphenicol and cultured. Then the plasmid was extracted for PCR experiment, and the results were shown in Fig. 1. In the first lane, the bands matching the size of the target genes were exhibited (303 bp of UreA, 381 bp of UreB, 1713 bp of UreC, 774 bp of UreD, 447 bp of UreE, 609 bp of UreF, and 636 bp of UreG, respectively), indicating that the recombinant plasmid pRADK-Ure had been successfully transformed into D.radiodurans. Then the PCR products were sequenced and compared by NCBI’s nucleic acid Blast alignment, which illustrated that the pRADK-Ure recombinant plasmid had been successfully transferred into D.radiodurans to construct a genetically engineered bacterium with radiation tolerance and the urease gene Ure.
Study on the growth and urease activity of Deino-Ure
Single colonies of wild D.radiodurans and Deino-Ure recombinant strains on TGY solid medium were selected and cultured to OD600≈1 at 30 °C in TGY liquid medium. Then bacterial solutions were transferred to fresh TGY liquid with an inoculum size of 2%. OD600 was measured every 2 h to obtain growth curves for Deino-wt and Deino-Ure, as shown in Fig. 2. The result illustrated that the growth curves of Deino-wt and Deino-Ure were highly overlapping and there was no significant difference, indicating that the Ure gene would not affect the growth of recombinant strains.
1.5 mL of different OD600 solutions were added to 13.5 mL of 0.5 M urea solution, and the conductivity of the mixture was measured at certain intervals. As elucidated in Fig. 3a, the increase in bacterial concentration could significantly accelerate the urea hydrolysis process, and the decomposition rate of urea was the fastest when the OD600 of the bacterial solution equaled 1.0. In addition, the conductivity of the four concentrations reached a peak at about 72 h, indicating that the urea in the solution had been completely decomposed at this time. The yield of CaCO3 increased with the increase in bacterial concentrations (Fig. 3b), reached a higher value within 3 days with the increase in reaction time, and then slowly and gradually increased. Therefore, increasing the concentration of bacteria at a certain concentration of urea and Ca2+ could effectively enhance the production of CaCO3.
Effects of different substrate concentrations on the growth and urease activity of recombinant bacteria Deino-Ure
Effect of Ni2+ concentration on the growth and urease activity of Deino-Ure
Urease is a metal-dependent enzyme whose active center requires the involvement of Ni2+ [32, 33]. The growth curve and urease activity of the recombinant strain Deino-Ure under different concentrations of Ni2+ were exhibited in Fig. 4. With the increase in Ni2+ concentration, the OD600 value of the recombinant bacteria and the growth rate decreased significantly, and the recombinant bacteria could hardly grow when the concentration of Ni2+ reached 1 mmol/L. However, as presented in Fig. 4b, Ni2+ played a significant role in enzyme activity, and with the increase in Ni2+ concentration, urease activity increased significantly, reaching a peak at 0.5 mmol/L of Ni2+. When Ni2+ surpassed 0.5 mmol/L, urease activity could decrease. Combined with the growth curve of Ni2+ concentration, it was inferred that the appropriate concentration of Ni2+ could be conducive to the production of active urease by Deino-Ure, while the decrease in urease activity may be due to the cytotoxicity and the inhibitory effect on the growth of recombinant bacteria caused by the overly high level of nickel [34, 35]. Therefore, 0.5 mmol/L of Ni2+ was determined to be the optimal condition for the following mineralization.
Effect of urea concentration on growth and urease activity of Deino-Ure
Urea is an essential substrate for MICP, which could provide carbon and nitrogen source for bacteria, but excessive concentration of it could also reduce the deposition efficiency of MICP. As shown in Fig. 5a, when the urea concentration exceeded a certain range, the growth of recombinant strains was inhibited, which might be attributed to a large amount of CO32− and NH4+ produced by the excessively hydrolyzed urea, which could affect the ion balance in the intracellular and extracellular environment and raise the pH, which was not conducive to the survival of bacteria. The urease activity at different urea concentrations was illustrated in Fig. 5b. The urease activity of the strain increased significantly with the increase in urea concentration, and when the urea concentration was 30 g/L, the urease activity reached a maximum value of 3.74 mmol/L hydrolyzed urea/min. Combined with the growth curve of urea concentration, 30 g/L of urea was selected as the optimal mineralization condition.
