Abstract
Laryngeal squamous cell carcinoma (LSCC), one of the most common malignant solid tumors in the world, has a high rate of mortality, recurrence, and metastasis. Recombinant human arginase (rhArg) recently has been developed in arginine deprivation therapy for a number of malignant tumors. In the present study, we observed that rhArg triggered significant cytotoxicity in human laryngeal squamous cell carcinoma Tu212 cells. Meanwhile, we observed that rhArg simultaneously activated autophagic flux in Tu212 cells, which was demonstrated by the accumulation of autophagosome and light chain 3 II (LC3-II). And, we explored the role of autophagy in cytotoxicity induced by rhArg in Tu212 cells. Autophagy inhibitors including chloroquine (CQ) and bafilomycin A1 (Baf A1) enhanced cytotoxicity induced by rhArg, implying the protective role of autophagy in rhArg-treated Tu212 cells. Moreover, Akt/mTOR signaling pathway was most possibly to participate in the rhArg-induced autophagy. Meanwhile, rhArg could upregulate the phosphorylation of ERK1/2 in a time-dependent manner. Therefore, all the results illuminated the cytoprotective role of autophagy in the treatment of rhArg in laryngeal squamous carcinoma Tu212 cells and provided a potential approach for LSCC therapy by rhArg combined with autophagy inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the major threats to human health. More than 500,000 cases of HNSCC occur each year (Perl et al. 2013). Laryngeal squamous cell carcinoma (LSCC) accounts for a large proportion of HNSCC (Machiels et al. 2014). Although the diagnosis and treatment of LSCC, including surgery, chemotherapy, and radiotherapy, have been rapidly developed, 5-year overall survival rate of LSCC is still relatively low. Thus, it is imperative to find a novel therapy for LSCC.
Arginine, a conditionally essential amino acid, was known to be a requirement of cell division, wound healing, and tumor growth (Garcia-Navas et al. 2012; Wang et al. 2014b). Arginine is an integral part of ornithine cycle, and in this cycle, arginase catalyzes the conversion of arginine to ornithine and urea. Many reports indicated that arginine deficiency could serve as an effective approach for the treatment of malignant solid tumors. Recombinant human arginase (rhArg) is an arginine-degrading enzyme, which has been used as anti-cancer drug for the treatment of arginine-auxotrophic tumors, such as liver cancer, melanomas, and hepatocellular carcinomas (Cheng et al. 2005; Li et al. 2013), indicating the potential approach of rhArg for tumor therapies. Nevertheless, whether it is efficient for LSCC still remains unclear.
Autophagy is a process by which the cell eliminates damaged cellular proteins or organelles and recycles obsolete cellular constituents. Autophagy maintains cell homeostasis and integrity (Chen et al. 2014; Ding and Choi 2015). Studies indicated that autophagy can be induced when cells are faced with growth pressure such as metabolic disturbance, cellular stress, or hypoxia (Trendeleva et al. 2014; Rabinowitz and White 2010). The premier step in the process of autophagy is the formation of double membrane vesicle, known as autophagosome, which encircles cytoplasmic components. Then, the autophagosome fuses with the lysosome to form autolysosome. After digestion of the autophagosome contents by hydrolytic enzymes, the metabolites could be used for new synthesis (Hackenberg et al. 2014). Regulation of autophagy is highly related to inputs from the cellular environment (Kim et al. 2009). Autophagy mostly plays a protective role in cancer therapy in the pathogenesis process (Chen et al. 2013; Nakai et al. 2007). However, several studies reported that autophagy was associated with cancer cell death following chemotherapy in a series of cancer cells, including hepatocellular carcinoma cells (Pan et al. 2014), breast cancer cells (Shajahan-Haq et al. 2014), and ovarian cancer cells (Jin et al. 2014). The relationship between autophagy and cytotoxicity induced by rhArg in LSCC still remains unclear.
