Abstract
Interferon-beta (IFN-β) is a cytokine with anti-viral, anti-proliferative, and immunomodulatory effects. In this study, we investigated the effects of IFN-β on the induction of autophagy and the relationships among autophagy, growth inhibition, and apoptosis induced by IFN-β in human glioma cells. We found that IFN-β induced autophagosome formation and conversion of microtubule associated protein 1 light chain 3 (LC3) protein, whereas it inhibited cell growth through caspase-dependent cell apoptosis. The Akt/mTOR signaling pathway was involved in autophagy induced by IFN-β. A dose- and time-dependent increase of p-ERK 1/2 expression was also observed in human glioma cells treated with IFN-β. Autophagy induced by IFN-β was suppressed when p-ERK1/2 was impaired by treatment with U0126. We also demonstrated that suppression of autophagy significantly enhanced growth inhibition and cell apoptosis induced by IFN-β, whereas inhibition of caspase-dependent cell apoptosis impaired autophagy induced by IFN-β. Collectively, these findings indicated that autophagy induced by IFN-β was associated with the Akt/mTOR and ERK 1/2 signaling pathways, and inhibition of autophagy could enhance the growth inhibitory effects of IFN-β and increase apoptosis in human glioma cells. Together, these findings support the possibility that autophagy inhibitors may improve IFN-β therapy for gliomas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant gliomas are the most common and lethal primary brain tumors in the central nervous system, and account for 30 % of adult primary brain tumors [1]. Even though multi-modality treatments—including surgery, radiotherapy, and chemotherapy—have been developed to improve therapeutic efficacy, over the past 30 years, no significant improvements in the survival of patients suffering from this disease have been achieved [2]. Because malignant glioma cells usually infiltrate deeply into normal tissues, and the complete removal of glioma is almost impossible, leading to a high incidence of tumor recurrence [3], the median survival time from diagnosis for those with a glioma is still only 14.5 months [4]. Therefore, the development of novel therapeutic approaches is urgently needed.
IFN-β is a type I interferon that exhibits anti-viral, immunomodulating, and anti-tumor activities. It is widely used alone or in combination with other anti-tumor agents, such as nitrosoureas and temozolomide, in the treatment of malignant gliomas [5]. Although IFN-β can inhibit growth or induce apoptosis in human glioma cells [6], its therapeutic efficacy is still limited. Increasing evidence suggests that malignant glioma cells can be resistant to chemotherapy and radiotherapy because of resistance to apoptosis [7]. Studies of multi-drug resistance in chronic lymphocytic leukemia and breast cancer cells suggested that autophagy plays a key role in tumor resistance [8, 9].
In the present investigation, we hypothesized that autophagy could partly explain the resistance of glioma cells to IFN-β therapy, and that abrogation of autophagy induced by IFN-β may restore drug sensitivity and enhance the effects of IFN-β on growth inhibition and apoptosis in human glioma cells. Autophagy is an evolutionarily conserved process that is involved in aging, neurodegenerative diseases, and cancer [10]. It is characterized by the formation of double membranes, and is often activated during cell starvation and other stresses [11]. The triggering of autophagy is mostly associated with inhibition of the mammalian target of rapamycin complex 1 (mTORC1), which causes the activation of autophagy-related proteins (Atgs) and signaling pathways [12, 13]. Though activation of the autophagy can either protect cells or initiate type II programmed cell death [14, 15], greater evidence supports the idea that autophagy is cytoprotective, particularly in cancer therapy [16–18].
In this study, we first demonstrated IFN-β-induced autophagy in U251MG and U87MG glioma cells. The Akt/mTOR and MEK 1/2 signaling pathways were involved in autophagy induced by IFN-β. Suppression of autophagy significantly enhanced growth inhibition and cell apoptosis induced by IFN-β, whereas inhibition of caspase-dependent cell apoptosis impaired autophagy induced by IFN-β. This is the first report showing that autophagy induced by IFN-β plays a critical cytoprotective role in the inhibition of glioma cell growth and cell apoptosis. This finding may contribute to the application of autophagy inhibitors for the improvement of IFN-β therapy for gliomas in clinic.
Materials and Methods
Cell Culture
The human glioma cell lines U251MG and U87MG were obtained from the Cell Bank of the Shanghai Institutes for Biological Sciences, Chinese Academy of Science (Shanghai, China). Both cells were cultured in Dulbecco's modified eagle's medium (DMEM) (Invitrogen, San Diego, CA, USA) supplemented with 10 % heat-inactivated fetal bovine serum (Invitrogen, San Diego, CA, USA), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in air containing 5 % CO2.