Effect of Ca2+ concentration on growth and urease activity of Deino-Ure
In the process of MICP, the concentration of Ca2+ has a great influence on the growth of strains and the precipitation amount of calcium carbonate [36], because Ca2+ continuously reacts with CO32− to form CaCO3 precipitation during the reaction, which will affect the change in conductivity, so the concentration change of NH4+–N was determined by the indigophenol blue colorimetric method to reflect the urease activity. Figure 6 presented that high concentrations of Ca2+ had a significant inhibitory effect on urease activity, attributable to the fact that Ca2+ could change the permeability of bacteria, which was embodied in the preparation of competent cells [37]. In addition, Ca2+ functioned as a second messenger in cells to promote or regulate a variety of biochemical activities [38], while excessive amounts of calcium ions will lead to intracellular calcium overload, resulting in irreversible damage to cells and ultimately apoptosis [39, 40]. Therefore, the optimal mineralization condition of Ca2+ concentration was set at 10 g/L in subsequent experiments.
Urease activity and calcium carbonate yield of recombinant strain optimized by the response surface
Urease activity
According to the 17 sets of experiments simulated by Design-Expert 12 software and the linear fitting of multiple regression from the experimental data in Table 3, the linear equation of multiple regression of urease activity was obtained as Eq. (3):
According to the results of ANOVA (Table 4), A, B, and C all showed extremely significant effects on urease activity (P < 0.01), and the single factor effect was Ca2+ > Ni2+ > urea, with F = 91.28 and P < 0.01 indicating that the regression equation model fitted well in the entire studied regression region. Moreover, the complex correlation coefficient R2 = 0.9916 inferred that the regression equation model had a good correlation, and the corrected absolute coefficient Radj2 = 0.9938 informed that 98.07% of the variability of the experimental data could be explained by this regression model. Therefore, the model of the obtained experiments was reasonable, and it could be used to analyze and predict urease activity.
With an elliptical shape, the contour shape of Fig. 7 showed vertices in both the response surface and the contour map, indicating that there were extreme values in the selected range and that the interaction between Ca2+ concentration, Ni2+ concentration, and urea concentration was significant, which was basically consistent with the results of ANOVA. Through the analysis of the response surface and contour map, the regression model was calculated, and the optimal conditions for predicting urease activity were Ca2+ = 7.86 g/L, Ni2+ = 0.54 mmol/L, and urea = 32.67 g/L, with the urease activity calculated to be 3.54 mmol/L hydrolyzed urea/min.
Effect of interaction of various factors on urease activity. a Response surface curves and contour plots of urea concentration and Ni2+ concentration on urease activity, b Response surface curves and contour plots of Ca2+ and Ni2+ concentrations on urease activity, c Response surface curves and contour plots of Ca2+ and urea concentration on urease activity
Effect of interaction of various factors on calcium carbonate yield. a Response surface curves and contour plots of urea concentration and Ni2+ concentration on calcium carbonate yield, b Response surface curves and contour plots of Ca2+ and Ni2+ concentrations on calcium carbonate yield, c Response surface curves and contour plots of Ca2+ and urea concentration on calcium carbonate yield
Calcium carbonate yield
According to Design-Expert 12 and the fitting of the experimental data in Table 3, the multiple regression linear equation of calcium carbonate yield was obtained as Eq. (4):
From the ANOVA results (Table 5), A,B,C showed significant effects on calcium carbonate yield (P<0.05), indicating that Ca2+, Ni2+, and urea concentration are important key control factors for calcium carbonate yield. By analysing the response surface and contour map of the interaction of each response factor on calcium carbonate yield (Fig. 8), and combining with the software to calculate the regression model can be predicted that the optimal conditions for urease activity are when the Ca2+ = 9.86 g/L, Ni2+ = 0.54 mmol/L, and urea = 34.65 g/L. By substituting the three factors into the regression equation, it was calculated that at this time, the calcium carbonate yield was 85.59%.