In the report, we studied whether rhArg could be a promising therapy for LSCC. First, we found that rhArg triggered cytotoxicity of LSCC Tu212 cells. Meanwhile, rhArg induced autophagy in Tu212 cells. Secondly, the critical role of autophagy in cytotoxicity induced by rhArg in Tu212 cells was studied. Interestingly, inhibiting autophagy could enhance cytotoxicity induced by rhArg, implying that autophagy played a cytoprotective character in the treatment of Tu212. Collectively, rhArg, either alone or combined with autophagy inhibitor, might be a novel promising therapy for the treatment of LSCC.
Materials and methods
Reagents and antibodies
3-(4, 5-Dimetrylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and lysosomal inhibitor chloroquine (CQ) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Rapamycin and bafilomycin A1 (Baf A1) were obtained from Sangon Biotech (Shanghai, China). Cyto-ID® was obtained from ENZO Life Sciences, Inc. (Farmingdale, NY, USA). LysoTracker® was from Invitrogen (San Diego, CA, USA). Anti-phospho-p70 S6 Kinase (Ser371) and anti-phospho-4E-BP1/2/3 (pT45) were acquired from Epitomics (Burlin-game, CA, USA). Anti-tubulin was from Proteintech Group (Chicago, IL, USA). All the other primary antibodies including the antibodies against phospho-mTOR (Ser2448), phospho-Akt (Ser473), LC3, and β-actin were obtained from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were supplied by MingRui Biotech (Shanghai, China).
Preparation of rhArg
RhArg 1 (accession number: NM_000045.3) was obtained by Escherichia coli BL21 expression system (vector: pET30a) (Huan and Xian 2008). QuantiChrom™ Arginase Assay Kit was used to measure arginase activity. The specific activity of the arginase was approximately 200 U/ml.
Tumor cells and culture conditions
Tu212 cells were obtained from Cell Bank of Xiangya Central Experiment Laboratory (Changsha, China). Cells were cultivated in RPMI-1640 (Invitrogen, San Diego, CA, USA) with supplementing 10 % (v/v) of fetal bovine serum (FBS) (Invitrogen, San Diego, CA, USA), 100 μg/ml of streptomycin, and 100 U/ml of penicillin. Cells were incubated at 37 °C in 5 % (v/v) CO2.
Cell viability assay
The cell proliferation assay was performed using 3-(4.5-dimeth ylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Cells were seeded in 96-well flat bottom plates at a density of 5 × 103 cells per well. After 24 h of incubation, serial dilutions of rhArg with or without CQ and rapamycin were added to the cultures for 48 h. Then, the cells were incubated with MTT (0.5 mg/ml) at 37 °C for 4 h. After that, the medium was carefully removed and formazan crystal was solubilized with dimethyl sulphoxide (DMSO). The absorbance was measured with microplate reader at the wavelength of 595 nm.
Western blotting analysis
Tu212 cells, growing in six-well plates, were incubated with different concentrations of rhArg. Cells were harvested and gently washed with cold phosphate-buffered saline (PBS) two times and then lysed in RIPA buffer (Beyotime Biotechnology, China) on ice for 30 min. The procedure of Western blot for the collected lysate was as described by Wang et al. (2014a).
Transmission electron microscopy
Tu212 cells were cultured with or without rhArg (0.12 U/ml) for 24 h, and Tu212 cells were collected (Li et al. 2013). After being sliced, cells were stained by uranylacetate and lead citrate. The cells were observed by JEM 1410 TEM (JEOL USA, Inc.).
Confocal microscopy
Tu212 cells were treated with or without rhArg (0.12 U/ml). Rapamycin (50 nM), an autophagy inducer, was used as a positive control. Afterward, cells were incubated with Cyto-ID (ENZO Life Science, Farmingdale, NY, USA) and/or LysoTracker (Invitrogen, San Diego, CA, USA). And, then, cells were incubated in an atmosphere of 37 °C for 30 min. According to the operating instructions of confocal microscopy (Carl Zeiss L SM710, Carl Zeiss, Germany), the stained Tu212 cells were detected.
Statistics analysis
Data were carried out for statistical analysis with IBM SPSS Statistics 19. P value <0.05 was considered to be statistically significant. The results were expressed in the form of the means ± standard deviations (SDs). Significance of differences was calculated by Student’s t test and one-way ANOVA.