Reagents and Antibodies
IFN-β was purchased from Bayer Sharing Pharma AG (Leverkusen, Germany). The specific activity of the protein was 8 × 106 IU/ml. The PI3K inhibitor 3-methyladenine (3-MA) was obtained from EMD Chemicals, Inc. (San Diego, CA, USA). The lysosomal inhibitor chloroquine (CQ) was obtained from Sigma (St Louis, MO, USA). For experimental use, IFN-β, 3-MA, and CQ were prepared and diluted with DMEM. Cyto-ID Green dye was purchased from ENZO Life Sciences, Inc. (Farmingdale, NY, USA). Z-VAD-fmk was provided by Beyotime Institute of Biotechnology (Haimen, China), and the MEK1/2 inhibitor U0126 was obtained from Cell Signaling Technology (Danvers, MA, USA). The antibodies anti-LC3B, anti-caspase-3, anti-cleaved caspase-3, anti-phospho-Akt (Ser473), anti-Akt1, anti-phospho-mTOR (Ser2448), anti-mTOR, anti-phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204), anti-p44/42 MAPK (Erk1/2), anti-β-actin, and anti-tubulin were obtained from Cell Signaling Technology. Anti-p70 S6 Kinase Phospho (pS371) was purchased from Epitomics (Burlingame, CA, USA). The secondary antibodies horseradish peroxidase (HRP)-conjugated goat anti-mouse and anti-rabbit immunoglobulin G (IgG) were obtained from MR Biotech (Shanghai, China).
Cell Proliferation Assay
Cells were plated in 96-well flat bottom plates at a density of 1 × 104 cells/ml. After 24 h of incubation, different concentrations of IFN-β were added for additional time periods. The cell proliferation assay was performed using a 3-(4.5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (0.5 mg/ml). The plates were incubated in a humidified incubator at 37 °C for 4 h, then the medium was removed and formazan dye was solubilized with DMSO; the optical density (OD) was measured at an absorbance wavelength of 570 nm.
Western Blot Analysis
U251MG and U87MG cells were harvested, washed in cold phosphate-buffered saline (PBS) twice, and re-suspended in lysis buffer (Beyotime Biotechnology, China) for 30 min on ice. The lysate was then centrifuged at 13,000 × g for 5 min at 4 °C. Protein concentrations were determined by the bicinchoninic acid (BCA) method. Equivalent amounts of protein were loaded onto gels and separated by SDS–PAGE, and then electro-transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in TBST containing 5 % nonfat milk and incubated with an appropriate antibody at 4 °C overnight. After incubation with the second antibody conjugated with horseradish peroxidase, membranes were visualized using an enhanced chemiluminescent detection kit (Pierce, Rockford, IL, USA). Densitometric values of protein bands were quantified by the IQuantTL software (GE Healthcare, USA).
Transmission Electron Microscopy Analysis
U251MG and U87MG cells were fixed with 2 % glutaraldehyde in DMEM medium for 15 min, and then fixed in 2 % glutaraldehyde with 0.1 M Na cacodylate/HCl (pH 7.4) for 30 min. After extensive wash in 0.2 M Na cacodylate/HCl (pH 7.4) for three times, cells were fixed with 1 % OsO4–0.15 M Na cacodylate/HCl (pH 7.4) for 30 min. The cells were then dehydrated in an increasing gradient of ethanol and polymerized at 60 °C for 48 h [19]. Samples were prepared and analyzed with a JEM 1230 transmission electron microscope (JEOL, USA, Inc.) at 60 kV. Micrographs were taken at × 5,000 or × 10,000 magnification.
Confocal Immunofluorescence
U251MG and U87MG cells were plated in cell culture dishes with glass bottoms. After 24 h of incubation, cells were treated with 2,000 IU/ml of IFN-β for another 48 h. Positive controls were treated with the autophagy inducer rapamycin at 50 nM for 18 h. The cells were disposed with Cyto-ID® Autophagy Detection Kit according to the manufacturer’s protocol [20]. Briefly, cells were washed twice with 1× assay buffer, and then treated with Cyto-ID® Green dye and Hoechst 33342 at 37 °C for 30 min. After incubation, cells were washed with 1× assay buffer and immediately analyzed with an Olympus fluorescence microscope.
Flow Cytometry Analysis
Apoptosis was detected using the Annexin V–FITC/PI Apoptosis Detection Kit (BD Biosciences, San Diego, CA, USA). U251MG and U87MG cells were treated with IFN-β and different autophagy inhibitors for 48 h, then adherent and floating cells were harvested and washed with cold PBS, and re-suspended in 1× binding buffer at a concentration of 1 × 106 cells/ml. Cells were incubated with Annexin V–fluorescein isothiocyanate in the dark for 30 min, and then incubated with propidium iodide for 2 min and immediately analyzed by flow cytometry according to the manufacturer’s instructions. Analysis was performed using a FACSCalibur flow cytometer (Becton-Dickinson, Fullerton, CA, USA).
siRNA Transfection
U251MG and U87MG cells were seeded in 6-well plate or 96-well plate at 1×105/ml. After 24h of incubation, cells were transfected with 50nM siRNA using X-tremeGENE siRNA Transfection Reagent (Roche). Forty-eight hours post-transfection cells were treated with control medium, IFN-β for 48h as indicated, and the relevant assays performed. The siRNA used were all synthesized by Ribobio (Guangzhou, China). The sequences were as follows: ATG5a: 5'-GTGAGATATGGTTTGAATA-3'; ATG5b: 5'-GCAACTCTGGATGGGATTG-3'; ATG5c: 5'-GGAACATCACAGTACATTT-3'.