Comparison of urease activity and calcium carbonate yield under different conditions
In light of Design-Expert 12, the optimal urease activity was analyzed under the following conditions: concentrations of Ca2+, Ni2+, and urea were 7.86 g/L, 0.54 mmol/L, and 32.67 g/L, respectively. The conditions for the optimal calcium carbonate yield were 9.86 g/L of Ca2+, 0.54 mmol/L for Ni2+, and 34.65 g/L for urea. Considering the limitations of the experimental conditions, the experimental conditions of the former were adjusted to 0.5 mmol/L of Ni2+, 33 g/L of urea, and 8 g/L of Ca2+, and those of the latter were adjusted to 0.5 mmol/L, 35 g/L, and 10 g/L for Ni2+, urea, and Ca2+, respectively. Under these conditions, urease activities were 3.5 ± 0.05 mmol/L hydrolyzed urea/min and 3.5 ± 0.01 mmol/L hydrolyzed urea/min, respectively, and the calcium carbonate yields were 82 ± 1% and 85 ± 0.6%, respectively. The experimental results were close to the theoretical values, indicating that the model was reliable and suitable for the optimization of urease activity and calcium carbonate yield.
Based on the above results, 0.5 mmol/L of Ni2+, 35 g/L of urea, and 10 g/L of Ca2+, were selected as the subsequent mineralization experimental conditions (Table 4).
Effects on minerilization of uranium by Denio-Ure.
Shaking flask experiments were utilized to investigate the effects of changes in time, pH, and initial uranium concentration on the uranium mineralized by Deino-Ure, as shown in Figs. 9, 10, 11. On the first day of enrichment (Fig. 9), both Deino-wt and Deino-Ure provided extremely fast rates of enriching uranium, slowing down over time without significant change in uranium concentrations beyond the second day, possibly due to the large number of available adsorption sites on the surface of both bacteria at the beginning of enrichment to bind uranium to their surfaces. With the passage of time, the adsorption sites on the surface of Deino-wt decreased until they were completely occupied and finally reached enrichment equilibrium, with a maximum enrichment rate of only 70%. However, the Deino-Ure strain did not reach the enrichment equilibrium until the seventh day, which might be due to the fact that the urease produced by Deino-Ure continuously decomposed the urea in the solution environment to produce carbonate ions and formed calcium carbonate precipitation with Ca2+ in the solution, which promoted the continuous co-precipitation of uranium and calcium with a maximum enrichment rate of 90%.
pH could affect the mineralization process by influencing the chemical properties (charge distribution) of uranium and the urease activity of Deino-ure. The results (Fig. 10) showed that the Deino-Ure strain had a high level of uranium mineralization efficiency in the pH range of 6 ~ 9, with a maximum enrichment rate of 90%, indicating that it was more prone to mineralization under alkaline conditions, which might be attributable to the optimal pH range of bacterial urease in the pH range of 6 ~ 9, in which it could exert its best activity [41]. Meanwhile, H+ in the acidic environment could produce an acid–base neutralization reaction with carbonate and ammonia, which was not conducive to the formation of calcium carbonate crystals (Table 5).