Results
RhArg elicited cytotoxicity of laryngeal squamous cell carcinoma Tu212 cells in vitro
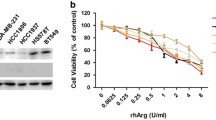
It was reported that the tumors which were deficient of ornithine transcarbamylase (OTC) or argininosuccinate synthetase (ASS) were suitable for arginine derivation therapy by rhArg (Cheng et al. 2007). In our study, we found that LSCC Tu212 cells did not express OTC protein, whereas the protein of ASS was observed in Western blot (Fig. 1a), indicating that Tu212 cells were sensitive to rhArg therapy. A549 was used as a positive control. The cytotoxic efficiency of rhArg on Tu212 cells was thus detected by MTT assay in vitro. Following exposure to different concentrations of rhArg for 48 h, we found that rhArg inhibited the growth of Tu212 cells in a dose-dependent way (Fig. 1b). RhArg could not inhibit the growth of A549 cells (Supplementary Fig. S1). Taken together, our data above suggested that rhArg could induce cytotoxicity in LSCC Tu212 cells.
Cytotoxocity induced by rhArg in Tu212 cells. a A549 and Tu212 cells were examined for OTC and ASS protein expression by Western blot analysis. A549 cells served as positive control. b Tu212 cells (5 × 103 cells/well) were incubated in the presence or absence of differrent concentrations of rhArg for 48 h. Student’s t test was used for the significance of differences (N = 3, means ± SD, *p < 0.05, **p < 0.01)
RhArg triggered an accumulation of autophagosomes in Tu212 cells
Autophagy is a stress adaptive response in eukaryotes. Previous research demonstrated that deprivation of amino acid could result in autophagy activation. In this study, we determined whether autophagy was induced in Tu212 cells following the treatment with rhArg. We observed an accumulation of autophagosomes in Tu212 cells following exposure to 0.12 U/ml of rhArg for 24 h. As shown in Fig. 2a, compared to untreated controls, Tu212 cells treated with rhArg presented many electron-dense inclusions. Upon the magnified view, they presented the double membrane structures, which were known as autophagosomes. Meanwhile, we used Cyto-ID® autophagy detection kit to verify whether autophagy was involved in rhArg-treated Tu212 cells. Compared with positive controls (rapamycin-treated Tu212 cells), as shown in Fig. 2b, intensive green fluorescence could be observed under fluorescence confocal microscopy after rhArg-treated cells (0.12 U/ml) at 24 and 48 h, whereas minimal fluorescence could be found in the untreated cells. Furthermore, immunoblotting showed LC3-II, membrane-associated autophagosome protein, significantly increased in Tu212 cells (Fig. 2c, d). Our data suggested that rhArg induced remarkable autophagy in Tu212 cells.
Autophagy was induced by rhArg in Tu212 cells. a Ultrastructural analysis of Tu212 cells either untreated or treated with rhArg (0.12 U/ml) for 24 h by TEM. The magnified view of the electron photomicrograph shows autophagosome. b Tu212 cells were untreated or treated with rhArg (0.12 U/ml) for 24 and 48 h; green fluorescence significantly enhanced could be observed by confocal fluorescence microscopy. c Tu212 cells were incubated with rhArg at 0.12 U/ml, the level of LC3-I/II was analyzed by Western blot. Tubulin was used as a protein-loading control. d Relative protein expression of LC3-II/tubulin was qualified by ImageJ software. Student’s t test was used for the significance of differences (N = 3; mean ± SD, **p < 0.01)
RhArg triggered autophagic flux in Tu212 cells
To further illustrate autophagy induced by rhArg in Tu212 cells, we assessed whether autophagic flux was triggered in Tu212 cells after rhArg treatment. Bafilomycin A1 (Baf A1), a lysosomal inhibitor, prevents the fusion of autophagosomes with lysosomes and blocks the autophagy at its maturation stage. Tu212 cells were incubated with different concentrations of rhArg for 20 h. Tu212 cells were incubated in the presence or absence of Baf A1 (40 nM) for a further 4 h. Western blot showed that the cells treated with rhArg and Baf A1 induced more expression of LC3-II compared to the cells treated with rhArg alone (Fig. 3a). We also used Cyto-ID and Lyso-Tracker to stain the Tu212 cells after cell incubation with rhArg (0.12 U/ml) for 0, 6, 12, 24, and 48 h. The enhancement of green fluorescence represented the formation of autophagosomes, which suggested an increased autophagic activity. LysoTracker marks autophagolysosomes and lysosomes. We found green fluorescence accumulation increased within 12 h and then decreased. The red fluorescence in cells represented the degradation of autophagosomes (Fig. 3b). There was a striking increase in yellow fluorescence after the treatment of rhArg for 24 h, suggesting that autophagosomes transformed to autolysosomes. All the data indicated that rhArg induced autophagic flux in Tu212 cells.