Statistical Analysis
GraphPad Prism 5 was used for all statistical analyses. All data are presented as means ± standard deviations (SD). All comparisons were performed using the Student’s t test (two-tailed), and values of P <0.05 were considered statistically significant.
Results
Autophagy Induced by IFN-β in Human Glioma Cells
To investigate the induction of autophagy by IFN-β, we first examined the morphology of U251MG and U87MG cells after exposure to 2,000 IU/ml of IFN-β for 48 h by transmission electron microscopy (TEM). As shown in Fig. 1a, cells treated with IFN-β presented numerous vacuolizations and electron-dense inclusions. Upon magnification, these inclusions were clearly double-layered membrane structures that appeared to be autophagosomes.
Autophagy induced by IFN-β in human glioma cells. a IFN-β-induced formation of autophagosomes. U251MG and U87MG cells were either untreated or treated with 2,000 IU/ml of IFN-β for 48 h. Cell samples were prepared for transmission electron microscopy analysis as described in “Materials and Methods”. A magnified view of the electron photomicrograph shows a characteristic autophagosome. b U251MG or U87MG cells were treated with 2,000 IU/ml of IFN-β for 48 h or with 500 nM of rapamycin for 12 h, and then the cells were stained with Cyto-ID® Green autophagy dye and analyzed by confocal microscopy. c IFN-β induced the accumulation of LC3-II. U251MG and U87MG cells were treated with 2,000 IU/ml of IFN-β for 48 h. Cell lysates were analyzed by Western blot. β-Actin was used as a protein-loading control. d Densitometric values of LC3-II and LC3-I were quantified using the IQuantTL software, and the data are presented as the means ± SD of three samples. *P < 0.05 vs. control
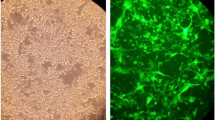
We also employed an autophagy detection kit to detect autophagy by fluorescence microscopy [20]. As shown in Fig. 1b, green fluorescence was detected in the cytoplasm of the IFN-β-treated U251MG and U87MG cells, but not in the negative control samples. Cells treated with rapamycin served as the positive controls.
In addition, we examined the expression of the microtubule-associated protein 1 light chain 3 (LC3-I) and its membrane-bound lipidated form LC3-II by Western blot analysis because LC3-II is expressed on the membranes of autophagosomes. The expression of LC3-II increased obviously in IFN-β-treated U251MG and U87MG cells (Fig. 1c), confirming the induction of autophagy by IFN-β. We also determined the values of LC3-II/LC3-I by counting relative intensities of LC3-II and LC-I, as shown in Fig. 1d, and the values of LC3-II/LC3-I were significantly higher in IFN-β-treated glioma cells (P < 0.05).
Collectively, our data indicate that IFN-β induced significant autophagy in human glioma U251MG and U87MG cells.
Suppression of Autophagy Enhanced Growth Inhibition Induced by IFN-β
To study the role of autophagy in the growth-inhibiting effects of IFN-β on human glioma cells, we used two autophagy inhibitors, 3-MA and CQ. 3-MA is a phosphatidylinositol 3- phosphate kinase inhibitor and decreases autophagosomic LC3 (LC3-II). CQ inhibits autophagy at a later stage by inhibiting fusion between the autophagosome and lysosome, which can significantly elevate the protein level of LC3-II [21, 22]. Western blot analysis showed that 3-MA inhibited autophagy induced by IFN-β in U251MG and U87MG cells, and decreased LC3-II formation. In contrast, the addition of CQ to IFN-β significantly enhanced LC3-II protein levels when compared with IFN-β alone (Fig. 2a). Compared with IFN-β treatment alone, the combination of 2,000 IU/ml of IFN-β with 1 mM of 3-MA or 5 μM of CQ significantly enhanced growth inhibition in both U251MG and U87MG cells (Fig. 2b).