Finally, the effect of different initial uranium concentrations on the mineralized uranium of radiation-tolerant D.radiodurans Deino-Ure was investigated, and the results were stated in Fig. 11. When the initial concentrations of uranium were 20 mg/L and 30 mg/L, the uranium mineralization efficiency was higher, which may be due to the fact that when the initial concentration of uranium was low, the solution contained fewer uranyl ions, so there was enough Ca2+ in the solution to support the continuous co-precipitation of uranium and calcium, triggering higher mineralization efficiency. With the increase in the initial concentration of uranium, the amount of Ca2+ in the solution remained unchanged, and when Ca2+ in the solution was consumed, the uranium mineralization efficiency gradually decreased with the increase in the amount of uranyl remaining in the solution. In addition, due to the radiation resistance of DR, the enrichment rate of uranium was still more than 60% when the uranium concentration was high, which had important potential value in exploring the bioaccumulation of high-concentration uranium wastewater.
SEM, EDS, XPS, and XRD analyses
Figure 12 verbalized the SEM images of the samples before and after the mineralization experiment. Before the experiment, the surface of the sample was relatively smooth and flat (a). After mineralization, the morphology changed gravely. The surface structure of the bacteria was destroyed, and clear patterns of crystals appeared on the surface after mineralization (b–c). Calcium carbonate is generally divided into three crystal forms: rhombohedral calcite, rod-shaped aragonite, and spherical vaterite. Calcite is the most stable form. As exhibited in the figure, clear crystals appeared after mineralization, among which were similar to the globular configuration of vaterite and the tetrahedral configuration of calcite.
The EDS spectra of the Deino-Ure recombinant strain before and after uranium mineralization (Fig. 13) elucidated that the recombinant Deino-Ure strain after mineralization presented obvious medium calcium and uranium peaks at a binding energy of 3 ~ 4 keV (Fig. 13b), confirming that uranium ions could enter the lattice of calcium carbonate to occupy the Ca2+ position and form (U,Ca)CO3 crystals.
In order to further study the mechanism of uranium mineralization by Deino-Ure, XRD analysis was performed. The results in Fig. 14 illustrated that the sample after mineralization of U(VI) by Deino-Ure contained Ca, U, and O (A), proving that the sample might be a combination mineral of Ca, U, and O elements. (B) is the U4f spectrum after Deino-Ure mineralized U(VI). The U4f5/2 peak appeared at 388.26 eV, and the U4f7/2 peak was exhibited at 384.37 eV. The peaks of U4f5/2 peak at 391.98 eV and U4f7/2 peak at 384.78 eV were represented as U(VI), and the peaks of U4f5/2 peak and U4f7/2 peak at 388.67 eV and 380.98 eV were represented as U(IV), elucidating that after Deino-Ure mineralized U(VI), its reaction products were coexisting substances of U(IV) and U(VI).
Finally, the composition of the Deino-Ure-enriched uranium product was analyzed by X-ray diffraction (XRD), as shown in Fig. 14c. According to the relevant data of the PDF standard card (PDF#72-1937), the characteristic peaks of calcite appeared in the sample, describing that the products after the mineralization of U(VI) by Deino-Ure mainly existed in the form of calcite, and combined with the results of SEM, EDS, and XPS analyses of the sample, which inferred that uranium might have co-precipitated with calcium during the mineralization of U(VI) at Deino-Ure, forming a stable U(VI)/U(IV)-calcite mineral precipitation.
Conclusion
In this study, a genetically engineered bacterium Deino-Ure with prominent radiation resistance and mineralization ability was successfully constructed, and the transfer of Ure gene did not affect the growth of the proposed bacterium. The urease activity and mineralization ability of Deino-Ure were successfully verified, and its urease activity was relatively outstanding under 0.5 mmol/L of Ni2+, 10 g/L of Ca2+, and 30 g/L of urea. After the experimental analysis of the response surface, the optimal concentrations for mineralization of Ni2+, Ca2+, and urea were calculated to be 0.5 mmol/L, 10 g/L, and 35 g/L, respectively. Moreover, the enrichment rate of uranium by Deino-Ure could reach 90% when the mineralization time was beyond 7 days, the pH was in the range of 6 ~ 9, and the initial concentration of uranium was 30 mg/L. During the mineralization of U(VI) by Deino-Ure, uranium might co-precipitate with calcium to form mineralized crystallization and attachment to the surface of the thallus, forming a stable U(VI)/U(IV)-calcite mineral precipitation. This study provided a new strategy for the remediation of uranium in soil, and the use of genetically engineered bacteria to mineralize uranium into calcite structures, so as to reduce the harmful and toxic effects of uranium in soil on the environment and organisms.