Autophagic flux was triggered by rhArg in Tu212 cells. a After incubation with rhArg at 0, 0.015, 0.03, 0.06, and 0.12 U/ml for 20 h, Tu212 cells were incubated with or without Baf A1 (40 nM) for a further 4 h. The expression of LC3-II was detected by Western blot analysis. b Representative fluorescence images of Tu212 cells co-stained with Hoechst, Cyto-ID®, and LysoTracker® after incubated with rhArg (0.12 U/ml) for 0, 6, 12, 24, and 48 h
Blocking autophagy enhanced growth inhibitory effect of rhArg in Tu212 cells
To explore the role of autophagy in the cytotoxicity induced by rhArg, we chose two autophagy inhibitors, CQ and Baf A1, which inhibit the fusion of autophagosomes with lysosomes at late stage and block LC3-II degradation. As shown in Fig. 4a, b, the combination CQ or Baf A1 with rhArg significantly enhanced the formation of LC3-II in Tu212 cells compared with the cells treated with rhArg alone. We used MTT assay to explore the role of autophagy in rhArg-induced cytotoxicity, the results revealed that inhibition of autophagy by CQ or Baf A1 potentiated rhArg-induced cytotoxicity of Tu212 cells (Fig. 4c, d). In addition, combining with autophagy inducer rapamycin did not show obvious impact on the cytotoxicity induced by rhArg in Tu212 cells (Supplementary Fig. S2). Collectively, the results revealed that autophagy played a cytoprotective role in the treatment of rhArg in Tu212 cells.
Combination with autophagy inhibitors increased cytotoxicity of Tu212 cells induced by rhArg. a, b Tu212 cells were treated with or without rhArg (0.12 U/ml), in the absence or presence of autophagy inhibitor CQ (5 μM) or Baf A1 (2.5 nM) for 24 h. Western blot analysis was employed to detect the expression of LC3. c, d Tu212 cells were treated with or without 0.12 U/ml of rhArg in the absence or presence of autophagy inhibitor CQ (5 μM) or Baf A1 (2.5 nM) for 48 h. Cell growth inhibition was analyzed by MTT assay. One-way ANOVA was used for the significance of differences (N = 3, means ± SD, *p < 0.05, **p < 0.01)
The Akt/mTOR signaling pathway participated in autophagy in rhArg-induced Tu212 cells
The PI3K/Akt/mTOR pathway is an important intracellular signaling pathway regulating autophagy in eukaryotic cells (Chang et al. 2014; Guertin and Sabatini 2007). Mammalian target of rapamycin (mTOR) exists in a phosphorylation type in the normal environment (Takeuchi et al. 2005). As shown in Fig. 5a, exposure of Tu212 cells to rhArg resulted in diminished levels of the phosphorylated form of mTOR (Ser2448) in a dose-dependent manner. The expression of LC3-II increased in a dose-dependent manner. RhArg also reduced the phosphorylation of Akt (an upstream inducer of mTOR), and 4E-BP1 and p70S6K (two downstream effectors of mTOR). Following the treatment of rhArg, the level of phosphorylated Akt was significantly downregulated. Extracellular-signal-regulated kinase 1/2 (ERK1/2) activation has been previously reported to promote cell survival (Kao et al., 2014; Ogier-Denis et al., 2000). We also found an increase of LC3-II and ERK1/2 phosphorylation in a time-dependent manner in rhArg-treated Tu212 cells (Fig. 5b). Collectively, the Akt/mTOR and ERK1/2 signaling pathways might be involved in autophagy induced by rhArg in Tu212 cells.