Suppression of autophagy enhanced growth inhibition induced by IFN-β. a U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of the autophagy inhibitors 3-MA or CQ for 48 h. The whole protein was extracted, and LC3 was analyzed by Western blot. b U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of the autophagy inhibitors 3-MA (1 mM) or CQ (5 μM) for 48 h. Cell growth inhibition was analyzed by MTT. The data are presented as the means ± SD of four samples. *P < 0.05 vs. IFN-β. c U251MG or U87MG cells were cultured for 24 h and transfected the next day with siRNA targeting core-autophagy protein ATG5. Forty-eight hours post-transfection, control medium or 2,000 IU/ml of IFN-β was added for another 48 h. The whole cell protein was extracted, and ATG5 and LC3 were analyzed by Western blot. d U251MG or U87MG cells were cultured for 24 h and transfected the next day with siRNA targeting core-autophagy proteins ATG5. Forty-eight hours post-transfection, control medium or 2,000 IU/ml of IFN-β was added for another 48 h. The cell growth inhibition was analyzed by MTT. The data were presented as the means ± SD of four samples. *P < 0.05 vs. IFN-β
We also used siRNA transfection to inhibiting autophagy induced by IFN-β through against the key autophagy regulator ATG5. To evaluate the impact of ATG5 silencing on autophagy induced by IFN-β, we compared IFN-β (2,000 IU/ml) induced autophagy in the face of nonsilencing scrambled control (SCR) or ATG5 siRNA transfection by means of LC3 conversion. Western blot analysis showed that ATG5 protein levels were robust downregulated after ATG5 siRNA transfection compared with SCR siRNA transfection (Fig. 2c). Meanwhile, compared with SCR transfected cells treated by IFN-β, LC3-II protein levels were strongly impaired after IFN-β treatment in ATG5 depleted glioma cells (Fig. 2c).
Compared with SCR transfected glioma cells, 2,000 IU/ml of IFN-β significantly enhanced growth inhibition in U251MG and U87MG cells transfected by ATG5 siRNA (Fig. 2d).
Suppression of Autophagy Enhanced IFN-β-Induced Apoptosis
To elucidate apoptotic effects of inhibiting autophagy by IFN-β, we analyzed apoptosis using the Annexin V/PI assay with flow cytometry. As shown in Fig. 3a, the proportion of Annexin V-positive U251MG and U87MG cells was moderately increased after treated with 2,000 IU/ml of IFN-β for 96 h. Compared with cells treated with IFN-β, the proportion of Annexin V-positive cells was significantly higher in cells treated with IFN-β combined with 3-MA and CQ (Fig. 3b).
Suppression of autophagy enhanced IFN-β-induced apoptosis. a U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of 3-MA (1 mM) or CQ (5 μM), cells were stained with Annexin V/PI and analyzed by flow cytometry after 96 h, and the percentage of Annexin V-positive cells is shown in the bar charts (b), *P < 0.05 vs. control and #P < 0.05 vs. IFN-β. c U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of the autophagy inhibitors 3-MA or CQ for 48 h. The whole protein was extracted, and LC3, caspase-3, and cleaved caspase-3 were analyzed by Western blot. d U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of the pan-caspase inhibitor Z-VAD-fmk for 48 h. Whole cell protein was extracted, and LC3, caspase-3, and cleaved caspase-3 were analyzed by Western blot. e U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of pan-caspase inhibitor Z-VAD-fmk for 48 h. Cell samples were prepared for transmission electron microscopy analysis as described in “Materials and Methods”. f U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of pan-caspase inhibitor Z-VAD-fmk for 48 h; cells were stained with Cyto-ID® Green autophagy dye and analyzed by confocal microscopy
We also assessed the expression of the autophagy-related protein LC3 and the apoptosis-related protein caspase-3 after inhibition of IFN-β-induced autophagy by 3-MA or CQ. We have previously found that the expression of cleaved caspase-3 significantly increased in IFN-β-treated U251MG and U87MG cells in a dose- and time-dependent manner. In contrast, inhibition of caspase activity by Z-VAD-fmk impaired the apoptotic effects of IFN-β (data not shown). The activation of caspases plays a central role in cell apoptosis. Apoptosis is known to require activation of executioner caspases, such as caspase-3, -6, and −7, and cleaved caspase-3 is therefore a marker of apoptosis [23]. Compared with U251MG cells treated with IFN-β, more caspase-3 was cleaved in cells treated with IFN-β combined with 3-MA or CQ (Fig. 3c).
To further study the relationships between autophagy induced by IFN-β and apoptosis, we used the pan-caspase inhibitor Z-VAD-fmk to inhibit caspase-dependent apoptosis. As shown in Fig. 3d, cleaved caspase-3 notably decreased in U251MG and U87MG cells treated with IFN-β in combination with 20 μM of Z-VAD-fmk when compared with IFN-β treatment alone. The level of LC3-II was also impaired after inhibition of apoptosis, suggesting suppression of autophagy in U251MG and U87MG human glioma cells. We also examined the autophagosome formation after exposure to 20 μM of Z-VAD-fmk with or without IFN-β (2,000 IU/ml) by the TEM. As shown in Fig. 3e, cells treated with IFN-β presented numerous vacuolizations, which were similar to the results in Fig. 1a, whereas cells pretreated with Z-VAD-fmk showed less double-layered membrane structures after exposure to 2,000 IU/ml of IFN-β for 48 h. Meanwhile, we used an autophagy detection kit to detect autophagy after cells’ exposure to IFN-β with Z-VAD-fmk. Compared with IFN-β-treated glioma cells, green fluorescence was much less in the cytoplasm of IFN-β and Z-VAD-fmk-treated cells (Fig. 3f).