References
Liu S, Pan G, Zhang Y, Xu J, Ma R, Shen Z, Dong S (2019) Risk assessment of soil heavy metals associated with land use variations in the riparian zones of a typical urban river gradient. Ecotoxicol Environ Saf 181:435–444. https://doi.org/10.1016/j.ecoenv.2019.04.060
Niu Y, Chen F, Li Y, Ren B (2021) Trends and sources of heavy metal pollution in global river and lake sediments from 1970 to 2018. Rev Environ Contam Toxicol 257:1–35. https://doi.org/10.1007/398_2020_59
Dhuldhaj UP, Singh R, Singh VK (2023) Pesticide contamination in agro-ecosystems: toxicity, impacts, and bio-based management strategies. Environ Sci Pollut Res Int 30:9243–9270. https://doi.org/10.1007/s11356-022-24381-y
Qin G, Niu Z, Yu J, Li Z, Ma J, Xiang P (2021) Soil heavy metal pollution and food safety in China: effects, sources and removing technology. Chemosphere 267:129205. https://doi.org/10.1016/j.chemosphere.2020.129205
Foong SY, Cheong KY, Kong SH, Yiin CL, Yek PNY, Safdar R, Liew RK, Loh SK, Lam SS (2023) Recent progress in the production and application of biochar and its composite in environmental biodegradation. Biores Technol 387:129592. https://doi.org/10.1016/j.biortech.2023.129592
Ma M, Wang R, Xu L, Xu M, Liu S (2020) Emerging health risks and underlying toxicological mechanisms of uranium contamination: lessons from the past two decades. Environ Int 145:106107. https://doi.org/10.1016/j.envint.2020.106107
Adeola AO, Iwuozor KO, Akpomie KG, Adegoke KA, Oyedotun KO, Ighalo JO, Amaku JF, Olisah C, Conradie J (2023) Advances in the management of radioactive wastes and radionuclide contamination in environmental compartments: a review. Environ Geochem Health 45:2663–2689. https://doi.org/10.1007/s10653-022-01378-7
Yuan Y, Xiang M, Liu C, Theng BKG (2019) Chronic impact of an accidental wastewater spill from a smelter, China: a study of health risk of heavy metal(loid)s via vegetable intake. Ecotoxicol Environ Saf 182:109401. https://doi.org/10.1016/j.ecoenv.2019.109401
Cordova-Marks FM, Carson WO, Monetathchi A, Little A, Erdrich J (2022) Native and indigenous populations and gastric cancer: a worldwide review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph19095437
Cheng C, Chen L, Guo K, Xie J, Shu Y, He S, Xiao F (2022) Progress of uranium-contaminated soil bioremediation technology. J Environ Radioact 241:106773. https://doi.org/10.1016/j.jenvrad.2021.106773
Azhar U, Ahmad H, Shafqat H, Babar M, Shahzad Munir HM, Sagir M, Arif M, Hassan A, Rachmadona N, Rajendran S, Mubashir M, Khoo KS (2022) Remediation techniques for elimination of heavy metal pollutants from soil: a review. Environ Res 214:113918. https://doi.org/10.1016/j.envres.2022.113918
Rajasekar A, Wilkinson S, Moy CKS (2021) MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: a review. Environ Sci Ecotechnol 6:100096. https://doi.org/10.1016/j.ese.2021.100096
Peng D, Qiao S, Luo Y, Ma H, Zhang L, Hou S, Wu B, Xu H (2020) Performance of microbial induced carbonate precipitation for immobilizing Cd in water and soil. J Hazard Mater 400:123116. https://doi.org/10.1016/j.jhazmat.2020.123116
Khadim HJ, Ammar SH, Ebrahim SE (2019) Biomineralization based remediation of cadmium and nickel contaminated wastewater by ureolytic bacteria isolated from barn horses soil. Environ Technol Innov. https://doi.org/10.1016/j.eti.2019.100315