The Akt/mTOR signaling pathways were involved in autophagy induced by rhArg in Tu212 cells. a Tu212 cells were exposed to various concentrations of rhArg (0, 0.015, 0.03, 0.06, 0.12 U/ml) for 24 h. The expression of p-Akt, p-mTOR, p-p70S6K, p-4E-BP1, and LC3 were examined by western blot analysis. b Tu212 cells were incubated with 0.12 U/ml of rhArg for the indicated times (0, 6, 12, 24, 48 h). The expression of Erk 1/2, phospho-Erk 1/2 (Thr202/Tyr204), and LC3 was examined by Western blot analysis
Discussion
The absence of OTC or ASS as bio-markers for rhArg efficacy has previously been studied in a number of malignant solid tumors, including hepatocellular carcinoma, melanoma, and prostate cancer (Cheng et al. 2007; Hsueh et al. 2012; Lam et al. 2009). This study demonstrated for the first time that rhArg exhibited anticancer efficacy in human laryngeal carcinoma cells. It has been reported that the sensitivity of rhArg was linked to deficiency of OTC or ASS, while the sensitivity of arginine deiminase (ADI), another arginine-degrading anticancer drug, was only linked to the deficiency of ASS. ADI is in clinical phase II studies of melanoma and hepatocellular carcinoma. In this study, we found that Tu212 cells did not express OTC protein but expressed ASS protein. Simultaneously, rhArg induced cytotoxicity of Tu212 cells as determined by MTT cytotoxicity assay. Furthermore, we found that obvious cell morphology changes following treatment of rhArg, including smaller cell size and irregular shape, while control cells showed normal cell morphology.
Numerous studies showed that autophagy plays an important role in the anticancer processes, and autophagy could partly illuminate the resistance of cancer cells to therapy. We hypothesized that the regulation of rhArg-induced autophagy might increase sensitivity and improve the cytotoxicity of rhArg on LSCC. In this paper, we reported for the first time that autophagy was induced by rhArg in LSCC Tu212 cells. We used several methods to detect rhArg-induced autophagy. For example, we used confocal microscopy to observe green fluorescence which presented autophagic vacuoles. The formation of LC3-II was also detected by Western blot analysis. Moreover, the stimulation of characteristic autophagosomes was observed by transmission electron microscopy. All these results suggested that rhArg could induce autophagy in Tu212 cells.
For understanding autophagy more clearly, we investigated whether rhArg could induce autophagic flux. Three stages of autophagy were observed using confocal microscopy, including formation of autophagosomes, fusion of autophagosomes with lysosomes, and clearance of autophagosomes. When rhArg was employed in combination with Baf A1, the formation of LC3-II significantly increased. These results strongly indicated that rhArg triggered autophagic flux in Tu212 cells.
Previous investigations demonstrated that autophagy might be cytoprotective in drug resistance of cancer cells (Li et al. 2010; Li et al. 2013). However, there were also several studies reporting that autophagy might mediate a type II form of programmed cell death (Heckmann et al. 2013; Lomonaco et al. 2011). Both the autophagy inhibitors (Baf A1 and CQ) enhanced cytotoxicity in Tu212 cells. Therefore, the activation of autophagy was cytoprotective in rhArg-induced cytotoxicity in Tu212 cells.