Taken together, these data indicate that inhibition of autophagy enhances IFN-β-induced apoptosis, and autophagy may be a response to IFN-β-induced apoptosis in human glioma cells.
Involvement of the Akt/mTOR Signaling Pathway in Autophagy Induced by IFN-β in Human Glioma Cells
The Akt/mTOR signaling pathway is one of the major pathways regulating autophagy in eukaryotic cells. This pathway plays a variety of physiological roles, including regulation of cell growth and cell survival [24, 25]. AKT1 and mTOR signaling molecules are also major regulators of autophagy [26, 27]. As shown in Fig. 4, treatment with IFN-β decreased phosphorylation (at Ser473) of the Akt protein in U251MG cells in a dose- and time-dependent manner. Similar responses were observed in U87MG cells.
The Akt/mTOR signaling pathway was involved in autophagy induced by IFN-β in human glioma cells. a IFN-β inhibited the Akt/mTOR signaling pathway in a dose-dependent manner. U251MG or U87MG cells were treated with different concentrations of IFN-β for 48 h. Changes in LC3, pAkt, pmTOR, and pP70S6K were examined by Western blot. b IFN-β inhibited the Akt/mTOR signaling pathway in a time-dependent manner. U251MG or U87MG cells were treated with 2,000 IU/ml of IFN-β from 24 h to 96 h. Whole cell protein was extracted, and changes in LC3, pAkt, pmTOR, and pP70S6K were examined by Western blot
We also investigated the effects of IFN-β treatment on mTOR activity in glioma cells. Exposure of U251MG and U87MG cells to IFN-β resulted in diminished levels of the phosphorylated form of mTOR (Ser2448) in a dose- and time-dependent manner. IFN-β treatment also decreased phosphorylation of the mTOR targets p70 ribosomal protein S6 kinase, revealing a potent inhibitory effect of IFN-β treatment on the Akt/mTOR signaling pathway. The basal levels of AKT1 and MTOR phosphorylation decreased along the U251MG and U87MG cells treated by IFN-β in a dose- and time-dependent manner (Fig. 4). Because AKT1 and MTOR act as major autophagy inhibitors, their decreased activity leads to an increase in autophagy.
ERK 1/2 Signaling Pathway was Activated by Autophagy Induced by IFN-β in Human Glioma Cells
Extracellular signal-regulated kinase 1/2 (ERK1/2, MAPK1/3) activation has been previously reported to contribute to autophagy effects that promote cell survival [28, 29]; furthermore, direct ERK activation by overexpression of constitutively active MEK promotes autophagy even in the absence of other stimuli [30]. In this study, we detected an increase in ERK1/2 phosphorylation when U251MG and U87MG cells were incubated in 2,000 IU/ml of IFN-β for 24 h, 48 h, 72 h, and 96 h or in 125 IU/ml, 250 IU/ml, 500 IU/ml, 1,000 IU/ml, and 2,000 IU/ml of IFN-β for 48 h (Fig. 5a, b).
The ERK 1/2 signaling pathway was activated during autophagy induced by IFN-β. a IFN-β induced ERK 1/2 phosphorylation in a dose-dependent manner. U251MG or U87MG cells were treated with different concentrations of IFN-β for 48 h. Whole cell protein was extracted, and Erk 1/2 and phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204) were analyzed by Western blot. b IFN-β induced ERK 1/2 phosphorylation in a time-dependent manner. U251MG and U87MG cells were treated with or without 2,000 IU/ml of IFN-β from 24 h to 96 h. Whole cell protein was extracted, and Erk 1/2 and phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204) were analyzed by Western blot. c Inhibition of MEK 1/2 signaling blocked autophagy induced by IFN-β. U251MG or U87MG cells were incubated with or without 2,000 IU/ml of IFN-β in the presence or absence of the MAPK 1/3 signaling pathway inhibitor U0126 (20 μM) for 48 h. Whole protein was extracted, conversion of LC3-I to LC3-II, Erk 1/2, and phospho-p44/42 MAPK (Erk 1/2) (Thr202/Tyr204) was determined by Western blot
To further investigate the role of ERK1/2 in autophagy induced by IFN-β, we used U0126, an inhibitor of both MEK1 and MEK2, to block the phosphorylation of ERK 1/2. U251MG and U87MG cells were pretreated with 20 μM of U0126 for 2 h, and then incubated with 2,000 IU/ml of IFN-β for 48 h. Western blot analysis showed that phosphorylation of ERK1/2 was inhibited by U0126. Inhibition of phosphorylation of ERK1/2 significantly decreased the protein level of LC3-II, indicating that inhibition of ERK 1/2 signaling blocked autophagy induced by IFN-β (Fig. 5c).