De Muynck W, De Belie N, Verstraete W (2010) Microbial carbonate precipitation in construction materials: a review. Ecol Eng 36:118–136. https://doi.org/10.1016/j.ecoleng.2009.02.006
Frederik H, Verstraete W (2002) Key roles of pH and calcium metabolism in microbial carbonate precipitation. Rev Environ Sci Biotechnol 1:3–7. https://doi.org/10.1023/A:1015135629155
Pei D, Liu Z, Wu W, Hu B (2021) Transcriptome analyses reveal the utilization of nitrogen sources and related metabolic mechanisms of Sporosarcina pasteurii. PLoS ONE 16:e0246818. https://doi.org/10.1371/journal.pone.0246818
Onal Okyay T, Frigi Rodrigues D (2013) High throughput colorimetric assay for rapid urease activity quantification. J Microbiol Methods 95:324–326. https://doi.org/10.1016/j.mimet.2013.09.018
Šovljanski O, Tomić A, Markov S (2022) Relationship between bacterial contribution and self-healing effect of cement-based materials. Microorganisms. https://doi.org/10.3390/microorganisms10071399
Chen HJ, Chang HL, Tang CW, Yang TY (2022) Application of biomineralization technology to self-healing of fiber-reinforced lightweight concrete after exposure to high temperatures. Materials. https://doi.org/10.3390/ma15217796
Tamaddon F, Arab D (2019) Urease covalently immobilized on cotton-derived nanocellulose-dialdehyde for urea detection and urea-based multicomponent synthesis of tetrahydro-pyrazolopyridines in water. RSC Adv 9:41893–41902. https://doi.org/10.1039/c9ra05240b
Basu B (2022) The radiophiles of Deinococcaceae family: resourceful microbes for innovative biotechnological applications. Current Res Microbial Sci 3:100153. https://doi.org/10.1016/j.crmicr.2022.100153
Liang L, Heveran C, Liu R, Gill RT, Nagarajan A, Cameron J, Hubler M, Srubar WV 3rd, Cook SM (2018) Rational control of calcium carbonate precipitation by engineered Escherichia coli. ACS Synth Biol 7:2497–2506. https://doi.org/10.1021/acssynbio.8b00194
Juminaga D, Baidoo EE, Redding-Johanson AM, Batth TS, Burd H, Mukhopadhyay A, Petzold CJ, Keasling JD (2012) Modular engineering of L-tyrosine production in Escherichia coli. Appl Environ Microbiol 78:89–98. https://doi.org/10.1128/aem.06017-11
Xia Z, Xiaoran J, Jie G, Jiahua B, Lunjie W, Yao N, Yan X (2022) Gene mining of urease and recombinant expression in Bacillus subtilis. J Food Sci Biotechnol 41:28–37. https://doi.org/10.3969/j.issn.1673-1689.2022.05.004
Liu Q, Jin X, Fang F, Li J, Du G, Kang Z (2019) Food-grade expression of an iron-containing acid urease in Bacillus subtilis. J Biotechnol 293:66–71. https://doi.org/10.1016/j.jbiotec.2019.01.012
Salis HM, Mirsky EA, Voigt CA (2009) Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol 27:946–950. https://doi.org/10.1038/nbt.1568
Chen J, Zhu R, Zhou J, Yang T, Zhang X, Xu M, Rao Z (2021) Efficient single whole-cell biotransformation for L-2-aminobutyric acid production through engineering of leucine dehydrogenase combined with expression regulation. Biores Technol 326:124665. https://doi.org/10.1016/j.biortech.2021.124665
Whiffin VS (2004) Microbial CaCO3 precipitation for the production of biocement. Murdoch University,
Weatherburn MW (1967) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