Several studies reported that autophagy has a complex signaling transduction pathway (Zhang et al. 2014). To explore the underlying molecular mechanisms in rhArg-induced autophagy, we investigated several signaling pathways in Tu212 cells. Expression of p-Akt and p-mTOR was downregulated in Tu212 cells after rhArg treatment. Meanwhile, the phosphorylation of p70S6K and 4E-BP1 decreased. They were downstream substrates of mTOR. Furthermore, the phosphorylation of ERK1/2 was upregulated. All these results strongly indicated that the inactivation of Akt-mTOR and activation of ERK1/2 signaling pathways participated in the treatment of rhArg in Tu212 cells.
In conclusion, we provided a novel potential therapy for LSCC. We also showed the possibility of making Tu212 cells more sensitive to rhArg by combining with autophagy inhibitor. First, we observed that remarkable cytotoxicity was triggered by rhArg. Second, we found that significant autophagy was induced by rhArg. And, autophagy played a cytoprotective role in Tu212 cells. Blocking autophagy can significantly potentiate cytotoxicity induced by rhArg in Tu212 cells. Finally, Akt/mTOR signaling pathway was most possibly to take part in autophagy induced by rhArg in Tu212 cells. The report might provide a promising therapeutic strategy for LSCC.
References
Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y (2014) PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis 5:e1437
Chen ML, Yi L, Jin X, Liang XY, Zhou Y, Zhang T, Xie Q, Zhou X, Chang H, Fu YJ, Zhu JD, Zhang QY, Mi MT (2013) Resveratrol attenuates vascular endothelial inflammation by inducing autophagy through the cAMP signaling pathway. Autophagy 9:2033–2045
Chen W, Sun Y, Liu K, Sun X (2014) Autophagy: a double-edged sword for neuronal survival after cerebral ischemia. Neural Regen Res 9:1210–1216
Cheng PN, Lam TL, Lam WM, Tsui SM, Cheng AW, Lo WH, Leung YC (2007) Pegylated recombinant human arginase (rhArg-peg5,000mw) inhibits the in vitro and in vivo proliferation of human hepatocellular carcinoma through arginine depletion. Cancer Res 67:309–317
Cheng PN, Leung YC, Lo WH, Tsui SM, Lam KC (2005) Remission of hepatocellular carcinoma with arginine depletion induced by systemic release of endogenous hepatic arginase due to transhepatic arterial embolisation, augmented by high-dose insulin: arginase as a potential drug candidate for hepatocellular carcinoma. Cancer Lett 224:67–80
Ding Y, Choi ME (2015) Autophagy in diabetic nephropathy. J Endocrinol 224:R15–R30
Garcia-Navas R, Munder M, Mollinedo F (2012) Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy 8:1557–1576
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Hackenberg S, Scherzed A, Gohla A, Technau A, Froelich K, Ginzkey C, Koehler C, Burghartz M, Hagen R, Kleinsasser N (2014) Nanoparticle-induced photocatalytic head and neck squamous cell carcinoma cell death is associated with autophagy. Nanomedicine (Lond) 9:21–33
Heckmann BL, Yang X, Zhang X, Liu J (2013) The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br J Pharmacol 168:163–171
Hsueh EC, Knebel SM, Lo WH, Leung YC, Cheng PN, Hsueh CT (2012) Deprivation of arginine by recombinant human arginase in prostate cancer cells. J Hematol Oncol 5:17
Huan Y, Xian Z (2008) Expression System for Recombinant Human Arginase I. US patent: US. 2008/0138858 A1.