Together, our experiments indicate that the ERK 1/2 signaling pathway is involved in autophagy induced by IFN-β.
Discussion
Previous studies demonstrated that IFN-β could reduce proliferative activity in numerous cell lines, including glioma, lung cancer, pancreatic cancer, and prostatic tumors [3, 31–33]. In this study, we studied the effects of IFN-β on the induction of autophagy and the relationships among autophagy, growth inhibition, and apoptosis induced by IFN-β in U251MG and U87MG human glioma cells. The major novel findings in this study are that IFN-β induced significant autophagy in U251MG and U87MG human glioma cells, the Akt/mTOR and ERK 1/2 signaling pathways were involved in the autophagy induced by IFN-β, and the inhibition of autophagy significantly enhanced growth inhibition and apoptosis induced by IFN-β.
IFN-β is a type-I interferon exhibiting anti-virus, anti-tumor, and immunomodulatory effects, and it is widely used for treating multiple sclerosis [34]. It is also one of the most commonly used cytokines for the treatment for malignant gliomas [35], although IFN-β can inhibit growth and induce apoptosis in human glioma cells when given alone or when combined with other anti-tumor agents. However, apoptosis-resistant glioma cells limit its therapeutic potential [6]. Increasing evidence shows that autophagy plays a key role in the multi-drug resistance of tumor cells [36, 37]. In the present investigation, we hypothesized that autophagy may be the cause of glioma cell resistance to IFN-β therapy, and that abrogation of autophagy induced by IFN-β may restore drug sensitivity. IFN-β has been found to induce autophagy in hepatitis C virus-infected hepatocytes and pancreatic cancer cells [32, 38, 39]. Previous investigations have demonstrated that activation of autophagy can either be cytoprotective or mediate a type II form of programmed cell death [40, 41]. In this study, we first reported that autophagy was activated in U251MG and U87MG cells after IFN-β treatment. Some experimental procedures were undertaken to verify the presence of autophagy, including autophagosome formation that was visualized on a transmission electron microscopy in IFN-β-treated cells and fluorescent staining for LC3 protein by an autophagy detection kit. Moreover, autophagy induced by IFN-β in U251MG and U87MG cells affected the formation of LC3-II protein in a dose- and time-dependent manner. Together, these data indicate that autophagy is induced by IFN-β in human glioma cells.
Signaling through the PI3K/Akt/mTOR pathway controls proliferation and apoptosis of cancer cells [25], whereas mTOR, PI3K, and AMPK directly regulate components of the autophagic machinery [42]. In this study, we showed that constitutive phosphorylation of Akt in U251MG and U87MG cells was decreased by IFN-β treatment in a dose- and time-dependent manner. We also noticed decreased phosphorylation of mTOR and its target p70 ribosomal protein S6 kinase. Because AKT1 and MTOR act as major autophagy inhibitors, their reduced activity leads to an increase in autophagy. It has been shown that the activation of the MAPK signaling pathway, which includes ERK 1/2, is involved in the formation of autophagy [43]. As shown in Fig. 5, the appearance of the LC3-II protein was correlated with the activation of ERK 1/2 phosphorylation. Furthermore, the levels of the LC3-II protein in IFN-β treated cells were lower after inhibition of ERK 1/2 phosphorylation with the specific MEK 1/2 inhibitor U0126, suggesting that the MEK/ERK signaling pathway is involved in IFN-β-induced autophagy.
It has been demonstrated that activation of autophagy can either be cytoprotective or mediate a type II form of programmed cell death in cancer chemotherapy, immunotherapy, and radiotherapy [15]. To explore the role of autophagy in the growth-inhibiting and apoptotic effects of IFN-β, we used two autophagy inhibitors, the PI3K inhibitor 3-MA and the autophagolysosome inhibitor CQ, to block IFN-β-induced autophagy. We found that IFN-β-induced cell growth inhibition was significantly enhanced by combined treatment with the autophagy inhibitors 3-MA and CQ, indicating that autophagy played a cytoprotective role in IFN-β-treated glioma cells. To further investigate the relationships between autophagy and apoptosis induced by IFN-β, we used FCM to detect cell apoptosis. Our data showed that inhibition of autophagy enhanced IFN-β-induced cell apoptosis, as reflected by an increased percentage of Annexin V-positive cells and caspase-3 cleavage, whereas 3-MA or CQ alone had little effect on glioma cells. These findings confirm the cytoprotective role of autophagy induced by IFN-β. We also found that inhibition of caspase-dependent apoptosis using the pan-caspase inhibitor Z-VAD-fmk reduced the level of protein LC3-II, indicating that autophagy induced after IFN-β treatment occurs as a response to IFN-β-induced apoptosis.