Qiuli L, Xiaoping L (2022) Spectrophotometric determination for the total uranium in high-level liquid waste. World Nucl Geosci 39:124–129. https://doi.org/10.3969/j.issn.1672-0636.2022.01.013
Alfano M, Cavazza C (2020) Structure, function, and biosynthesis of nickel-dependent enzymes. Protein Sci A Publ Protein Soc 29:1071–1089. https://doi.org/10.1002/pro.3836
Mazzei L, Musiani F, Ciurli S (2020) The structure-based reaction mechanism of urease, a nickel dependent enzyme: tale of a long debate. J Biol Inorg Chem JBIC Publ Soc Biol Inorg Chem 25:829–845. https://doi.org/10.1007/s00775-020-01808-w
Wang C, Hao L, Sun X, Yang Y, Yin Q, Li M (2022) Response mechanism of psychrotolerant Bacillus cereus D2 towards Ni (II) toxicity and involvement of amino acids in Ni (II) toxicity reduction. J Hazard Mater 430:128363. https://doi.org/10.1016/j.jhazmat.2022.128363
Bellouard M, Gasser M, Lenglet S, Gilardi F, Bararpour N, Augsburger M, Thomas A, Alvarez JC (2022) Toxicity and metabolomic impact of cobalt, chromium, and nickel exposure on HepaRG hepatocytes. Chem Res Toxicol 35:807–816. https://doi.org/10.1021/acs.chemrestox.1c00429
Okwadha GD, Li J (2010) Optimum conditions for microbial carbonate precipitation. Chemosphere 81:1143–1148. https://doi.org/10.1016/j.chemosphere.2010.09.066
Mengyao G, Jingfang W (2020) Research status on preparation and transformation of E. coli competent cell. J Hebei North Univ 36:44–48. https://doi.org/10.3969/j.issn.1673-1492.2020.08.017
Pan S, Ryu SY, Sheu SS (2011) Distinctive characteristics and functions of multiple mitochondrial Ca2+ influx mechanisms. Sci China Life Sci 54:763–769. https://doi.org/10.1007/s11427-011-4203-9
Xiao Y, Yin X, Zing M (2020) Research advance on molecular mechanism of apoptosis induced by heavy metal-associated calcium overload. Pract Prev Med 27:252–257. https://doi.org/10.3969/j.issn.1006-3110.2020.02.036
Walkon LL, Strubbe-Rivera JO, Bazil JN (2022) Calcium overload and mitochondrial metabolism. Biomolecules. https://doi.org/10.3390/biom12121891
Frankenberger WTF, Johanson JB (1982) Effect of pH on enzyme stability in soils. Soil Biol Biochem 14:433–437. https://doi.org/10.1016/0038-0717(82)90101-8
Acknowledgements
This work was supported by the Environmental Protection Research Project of Hunan Province (HBKYXM-2023021), the Independent Research Project of the China Academy of Radiation Protection (2022 Platform Project of the National Key Laboratory of Radiation Environment and Health for Environmental Protection -4), the National Natural Science Foundation of China (42377076), the Natural Science Foundation of Hunan Province (2023JJ50129), the Basic Applied Research Special Project of Hengyang (202002042158), and the Hunan Provincial Innovation Foundation for Postgraduates (QL20230242).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
We declare that no known conflict of interest that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, Y., Guo, K., Jiang, F. et al. Research on urease-modified Deinococcus radiodurans and the control mechanism of the contamination of U(VI). J Radioanal Nucl Chem 333, 4091–4106 (2024). https://doi.org/10.1007/s10967-024-09563-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09563-x