Jin Z, Zheng L, Xin X, Li Y, Hua T, Wu T, Wang H (2014) Upregulation of forkhead box O3 transcription is involved in C2-ceramide induced apoptosis and autophagy in ovarian cancer cells in vitro. Mol Med Rep 10:3099–3105
Kao C, Chao A, Tsai CL, Chuang WC, Huang WP, Chen GC, Lin CY, Wang TH, Wang HS, Lai CH (2014) Bortezomib enhances cancer cell death by blocking the autophagic flux through stimulating ERK phosphorylation. Cell Death Dis 5:e1510
Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ (2009) Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res 69:700–708
Lam TL, Wong GK, Chong HC, Cheng PN, Choi SC, Chow TL, Kwok SY, Poon RT, Wheatley DN, Lo WH, Leung YC (2009) Recombinant human arginase inhibits proliferation of human hepatocellular carcinoma by inducing cell cycle arrest. Cancer Lett 277:91–100
Li X, Lu Y, Pan T, Fan Z (2010) Roles of autophagy in cetuximab-mediated cancer therapy against EGFR. Autophagy 6:1066–1077
Li Y, Zhu H, Zeng X, Fan J, Qian X, Wang S, Wang Z, Sun Y, Wang X, Wang W, Ju D (2013) Suppression of autophagy enhanced growth inhibition and apoptosis of interferon-beta in human glioma cells. Mol Neurobiol 47:1000–1010
Lomonaco SL, Finniss S, Xiang C, Lee HK, Jiang W, Lemke N, Rempel SA, Mikkelsen T, Brodie C (2011) Cilengitide induces autophagy-mediated cell death in glioma cells. Neuro Oncol 13:857–865
Machiels JP, Lambrecht M, Hanin FX, Duprez T, Gregoire V, Schmitz S, Hamoir M (2014) Advances in the management of squamous cell carcinoma of the head and neck. F1000Prime Rep 6, 44
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624
Ogier-Denis E, Pattingre S, El Benna J, Codogno P (2000) Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275:39090–39095
Pan H, Wang Z, Jiang L, Sui X, You L, Shou J, Jing Z, Xie J, Ge W, Cai X, Huang W, Han W (2014) Autophagy inhibition sensitizes hepatocellular carcinoma to the multikinase inhibitor linifanib. Sci Rep 4:6683
Perl G, Ben-Aharon I, Popovtzer A, Stemmer SM, Vidal L (2013) Addition of taxane to induction therapy in head and neck malignancies: a systematic review and meta-analysis of randomized controlled trials. Chemotherapy 59:435–440
Rabinowitz JD, White E (2010) Autophagy and metabolism. Science 330:1344–1348
Shajahan-Haq AN, Cook KL, Schwartz-Roberts JL, Eltayeb AE, Demas DM, Warri AM, Facey CO, Hilakivi-Clarke LA, Clarke R (2014) MYC regulates the unfolded protein response and glucose and glutamine uptake in endocrine resistant breast cancer. Mol Cancer 13:239
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65:3336–3346
Trendeleva TA, Aliverdieva DA, Zvyagilskaya RA (2014) Mechanisms of sensing and adaptive responses to low oxygen conditions in mammals and yeasts. Biochemistry (Mosc) 79:750–760
Wang S, Li Y, Fan J, Wang Z, Zeng X, Sun Y, Song P, Ju D (2014a) The role of autophagy in the neurotoxicity of cationic PAMAM dendrimers. Biomaterials 35:7588–7597
Wang Z, Shi X, Li Y, Zeng X, Fan J, Sun Y, Xian Z, Zhang G, Wang S, Hu H, Ju D (2014b) Involvement of autophagy in recombinant human arginase-induced cell apoptosis and growth inhibition of malignant melanoma cells. Appl Microbiol Biotechnol 98:2485–2494
Zhang H, Guo M, Chen JH, Wang Z, Du XF, Liu PX, Li WH (2014) Osteopontin knockdown inhibits alphav, beta3 integrin-induced cell migration and invasion and promotes apoptosis of breast cancer cells by inducing autophagy and inactivating the PI3K/Akt/mTOR pathway. Cell Physiol Biochem 33:991–1002
Acknowledgments
The study was supported by the Biological Medicine Projects of the Shanghai Technology Commission (14431900200, 13431900303) and the key project of the Shanghai Health and Family Planning Commission (2013012).
Conflict of interest
The authors have declared no conflict of interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chen Lin, Ziyu Wang and Li Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 138 kb)
Rights and permissions
About this article
Cite this article
Lin, C., Wang, Z., Li, L. et al. The role of autophagy in the cytotoxicity induced by recombinant human arginase in laryngeal squamous cell carcinoma. Appl Microbiol Biotechnol 99, 8487–8494 (2015). https://doi.org/10.1007/s00253-015-6565-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6565-6