In summary, we found that significant autophagy was induced in glioma cells treated by IFN-β, and this autophagy played a cytoprotective role. Inhibition of autophagy by the autophagy inhibitors 3-MA and CQ can significantly enhance growth inhibition and cell apoptosis in glioma cells. This study provided a new strategy to enhance the efficacy of IFN-β for cancer treatment and may encourage the development of an autophagy inhibitor to improve IFN-β treatment for glioma.
References
Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, Yoshida J (2005) IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res 65(17):7573–7579. doi:10.1158/0008-5472.CAN-05-0036
Stewart LA (2002) Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 359(9311):1011–1018
Okazaki T, Kageji T, Kuwayama K, Kitazato KT, Mure H, Hara K, Morigaki R, Mizobuchi Y, Matsuzaki K, Nagahiro S (2012) Up-regulation of endogenous PML induced by a combination of interferon-beta and temozolomide enhances p73/YAP-mediated apoptosis in glioblastoma. Cancer Lett 323(2):199–207. doi:10.1016/j.canlet.2012.04.013
Auffinger B, Thaci B, Nigam P, Rincon E, Cheng Y, Lesniak MS (2012) New therapeutic approaches for malignant glioma: in search of the Rosetta stone. F1000 Med Rep 4:18. doi:10.3410/M4-18
Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, Ito M, Yoshida J (2008) A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol 61(4):653–659. doi:10.1007/s00280-007-0520-x
Saito R, Mizuno M, Hatano M, Kumabe T, Yoshimoto T, Yoshida J (2004) Two different mechanisms of apoptosis resistance observed in interferon-beta induced apoptosis of human glioma cells. J Neurooncol 67(3):273–280
Yoshino A, Ogino A, Yachi K, Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E (2009) Effect of IFN-beta on human glioma cell lines with temozolomide resistance. Int J Oncol 35(1):139–148
Mahoney E, Lucas DM, Gupta SV, Wagner AJ, Herman SE, Smith LL, Yeh YY, Andritsos L, Jones JA, Flynn JM, Blum KA, Zhang X, Lehman A, Kong H, Gurcan M, Grever MR, Johnson AJ, Byrd JC (2012) ER stress and autophagy: new discoveries in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Blood 120(6):1262–1273. doi:10.1182/blood-2011-12-400184
Zou Z, Yuan Z, Zhang Q, Long Z, Chen J, Tang Z, Zhu Y, Chen S, Xu J, Yan M, Wang J, Liu Q (2012) Aurora kinase A inhibition-induced autophagy triggers drug resistance in breast cancer cells. Autophagy 8(12):1798–810. doi:10.4161/auto.22110
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132(1):27–42. doi:10.1016/j.cell.2007.12.018
Mizushima N (2007) Autophagy: process and function. Genes Dev 21(22):2861–2873. doi:10.1101/gad.1599207
Corradetti MN, Guan KL (2006) Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25(48):6347–6360. doi:10.1038/sj.onc.1209885
He C, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. doi:10.1146/annurev-genet-102808-114910
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. doi:10.1126/science.1099993
White E, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15(17):5308–5316. doi:10.1158/1078-0432.CCR-07-5023
Kroemer G, Levine B (2008) Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol 9(12):1004–1010. doi:10.1038/nrm2529
Madeo F, Tavernarakis N, Kroemer G (2010) Can autophagy promote longevity? Nat Cell Biol 12(9):842–846. doi:10.1038/ncb0910-842
Marino G, Madeo F, Kroemer G (2011) Autophagy for tissue homeostasis and neuroprotection. Curr Opin Cell Biol 23(2):198–206. doi:10.1016/j.ceb.2010.10.001
Michallet AS, Mondiere P, Taillardet M, Leverrier Y, Genestier L, Defrance T (2011) Compromising the unfolded protein response induces autophagy-mediated cell death in multiple myeloma cells. PLoS One 6(10):e25820. doi:10.1371/journal.pone.0025820
Chan LL, Shen D, Wilkinson AR, Patton W, Lai N, Chan E, Kuksin D, Lin B, Qiu J (2012) A novel image-based cytometry method for autophagy detection in living cells. Autophagy 8(9):1371–1382. doi:10.4161/auto.21028
Seglen PO, Gordon PB (1982) 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A 79(6):1889–1892
Pliyev BK, Menshikov M (2012) Differential effects of the autophagy inhibitors 3-methyladenine and chloroquine on spontaneous and TNF-alpha-induced neutrophil apoptosis. Apoptosis 17(10):1050–1065. doi:10.1007/s10495-012-0738-x
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14(1):32–43. doi:10.1038/sj.cdd.4402060
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12(1):9–22. doi:10.1016/j.ccr.2007.05.008
Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S (2005) Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res 65(8):3336–3346. doi:10.1158/0008-5472.CAN-04-3640
Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett 584(7):1287–1295. doi:10.1016/j.febslet.2010.01.017
Ogier-Denis E, Pattingre S, El Benna J, Codogno P (2000) Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem 275(50):39090–39095. doi:10.1074/jbc.M006198200
Pattingre S, Bauvy C, Codogno P (2003) Amino acids interfere with the ERK1/2-dependent control of macroautophagy by controlling the activation of Raf-1 in human colon cancer HT-29 cells. J Biol Chem 278(19):16667–16674. doi:10.1074/jbc.M210998200
Cagnol S, Chambard JC (2010) ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J 277(1):2–21. doi:10.1111/j.1742-4658.2009.07366.x
Park MY, Kim DR, Jung HW, Yoon HI, Lee JH, Lee CT (2010) Genetic immunotherapy of lung cancer using conditionally replicating adenovirus and adenovirus-interferon-beta. Cancer Gene Ther 17(5):356–364. doi:10.1038/cgt.2009.78
Vitale G, Zappavigna S, Marra M, Dicitore A, Meschini S, Condello M, Arancia G, Castiglioni S, Maroni P, Bendinelli P, Piccoletti R, van Koetsveld PM, Cavagnini F, Budillon A, Abbruzzese A, Hofland LJ, Caraglia M (2012) The PPAR-gamma agonist troglitazone antagonizes survival pathways induced by STAT-3 in recombinant interferon-beta treated pancreatic cancer cells. Biotechnol Adv 30(1):169–184. doi:10.1016/j.biotechadv.2011.08.001
Olson MV, Lee J, Zhang F, Wang A, Dong Z (2006) Inducible nitric oxide synthase activity is essential for inhibition of prostatic tumor growth by interferon-beta gene therapy. Cancer Gene Ther 13(7):676–685. doi:10.1038/sj.cgt.7700941
Paty DW, Li DK (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 43(4):662–667
Ruotsalainen J, Martikainen M, Niittykoski M, Huhtala T, Aaltonen T, Heikkila J, Bell J, Vaha-Koskela M, Hinkkanen A (2012) Interferon-beta sensitivity of tumor cells correlates with poor response to VA7 virotherapy in mouse glioma models. Mol Ther 20(8):1529–1539. doi:10.1038/mt.2012.53
Paillas S, Causse A, Marzi L, de Medina P, Poirot M, Denis V, Vezzio-Vie N, Espert L, Arzouk H, Coquelle A, Martineau P, Del Rio M, Pattingre S, Gongora C (2012) MAPK14/p38alpha confers irinotecan resistance to TP53-defective cells by inducing survival autophagy. Autophagy 8(7):1098–112. doi:10.4161/auto.20268
Kong D, Ma S, Liang B, Yi H, Zhao Y, Xin R, Cui L, Jia L, Liu X (2012) The different regulatory effects of p53 status on multidrug resistance are determined by autophagy in ovarian cancer cells. Biomed Pharmacother 66(4):271–278. doi:10.1016/j.biopha.2011.12.002
Sun J, Desai MM, Soong L, Ou JH (2011) IFN-alpha/beta and autophagy: tug-of-war between HCV and the host. Autophagy 7(11):1394–1396. doi:10.4161/auto.7.11.17514
Desai MM, Gong B, Chan T, Davey RA, Soong L, Kolokoltsov AA, Sun J (2011) Differential, type I interferon-mediated autophagic trafficking of hepatitis C virus proteins in mouse liver. Gastroenterology 141(2):674–685. doi:10.1053/j.gastro.2011.04.060, 685 e671-676
Liang C (2010) Negative regulation of autophagy. Cell Death Differ 17(12):1807–1815. doi:10.1038/cdd.2010.115
Lomonaco SL, Finniss S, Xiang C, Lee HK, Jiang W, Lemke N, Rempel SA, Mikkelsen T, Brodie C (2011) Cilengitide induces autophagy-mediated cell death in glioma cells. Neuro Oncol 13(8):857–865. doi:10.1093/neuonc/nor073
Kondo Y, Kanzawa T, Sawaya R, Kondo S (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5(9):726–734. doi:10.1038/nrc1692
Sivaprasad U, Basu A (2008) Inhibition of ERK attenuates autophagy and potentiates tumour necrosis factor-alpha-induced cell death in MCF-7 cells. J Cell Mol Med 12(4):1265–1271. doi:10.1111/j.1582-4934.2008.00282.x
Acknowledgments
This work was supported by Shanghai Science and Technology Funds (11431920104, 09XD1421800), National Science and Technology Major Project for Drug Discovery of Ministry of Science and Technology of China (2011ZX09102-001-27), and the National Key Basic Research Program of China (2013CB932502).
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y.L. and H.Z. contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, Y., Zhu, H., Zeng, X. et al. Suppression of Autophagy Enhanced Growth Inhibition and Apoptosis of Interferon-β in Human Glioma Cells. Mol Neurobiol 47, 1000–1010 (2013). https://doi.org/10.1007/s12035-013-8403-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8403-0